Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Laure-Alix Clerbaux | -- | 2137 | 2022-10-03 11:18:53 | | | |
| 2 | Beatrix Zheng | + 3 word(s) | 2140 | 2022-10-04 16:03:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Clerbaux, L.; Fillipovska, J.; Muñoz, A.; Petrillo, M.; Coecke, S.; Amorim, M.; Grenga, L. Central Role of Gut Microbiota in COVID-19. Encyclopedia. Available online: https://encyclopedia.pub/entry/28229 (accessed on 07 February 2026).
Clerbaux L, Fillipovska J, Muñoz A, Petrillo M, Coecke S, Amorim M, et al. Central Role of Gut Microbiota in COVID-19. Encyclopedia. Available at: https://encyclopedia.pub/entry/28229. Accessed February 07, 2026.
Clerbaux, Laure-Alix, Julija Fillipovska, Amalia Muñoz, Mauro Petrillo, Sandra Coecke, Maria-Joao Amorim, Lucia Grenga. "Central Role of Gut Microbiota in COVID-19" Encyclopedia, https://encyclopedia.pub/entry/28229 (accessed February 07, 2026).
Clerbaux, L., Fillipovska, J., Muñoz, A., Petrillo, M., Coecke, S., Amorim, M., & Grenga, L. (2022, October 03). Central Role of Gut Microbiota in COVID-19. In Encyclopedia. https://encyclopedia.pub/entry/28229
Clerbaux, Laure-Alix, et al. "Central Role of Gut Microbiota in COVID-19." Encyclopedia. Web. 03 October, 2022.
Copy Citation
Alteration in gut microbiota has been observed in COVID-19 patients. However, the underlying mechanisms remain poorly understood. Here, the researchers outlined three potential interconnected pathways leading to gut dysbiosis as an adverse outcome following SARS-CoV-2 presence in the gastrointestinal tract.
SARS-CoV-2 infection
COVID-19
gut dysbiosis
microbiota
gastrointestinal disorders
1. Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is still a global public health emergency. A better understanding of the mechanisms underlying the progression and severity of the disease is needed. Particularly, COVID-19 is markedly heterogeneous in terms of clinical outcomes, with a high variation at the individual level. Poor clinical outcomes in COVID-19 patients were notably associated with elderliness and certain pre-existing medical conditions, including but not limited to diabetes, cardiovascular diseases, obesity, and high LDH levels [1][2][3][4][5]. Older age and the comorbidities mentioned above are associated with alterations in the gut microbiota [6][7][8]. Besides, COVID-19 patients exhibit fecal microbiome alterations compared to controls [9][10][11][12]. These changes correlated to COVID-19 severity [12]. Gut dysbiosis, defined as a reduction in gut microbiota diversity or the depletion of beneficial bacteria with an enrichment of the pathogenic ones, may alter susceptibility to SARS-CoV-2 infection [13][14][15]. This is aligned with the evidence that many pathophysiological dimensions of diseases are underpinned by the gut microbiota, especially in chronic inflammatory diseases [16] such as inflammatory bowel diseases (IBD). Although the exact etiologies of IBD remain uncertain, many studies have provided important insights into the central role of gut dysbiosis and barrier dysfunction in inflammatory status [17][18]. The gut microbiota plays an essential role in the education and functions of both the local and systemic immune systems. Besides, emerging evidence has demonstrated important cross-talks between the gut microbiota and many other organs via communication axes such as the gut–lung [19], gut–liver [20][21], and gut–brain [22] axes. Notably, gut dysbiosis during respiratory viral infection has been shown to worsen pulmonary symptoms [23]. Similarly, gut dysbiosis and disrupted intestinal barrier can cause neurological inflammation [22] or hepatic inflammation through the translocation of endotoxins and bacteria via the portal vein [24]. Consistently, taking into account gut microbiome-mediated mechanisms may help depict a comprehensive overview of COVID-19 pathogenesis. Exploring how gut dysbiosis as a pre-existing condition in some COVID-19 patients mechanistically influences the disease progression and impacts the clinical outcomes might help identify high-risk patients, and has been discussed elsewhere [5]. Here, the researchers aim to investigate how SARS-CoV-2 might directly alter the gut microbiota, thus considering gut dysbiosis as a direct consequence of the virus in the gastrointestinal (GI) tract. Recently, animal studies have provided evidence for a direct impact of SARS-CoV-2 infection on the gut microbiota. A study conducted in transgenic mice expressing human ACE2 showed that the gut microbiome is affected by SARS-CoV-2 in a dose-dependent manner after intranasal inoculation [25]. In Syrian hamsters, SARS-CoV-2 infection was associated with mild intestinal inflammation, relative alteration of the intestinal barrier property, and alteration of the gut microbiota [26]. SARS-CoV-2 infection in nonhuman primates was associated with changes in the gut microbiota composition and functional activity [27]. However, despite the dynamic research, the underlying pathways leading to gut dysbiosis in COVID-19 are still poorly understood.
To contribute to deciphering these mechanisms, the Joint Research Centre of the European Commission initiated an interdisciplinary project, the CIAO project, to model the pathogenesis of COVID-19 using the Adverse Outcome Pathway (AOP) framework [28][29][30][31]. The AOP approach is well established in regulatory toxicology [32] but is innovatively applied here to a viral disease of high societal relevance. The project relies on the assumption that an AOP-driven organization of the relevant knowledge will improve the integration of the tsunami of data on COVID-19 [28]. The AOP approach does not capture all the details in a biological pathway, but aims for a pragmatic identification of successively linked key events (KE) that represent essential steps in a pathway leading to an adverse outcome [33][34][35][36]. A key event describes a measurable and essential change in a biological system that can be quantified in experimental or clinical settings [32]. The AOP framework also provides a structured approach for the evaluation of the level of evidence currently available to ascertain the causal relationships between pairs of successive key events [37]. AOPs do not build on the correlation between two events but gather and weigh the evidence for their causal relationship. Because of this mechanistic and causal description of the pathways, AOPs help elucidate the pathophysiological mechanisms also by learning from other diseases, such as IBD or respiratory virus-related diseases presenting gut dysbiosis. Finally, an AOP integrates knowledge across the different biological levels (from molecular, cellular, tissue, organ, and up to organism level). While research tends to compartmentalize in silos, this pandemic calls for an interdisciplinary integration of data from the different experimental systems. Hence, the AOP approach allows the structured review and organization of rapidly growing relevant in vitro, in vivo, and clinical data. Assessing the evidence currently available using the AOP framework permits the identification of critical inconsistencies and knowledge gaps guiding future research needs. The AOPs are steered by the Organization for Economic Co-operation and Development (OECD), which maintains a centralized online platform called AOP wiki (https://aopwiki.org/ accessed on 29 June 2022), where information captured in AOPs is openly accessible. Numbers in the text refer to these AOP-wiki pages (Table 1).
Table 1. AOP-wiki pages.
| KER1739 | https://aopwiki.org/events/1739 | accessed on 29 June 2022 |
| KER1738 | https://aopwiki.org/events/1738 | accessed on 29 June 2022 |
| KER1847 | https://aopwiki.org/events/1847 | accessed on 29 June 2022 |
| KER1901 | https://aopwiki.org/events/1901 | accessed on 29 June 2022 |
| KER1493 | https://aopwiki.org/events/1493 | accessed on 29 June 2022 |
| KER1497 | https://aopwiki.org/events/1497 | accessed on 29 June 2022 |
| KER1954 | https://aopwiki.org/events/1954 | accessed on 29 June 2022 |
| KER2311 | https://aopwiki.org/events/2311 | accessed on 29 June 2022 |
2. Central Role of Gut Microbiota in COVID-19 and Potential Modulation
The three above proposed pathways leading to an alteration of gut microbiota following SARS-CoV-2 presence in the gut lumen are non-mutually exclusive but rather interconnected (Figure 1).
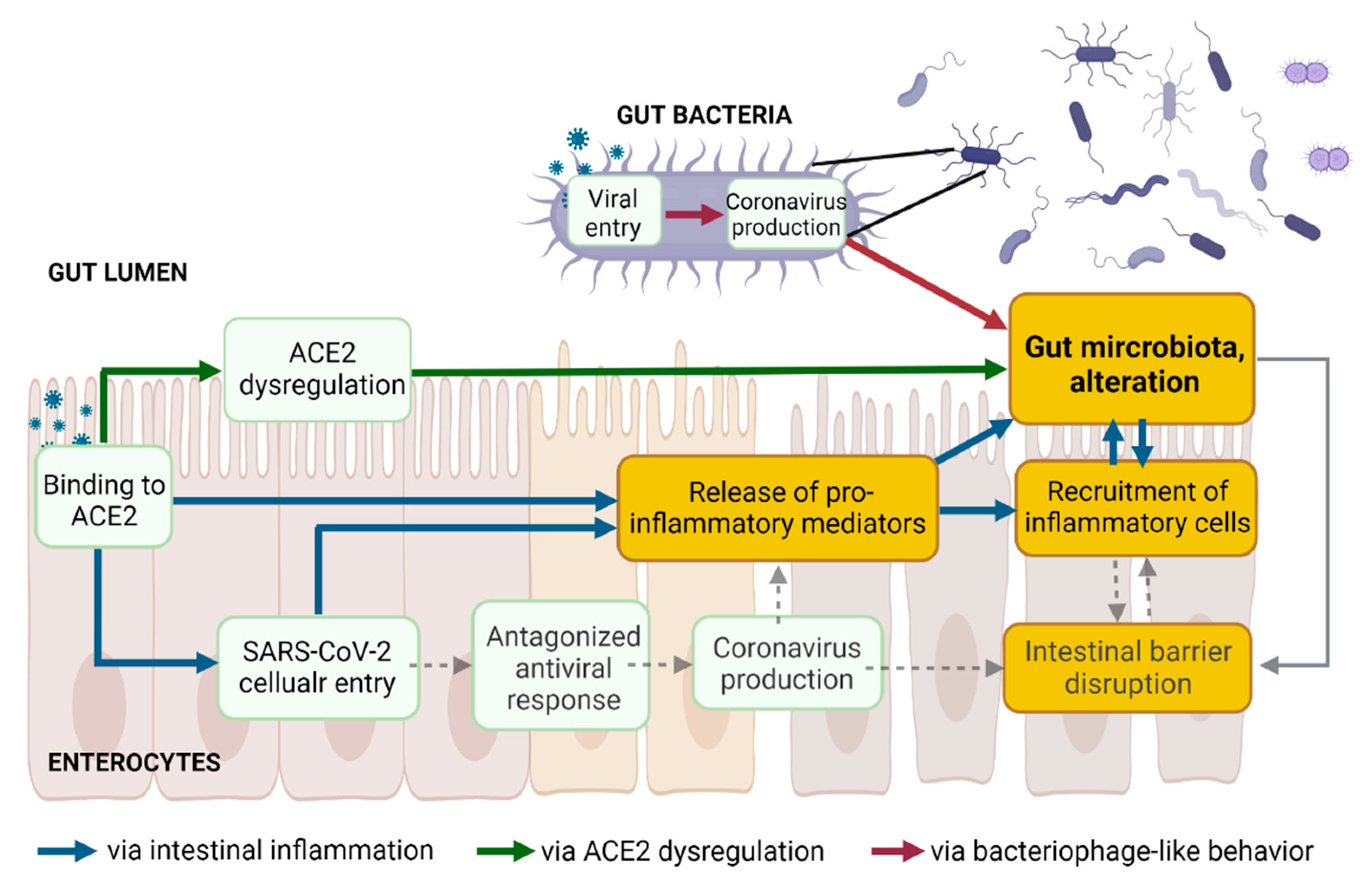
Figure 1. The three proposed pathways leading to gut dysbiosis following SARS-CoV-2 presence in the gut lumen are not mutually exclusive but might be interconnected. Created with Biorender.com.
2.1. Gut Microbiota and Intestinal Barrier Integrity in COVID-19
Together with the mucosal barrier and the cellular immune system, the intestinal epithelial cell monolayer and the tight junction proteins act simultaneously as a physical barrier against harmful external substances, as well as a selective barrier. Increased intestinal permeability, a sign of an impaired barrier function, enhances the translocation of gut bacteria and bacterial toxins from the intestinal lumen into the systemic circulation. The gut microbiota ensures intestinal barrier integrity through diverse mechanisms [38] (Figure 1, dashed grey lines). Beneficial butyrate-producing bacteria are proposed to maintain intestinal integrity, as butyrate, a short-chain fatty acid (SCFA), facilitates the regeneration of healthy colonocytes [39]. A reduced relative proportion of bacteria producing SCFA was observed in Syrian hamsters infected with SARS-CoV-2, compared to non-infected controls, with a transient decrease in systemic SCFA amounts [26]. Decreases in the abundance of butyrate-producing bacteria and a decline in SCFA were observed in severe COVID-19 [10][12][40]. Besides the reduction of beneficial bacteria, the overgrowth of pathobionts, such as Escherichia coli or Salmonella enterica, disrupts intestinal barrier function [41][42][43]. Outgrowth of pathogenic Prevotella has been associated with reduced mucus secretion, one crucial protective layer of the intestinal barrier [44]. Blooms of pathogenic bacteria have been observed in hospitalized COVID-19 patients, along with the translocation of gut bacteria into the blood [25]. Lowered levels of butyrate-producers and higher levels of opportunistic pathogens (including E. coli and S. enterica) were observed in COVID-19 patients compared with H1N1 patients and healthy controls [9]. In addition, gut microbiota composition correlated with plasma levels of tissue damage markers, altered tight junctions, and microbial translocation in COVID-19 patients [10]. Finally, the colonic mucus barrier is shaped by the composition of the gut microbiota [45]. Alteration of the gut microbiota might contribute to disrupting the mucus barrier.
Human intestinal organoid co-cultures with microbes could represent useful systems to investigate the protective function of bacteria on gut permeability upon SARS-CoV-2 infection [46]. In addition, similar to the treatment of other diseases, treating SARS-CoV-2 infected mice or Syrian hamsters with SCFA supplementation [26][47], prebiotics, or probiotics (such as Lactobacillus reuteri in rodents), [48] and evaluating the intestinal permeability (dextran and bacterial translocation) in parallel with microbiota omics could strengthen the researchers' understanding of the relationship between gut microbiota and the intestinal barrier in COVID-19 pathophysiology.
2.2. Central Role of the Gut (Microbiota) in COVID-19 and Long COVID
Dysbiosis, intestinal inflammation, and leaky gut are intimately interconnected (Figure 1) and intestinal homeostasis is increasingly recognized as an underpinning clinical driver in several noncommunicable diseases as well as in COVID-19. Accumulating evidence supports that altered gut microbiota and associated leaky gut may contribute to the GI symptoms and the cytokine storm and multiorgan complications in COVID-19 [49][50]. In critically ill patients with sepsis and respiratory distress, bacterial translocation is widely documented [51][52]. Higher plasma levels of gut permeability markers were found in COVID-19 patients, along with abnormal presence of gut bacteria in the blood [53][54]. These markers correlated with higher levels of C-reactive peptide (a marker of hyperinflammation) and with a higher mortality rate [54]. Serum levels of lipopolysaccharide-binding protein were higher in patients with severe COVID-19 and were associated with circulating inflammation biomarkers [55]. Altered intestinal homeostasis induces diarrhea [56], which is the digestive symptom most commonly reported in COVID-19 patients [57][58][59][60][61].
Despite the well-documented prevalence of GI symptoms and the high rate of SARS-CoV-2 fecal RNA shedding, the isolation of replication-competent virus from fecal samples has not been reproducibly and systematically demonstrated [62]. The biological, clinical, and epidemiological relevance of SARS-CoV-2 shedding remains unclear [63]. SARS-CoV-2 shedding in stools has been reported from one week to seven months after diagnosis [63][64]. The prolonged presence of viral RNA in feces [63], but not in respiratory samples, and its association with GI symptoms suggests that SARS-CoV-2 infects the GI tract, and that this infection can be prolonged in a subset of individuals with COVID-19. SARS-CoV-2 infection leading to perturbation of the gut microbiome may contribute to the underlying etiology of GI symptoms observed in COVID-19 and long COVID [65][66]. Alteration in the gut microbiome persists long after a patient recovers, suggesting that the gut microbiome may play an important role in long COVID [67]. Long COVID or post-acute COVID-19 syndrome (PACS) is rapidly emerging across the globe and many studies following patients who have recovered from the respiratory effects of COVID-19 identified persistent GI sequelae, including dysbiosis [63][64][68]. While the pathogenesis of long COVID is still under intense investigation, on the four current leading hypotheses [65], it is interesting to note that gut dysbiosis is considered as one of them [67][69]. A comprehensive understanding of the dynamics of fecal clearance of SARS-CoV-2 RNA and its link with gut dysbiosis is currently lacking. Further studies are needed as the gut microbiota could serve as a potential prognosis indicator and could be therapeutically valuable.
2.3. Potential Modulation of Gut Microbiota to Mitigate COVID-19
In light of the current insight into the central role of the gut in COVID-19 and long COVID, modulating the gut microbiota to improve disease prevention and management may be relevant. First, fecal microbiota transplantation (FMT) enables stool infusion from a healthy individual to a severely ill patient to restore intestinal microbial balance [70]. So far, FMT has been remarkably successful in the treatment of Clostridium difficile infection, but much less in treating other conditions, such as IBD or metabolic disorders. COVID-19 being an infectious disease and not an inflammatory disorder, FMT could be more successful [49]. However, COVID-19 could potentially be transmitted via FMT, particularly from asymptomatic donors who tested negative for the presence of the virus in their respiratory tract but positive in their stools [71]. No cases of COVID-19 transmission through FMT have been reported so far, but only FMT products generated from stools donated before December/November 2019 were used according to the FDA and Hong Kong recommendations, respectively. Secondly, gut microbiota modulation with probiotics, prebiotics, or diet and therapies preventing gut barrier defects may represent easy-to-implement strategies to mitigate COVID-19 [72]. Clinical trials of probiotics with expected anti-inflammatory effects for preventing or treating SARS-CoV-2 infection are currently ongoing [73]. Next-generation probiotics focusing on butyrate-producing bacteria, or simply increasing the daily intake of dietary fiber are proposed as potential beneficial approaches for COVID-19 patients [49]. A few reports cite indirect evidence for the association between probiotics and COVID-19, primarily based on previous coronaviruses and other viral infections [74][75]. The health benefits of prebiotics to the GI tract, including the inhibition of pathogens and stimulation of the immune system, are due to their ability to modulate the composition and activity of human microbiota [76][77][78]. However, to date, there is no information directly linking prebiotics to COVID-19 infections, although an indirect effect may be hypothesized [79]. Thus, using conventional probiotics is not currently warranted, but is considered promising, and a better understanding of SARS-CoV-2 pathogenesis and its mutual effect on gut microbiota is needed. More generally, diet is obviously a factor impacting gut microbiota [80][81][82][83]. Dietary adaptation may be the easiest method to be implemented in the preventive arsenal against COVID-19 and for general health improvement [49].
References
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436.
- Chidambaram, V.; Tun, N.L.; Haque, W.Z.; Majella, M.G.; Sivakumar, R.K.; Kumar, A.; Hsu, A.T.-W.; Ishak, I.A.; Nur, A.A.; Ayeh, S.K.; et al. Factors associated with disease severity and mortality among patients with COVID-19: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0241541.
- Mudatsir, M.; Fajar, J.K.; Wulandari, L.; Soegiarto, G.; Ilmawan, M.; Purnamasari, Y.; Mahdi, B.A.; Jayanto, G.D.; Suhendra, S.; Setianingsih, Y.A.; et al. Predictors of COVID-19 severity: A systematic review and meta-analysis. F1000Research 2020, 9, 1107.
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558.
- Clerbaux, L.-A.; Albertini, M.C.; Amigó, N.; Beronius, A.; Bezemer, G.F.G.; Coecke, S.; Daskalopoulos, E.P.; del Giudice, G.; Greco, D.; Grenga, L.; et al. Factors Modulating COVID-19: A Mechanistic Understanding Based on the Adverse Outcome Pathway Framework. J. Clin. Med. 2022, 11, 4464.
- Ragonnaud, E.; Biragyn, A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun. Ageing 2021, 18, 2.
- Bosco, N.; Noti, M. The aging gut microbiome and its impact on host immunity. Genes Immun. 2021, 22, 289–303.
- Vodnar, D.-C.; Mitrea, L.; Teleky, B.-E.; Szabo, K.; Călinoiu, L.-F.; Nemeş, S.-A.; Martău, G.-A. Coronavirus Disease (COVID-19) Caused by (SARS-CoV-2) Infections: A Real Challenge for Human Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 575559.
- Gu, S.; Chen, Y.; Wu, Z.; Chen, Y.; Gao, H.; Lv, L.; Guo, F.; Zhang, X.; Luo, R.; Huang, C.; et al. Alterations of the Gut Microbiota in Patients with Coronavirus Disease 2019 or H1N1 Influenza. Clin. Infect. Dis. 2020, 71, 2669–2678.
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706.
- Cheung, C.C.L.; Goh, D.; Lim, X.; Tien, T.Z.; Lim, J.C.T.; Lee, J.N.; Tan, B.; Tay, Z.E.A.; Wan, W.Y.; Chen, E.X.; et al. Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut 2022, 71, 226–229.
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients with COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e948.
- Petrillo, M.; Brogna, C.; Cristoni, S.; Querci, M.; Piazza, O.; Eede, G.V.D. Increase of SARS-CoV-2 RNA load in faecal samples prompts for rethinking of SARS-CoV-2 biology and COVID-19 epidemiology. F1000Research 2021, 10, 370.
- Sarkar, A.; Harty, S.; Moeller, A.H.; Klein, S.L.; Erdman, S.E.; Friston, K.J.; Carmody, R.N. The gut microbiome as a biomarker of differential susceptibility to SARS-CoV-2. Trends Mol. Med. 2021, 27, 1115–1134.
- Schult, D.; Reitmeier, S.; Koyumdzhieva, P.; Lahmer, T.; Middelhoff, M.; Erber, J.; Schneider, J.; Kager, J.; Frolova, M.; Horstmann, J.; et al. Gut bacterial dysbiosis and instability is associated with the onset of complications and mortality in COVID-19. Gut Microbes 2022, 14, 2031840.
- Spor, A.; Koren, O.; Ley, R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat. Rev. Microbiol. 2011, 9, 279–290.
- Zhang, Y.-J.; Li, S.; Gan, R.-Y.; Zhou, T.; Xu, D.-P.; Li, H.-B. Impacts of Gut Bacteria on Human Health and Diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519.
- Sartor, R.B. Mechanisms of Disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pr. Gastroenterol. Hepatol. 2006, 3, 390–407.
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut–lung axis. Mucosal Immunol. 2019, 12, 843–850.
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The gut–liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411.
- Manzoor, R.; Ahmed, W.; Afify, N.; Memon, M.; Yasin, M.; Memon, H.; Rustom, M.; Al Akeel, M.; Alhajri, N. Trust Your Gut: The Association of Gut Microbiota and Liver Disease. Microorganisms 2022, 10, 1045.
- Mitrea, L.; Nemes, S.A.; Szabo, K.; Teleky, B.E.; Vodnar, D.C. Guts Imbalance Imbalances the Brain: A Review of Gut Microbiota Association with Neurological and Psychiatric Disorders. Front. Med. 2022, 9, 813204.
- Manna, S.; Baindara, P.; Mandal, S.M. Molecular pathogenesis of secondary bacterial infection associated to viral infections including SARS-CoV-2. J. Infect. Public Health 2020, 13, 1397–1404.
- Chen, J.; Hall, S.; Vitetta, L. Altered gut microbial metabolites could mediate the effects of risk factors in COVID-19. Rev. Med. Virol. 2021, 31, 1–13.
- Venzon, M.; Bernard-Raichon, L.; Klein, J.; Axelrad, J.; Hussey, G.; Sullivan, A.; Casanovas-Massana, A.; Noval, M.; Valero-Jimenez, A.; Gago, J.; et al. Gut microbiome dysbiosis during COVID-19 is associated with increased risk for bacteremia and microbial translocation. Res. Sq. 2021.
- Sencio, V.; Machelart, A.; Robil, C.; Benech, N.; Hoffmann, E.; Galbert, C.; Deryuter, L.; Heumel, S.; Hantute-Ghesquier, A.; Flourens, A.; et al. Alteration of the gut microbiota following SARS-CoV-2 infection correlates with disease severity in hamsters. Gut Microbes 2022, 14, 2018900.
- Sokol, H.; Contreras, V.; Maisonnasse, P.; Desmons, A.; Delache, B.; Sencio, V.; Machelart, A.; Brisebarre, A.; Humbert, L.; Deryuter, L.; et al. SARS-CoV-2 infection in nonhuman primates alters the composition and functional activity of the gut microbiota. Gut Microbes 2021, 13, 1893113.
- Nymark, P.; Sachana, M.; Leite, S.B.; Sund, J.; Krebs, C.E.; Sullivan, K.; Edwards, S.; Viviani, L.; Willett, C.; Landesmann, B.; et al. Systematic Organization of COVID-19 Data Supported by the Adverse Outcome Pathway Framework. Front. Public Health 2021, 9, 638605.
- Wittwehr, C.; Amorim, M.J.; Clerbaux, L.A.; Krebs, C.; Landesmann, B.; Macmillan, D.S.; Nymark, P.; Ram, R.; Garcia-Reyero, N.; Sachana, M.; et al. Understanding COVID-19 through adverse outcome pathways–2nd CIAO AOP Design Workshop. ALTEX 2021, 38, 351–357.
- Clerbaux, L.-A.; Amigó, N.; Amorim, M.J.; Bal-Price, A.; Leite, S.B.; Beronius, A.; Bezemer, G.F.G.; Bostroem, A.-C.; Carusi, A.; Coecke, S.; et al. COVID-19 through Adverse Outcome Pathways: Building networks to better understand the disease—3rd CIAO AOP Design Workshop. ALTEX 2022, 39, 322–335.
- CIAO. Modelling the Pathogenesis of COVID-19 Using the Adverse Outcome Pathway Framework. Available online: www.ciao-covid.net (accessed on 29 June 2022).
- OECD. Users’ Handbook supplement to the Guidance Document for developing and assessing Adverse Outcome Pathways. In OECD Series on Adverse Outcome Pathways; OECD Publishing: Paris, France, 2018.
- Draskau, M.K.; Spiller, C.M.; Boberg, J.; Bowles, J.; Svingen, T.; Kam, M. Developmental biology meets toxicology: Contributing reproductive mechanisms to build adverse outcome pathways. Mol. Hum. Reprod. 2020, 26, 111–116.
- Siwicki, A.K.; Terech-Majewska, E.; Grudniewska, J.; Malaczewska, J.; Kazun, K.; Lepa, A. Influence of deltamethrin on nonspecific cellular and humoral defense mechanisms in rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 2010, 29, 489–491.
- Villeneuve, D.L.; Crump, D.; Garcia-Reyero, N.; Hecker, M.; Hutchinson, T.H.; Lalone, C.A.; Landesmann, B.; Lettieri, T.; Munn, S.; Nepelska, M.; et al. Adverse Outcome Pathway (AOP) Development I: Strategies and Principles. Toxicol. Sci. 2014, 142, 312–320.
- Villeneuve, D.L.; Crump, D.; Garcia-Reyero, N.; Hecker, M.; Hutchinson, T.H.; Lalone, C.A.; Landesmann, B.; Lettieri, T.; Munn, S.; Nepelska, M.; et al. Adverse Outcome Pathway Development II: Best Practices. Toxicol. Sci. 2014, 142, 321–330.
- Svingen, T.; Villeneuve, D.L.; Knapen, D.; Panagiotou, E.M.; Draskau, M.K.; Damdimopoulou, P.; O’Brien, J.M. A Pragmatic Approach to Adverse Outcome Pathway Development and Evaluation. Toxicol. Sci. 2021, 184, 183–190.
- Hu, J.; Zhang, L.; Lin, W.; Tang, W.; Chan, F.K.; Ng, S.C. Review article: Probiotics, prebiotics and dietary approaches during COVID-19 pandemic. Trends Food Sci. Technol. 2021, 108, 187–196.
- Salvi, P.S.; Cowles, R.A. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells 2021, 10, 1775.
- Grenga, L.; Pible, O.; Miotello, G.; Culotta, K.; Ruat, S.; Roncato, M.; Gas, F.; Bellanger, L.; Claret, P.; Dunyach-Remy, C.; et al. Taxonomical and functional changes in COVID -19 faecal microbiome could be related to SARS-CoV-2 faecal load. Environ. Microbiol. 2022.
- Dean, P.; Kenny, B. Intestinal barrier dysfunction by enteropathogenic Escherichia coli is mediated by two effector molecules and a bacterial surface protein. Mol. Microbiol. 2004, 54, 665–675.
- Viswanathan, V.K.; Koutsouris, A.; Lukic, S.; Pilkinton, M.; Simonovic, I.; Simonovic, M.; Hecht, G. Comparative Analysis of EspF from Enteropathogenic and Enterohemorrhagic Escherichia coli in Alteration of Epithelial Barrier Function. Infect. Immun. 2004, 72, 3218–3227.
- Tafazoli, F.; Magnusson, K.-E.; Zheng, L. Disruption of Epithelial Barrier Integrity by Salmonella enterica Serovar Typhimurium Requires Geranylgeranylated Proteins. Infect. Immun. 2003, 71, 872–881.
- Larsen, J.M. The immune response to Prevotellabacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374.
- Jakobsson, H.E.; Rodríguez-Piñeiro, A.M.; Schütte, A.; Ermund, A.; Boysen, P.; Bemark, M.; Sommer, F.; Bäckhed, F.; Hansson, G.C.; Johansson, M.E.V. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015, 16, 164–177.
- Puschhof, J.; Pleguezuelos-Manzano, C.; Martinez-Silgado, A.; Akkerman, N.; Saftien, A.; Boot, C.; de Waal, A.; Beumer, J.; Dutta, D.; Heo, I.; et al. Intestinal organoid cocultures with microbes. Nat. Protoc. 2021, 16, 4633–4649.
- Sencio, V.; Gallerand, A.; Machado, M.G.; Deruyter, L.; Heumel, S.; Soulard, D.; Barthelemy, J.; Cuinat, C.; Vieira, A.T.; Barthelemy, A.; et al. Influenza Virus Infection Impairs the Gut’s Barrier Properties and Favors Secondary Enteric Bacterial Infection through Reduced Production of Short-Chain Fatty Acids. Infect. Immun. 2021, 89, e0073420.
- Dicksved, J.; Schreiber, O.; Willing, B.; Petersson, J.; Rang, S.; Phillipson, M.; Holm, L.; Roos, S. Lactobacillus reuteri Maintains a Functional Mucosal Barrier during DSS Treatment Despite Mucus Layer Dysfunction. PLoS ONE 2012, 7, e46399.
- Kim, H.S. Do an Altered Gut Microbiota and an Associated Leaky Gut Affect COVID-19 Severity? mBio 2021, 12.
- Vignesh, R.; Swathirajan, C.R.; Tun, Z.H.; Rameshkumar, M.R.; Solomon, S.S.; Balakrishnan, P. Could Perturbation of Gut Microbiota Possibly Exacerbate the Severity of COVID-19 via Cytokine Storm? Front. Immunol. 2020, 11, 607734.
- Openshaw, P.J. Crossing barriers: Infections of the lung and the gut. Mucosal Immunol. 2009, 2, 100–102.
- Cardinale, V.; Capurso, G.; Ianiro, G.; Gasbarrini, A.; Arcidiacono, P.G.; Alvaro, D. Intestinal permeability changes with bacterial translocation as key events modulating systemic host immune response to SARS-CoV-2: A working hypothesis. Dig. Liver Dis. 2020, 52, 1383–1389.
- Giron, L.B.; Dweep, H.; Yin, X.; Wang, H.; Damra, M.; Goldman, A.R.; Gorman, N.; Palmer, C.S.; Tang, H.-Y.; Shaikh, M.W.; et al. Plasma Markers of Disrupted Gut Permeability in Severe COVID-19 Patients. Front. Immunol. 2021, 12, 686240.
- Prasad, R.; Patton, M.J.; Floyd, J.L.; Fortmann, S.; DuPont, M.; Harbour, A.; Wright, J.; Lamendella, R.; Stevens, B.R.; Oudit, G.Y.; et al. Plasma Microbiome in COVID-19 Subjects: An Indicator of Gut Barrier Defects and Dysbiosis. Int. J. Mol. Sci. 2022, 23, 9141.
- Sun, Z.; Song, Z.-G.; Liu, C.; Tan, S.; Lin, S.; Zhu, J.; Dai, F.-H.; Gao, J.; She, J.-L.; Mei, Z.; et al. Gut microbiome alterations and gut barrier dysfunction are associated with host immune homeostasis in COVID-19 patients. BMC Med. 2022, 20, 24.
- Maguire, M.; Maguire, G. Gut dysbiosis, leaky gut, and intestinal epithelial proliferation in neurological disorders: Towards the development of a new therapeutic using amino acids, prebiotics, probiotics, and postbiotics. Rev. Neurosci. 2019, 30, 179–201.
- Effenberger, M.; Grabherr, F.; Mayr, L.; Schwaerzler, J.; Nairz, M.; Seifert, M.; Hilbe, R.; Seiwald, S.; Scholl-Buergi, S.; Fritsche, G.; et al. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut 2020, 69, 1543–1544.
- Hayashi, Y.; Wagatsuma, K.; Nojima, M.; Yamakawa, T.; Ichimiya, T.; Yokoyama, Y.; Kazama, T.; Hirayama, D.; Nakase, H. The characteristics of gastrointestinal symptoms in patients with severe COVID-19: A systematic review and meta-analysis. J. Gastroenterol. 2021, 56, 409–420.
- Jin, X.; Lian, J.S.; Hu, J.H.; Gao, J.; Zheng, L.; Zhang, Y.M.; Hao, S.R.; Jia, H.Y.; Cai, H.; Zhang, X.L.; et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 2020, 69, 1002–1009.
- Nobel, Y.R.; Phipps, M.; Zucker, J.; Lebwohl, B.; Wang, T.C.; Sobieszczyk, M.E.; Freedberg, D.E. Gastrointestinal Symptoms and Coronavirus Disease 2019: A Case-Control Study from the United States. Gastroenterology 2020, 159, 373–375.e2.
- Redd, W.D.; Zhou, J.C.; Hathorn, K.E.; Mccarty, T.R.; Bazarbashi, A.N.; Thompson, C.C.; Shen, L.; Chan, W.W. Prevalence and Characteristics of Gastrointestinal Symptoms in Patients with Severe Acute Respiratory Syndrome Coronavirus 2 Infection in the United States: A Multicenter Cohort Study. Gastroenterology 2020, 159, 765–767.e2.
- Clerbaux, L.A.; Muñoz, A.; Soares, H.; Petrillo, M.; Albertini, M.C.; Lanthier, N.; Grenga, L.; Amorim, M.J. Gut as an alternative entry route for SARS-CoV-2: Current evidence and uncertainties of productive enteric infection in COVID-19. J. Clin. Med. 2022. submitted.
- Natarajan, A.; Zlitni, S.; Brooks, E.F.; Vance, S.E.; Dahlen, A.; Hedlin, H.; Park, R.M.; Han, A.; Schmidtke, D.T.; Verma, R.; et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med 2022, 3, 371–387.e9.
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435.
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127.
- Brooks, E.F.; Bhatt, A.S. The gut microbiome: A missing link in understanding the gastrointestinal manifestations of COVID-19? Cold Spring Harb. Mol. Case Stud. 2021, 7, a006031.
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.-Y.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S.-C.; et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022, 71, 544–552.
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’Em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine 2021, 38, 101019.
- Arostegui, D.; Castro, K.; Schwarz, S.; Vaidy, K.; Rabinowitz, S.; Wallach, T. Persistent SARS-CoV-2 Nucleocapsid Protein Presence in the Intestinal Epithelium of a Pediatric Patient 3 Months after Acute Infection. JPGN Rep. 2021, 3, e152.
- Kim, K.O.; Gluck, M. Fecal Microbiota Transplantation: An Update on Clinical Practice. Clin. Endosc. 2019, 52, 137–143.
- Kazemian, N.; Kao, D.; Pakpour, S. Fecal Microbiota Transplantation during and Post-COVID-19 Pandemic. Int. J. Mol. Sci. 2021, 22, 3004.
- Wang, X.; Zhang, P.; Zhang, X. Probiotics Regulate Gut Microbiota: An Effective Method to Improve Immunity. Molecules 2021, 26, 6076.
- Baindara, P.; Chakraborty, R.; Holliday, Z.M.; Mandal, S.M.; Schrum, A.G. Oral probiotics in coronavirus disease 2019: Connecting the gut–lung axis to viral pathogenesis, inflammation, secondary infection and clinical trials. New Microbes New Infect. 2021, 40, 100837.
- Kurian, S.J.; Unnikrishnan, M.K.; Miraj, S.S.; Bagchi, D.; Banerjee, M.; Reddy, B.S.; Rodrigues, G.S.; Manu, M.K.; Saravu, K.; Mukhopadhyay, C.; et al. Probiotics in Prevention and Treatment of COVID-19: Current Perspective and Future Prospects. Arch. Med. Res. 2021, 52, 582–594.
- Mak, J.W.Y.; Chan, F.K.L.; Ng, S.C. Probiotics and COVID-19: One size does not fit all. Lancet Gastroenterol. Hepatol. 2020, 5, 644–645.
- Guarino, M.P.L.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients 2020, 12, 1037.
- Peters, V.; Van De Steeg, E.; van Bilsen, J.; Meijerink, M. Mechanisms and immunomodulatory properties of pre- and probiotics. Benef. Microbes 2019, 10, 225–236.
- Shokryazdan, P.; Jahromi, M.F.; Navidshad, B.; Liang, J.B. Effects of prebiotics on immune system and cytokine expression. Med. Microbiol. Immunol. 2016, 206, 1–9.
- Khaled, J.M. Probiotics, prebiotics, and COVID-19 infection: A review article. Saudi J. Biol. Sci. 2021, 28, 865–869.
- Kalantar-Zadeh, K.; Ward, S.A.; Kalantar-Zadeh, K.; El-Omar, E. Considering the Effects of Microbiome and Diet on SARS-CoV-2 Infection: Nanotechnology Roles. ACS Nano 2020, 14, 5179–5182.
- Infusino, F.; Marazzato, M.; Mancone, M.; Fedele, F.; Mastroianni, C.M.; Severino, P.; Ceccarelli, G.; Santinelli, L.; Cavarretta, E.; Marullo, A.G.M.; et al. Diet Supplementation, Probiotics, and Nutraceuticals in SARS-CoV-2 Infection: A Scoping Review. Nutrients 2020, 12, 1718.
- Deschasaux-Tanguy, M.; Srour, B.; Bourhis, L.; Arnault, N.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, F.S.; Allègre, J.; Allès, B.; Andreeva, V.A.; et al. Nutritional risk factors for SARS-CoV-2 infection: A prospective study within the NutriNet-Santé cohort. BMC Med. 2021, 19, 290.
- Salazar-Robles, E.; Kalantar-Zadeh, K.; Badillo, H.; Calderón-Juárez, M.; García-Bárcenas, C.A.; Ledesma-Pérez, P.D.; Lerma, A.; Lerma, C. Association between severity of COVID-19 symptoms and habitual food intake in adult outpatients. BMJ Nutr. Prev. Health 2021, 4, 469–478.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Entry Collection:
COVID-19
Revisions:
2 times
(View History)
Update Date:
04 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




