Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ratko Milorad Lasica | -- | 2246 | 2022-10-02 01:00:05 | | | |
| 2 | Rita Xu | Meta information modification | 2246 | 2022-10-08 08:00:17 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lasica, R.; Asanin, M.; Djukanovic, L.; Radovanovic, N.; Savic, L.; Polovina, M.; Stankovic, S.; Ristic, A.; Zdravkovic, M.; Lasica, A.; et al. Risk Stratification in Patients with Pulmonary Embolism. Encyclopedia. Available online: https://encyclopedia.pub/entry/28202 (accessed on 06 March 2026).
Lasica R, Asanin M, Djukanovic L, Radovanovic N, Savic L, Polovina M, et al. Risk Stratification in Patients with Pulmonary Embolism. Encyclopedia. Available at: https://encyclopedia.pub/entry/28202. Accessed March 06, 2026.
Lasica, Ratko, Milika Asanin, Lazar Djukanovic, Nebojsa Radovanovic, Lidija Savic, Marija Polovina, Sanja Stankovic, Arsen Ristic, Marija Zdravkovic, Andjelka Lasica, et al. "Risk Stratification in Patients with Pulmonary Embolism" Encyclopedia, https://encyclopedia.pub/entry/28202 (accessed March 06, 2026).
Lasica, R., Asanin, M., Djukanovic, L., Radovanovic, N., Savic, L., Polovina, M., Stankovic, S., Ristic, A., Zdravkovic, M., Lasica, A., Kravic, J., & Perunicic, J. (2022, October 02). Risk Stratification in Patients with Pulmonary Embolism. In Encyclopedia. https://encyclopedia.pub/entry/28202
Lasica, Ratko, et al. "Risk Stratification in Patients with Pulmonary Embolism." Encyclopedia. Web. 02 October, 2022.
Copy Citation
Pulmonary thromboembolism is a very common cardiovascular disease, with a high mortality rate. This disease still represents a great challenge both in diagnosis and treatment. The heterogeneous clinical picture, often without pathognomonic signs and symptoms, represents a huge differential diagnostic problem even for experienced doctors. The decisions surrounding this therapeutic regimen also represent a major dilemma in the group of patients who are hemodynamically stable at initial presentation and have signs of right ventricular (RV) dysfunction proven by echocardiography and positive biomarker values (pulmonary embolism of intermediate–high risk).
acute pulmonary embolism
dilemmas
therapeutic treatment
recommendations
1. From Diagnostic Doubts to the Correct Diagnosis of Pulmonary Embolism
Venous thromboembolism (VTE) is the third most common cardiovascular disease (after myocardial infarction and cerebrovascular insult), with an estimated annual incidence in epidemiological studies of 1–2 cases per 1000 people [1][2]. The frequency of VTE largely depends on age, gender, and associated diseases.
Most episodes of VTE are provoked by the presence of a number of risk factors, and in some episodes of VTE it is not possible to establish a clear risk factor (unprovoked PE) [3].
Major traumatic injuries, surgery, lower extremity fractures, knee or hip arthroplasty, myocardial infarction (within the previous three months), previous VTE, as well as spinal cord injury are strong risk factors. Blood transfusions, the use of drugs that stimulate erythropoiesis, chemotherapy, autoimmune diseases, thrombophilia (Factor V Leiden gene mutation, activated protein C resistance, prothrombin gene mutation (G 20210 A), AT3 deficiency, protein C and S deficiency, elevated values of lipoprotein), the presence of a central venous catheter, heart failure, stroke, the postpartum period, as well as infections are classified as moderate risk factors [4]. Malignant diseases comprise a well-known predisposing factor for VTE. It has been shown that 20% of patients with VTE have an active malignant disease [5]. It has been shown that cancer patients have an increased risk of recurrence of PE, major bleeding, and risk of early and three-month mortality after PE [6]. The use of oral contraceptives is the most common risk factor in women during the reproductive period [7]. Weak risk factors for the development of PE are older age, diabetes, hypertension, obesity, varicose veins, pregnancy, laparoscopic surgery, immobilization for more than 3 days, and prolonged sitting [4]. A more recent division of risk factors for PE includes four groups: major transitory (traumas, major surgical interventions); major persistent (malignant diseases and antiphospholipid syndrome); minor transient (oral contraceptives, pregnancy, puerperium); minor persistent (congenital thrombophilias, autoimmune diseases). Strong transient risk factors are responsible for approximately 20% of all VTE episodes [8].
The diagnosis of this sometimes insidious disease is also complicated by the fact that the specificity and sensitivity of symptoms associated with PE is very low, so the presence or absence of any symptom is not enough to confirm or exclude the existence of PE [9]. The most common symptoms and signs of PE are dyspnea (30–88%) [10][11][12], a pleuritic type of chest pain (from 39–70%) [9][10][13], leg swelling, which is suspicious for deep vein thrombosis (DVT) (24%) [14], hemoptysis (2–11.8%) [15][16], cough (9–23%) [12][16] syncope (6–39%) [9][10][16], tachycardia (40%) [9][10][11], hypoxemia (70%) [17][18], and new-onset atrial fibrillation (24%) [8].
In recent times, the tendency to reduce the unnecessary costs of testing patients with suspected PE on the one hand and overlooking non-specific signs and symptoms of the disease on the other hand are the causes of the largest number of missed diagnoses of PE. By standardizing algorithms for the diagnosis and treatment of patients with suspected PE, the diagnostic accuracy of this disease has been improved [19]. These diagnostic algorithms include pre-test probability assessments for the existence of PE, the evaluation of D-dimer values as well as non-invasive radiographic techniques. However, in clinical practice, the non-specific clinical picture of PE represents a great challenge for a diagnosis of this disease. The initial presentation of patients with PE varies from asymptomatic cases (discovered incidentally during a diagnostic examination to detect other diseases) to high-risk PE resulting in RV dysfunction and the subsequent development of shock. The symptoms of the disease in PE can vary and gain in dynamics. Thus, patients initially misdiagnosed with PE may deteriorate clinically to such an extent that re-evaluation will lead to a definitive diagnosis of PE. On the other hand, patients who are hemodynamically unstable with cardiogenic shock may raise the suspicion of an initially cardiac cause of such a condition.
It is recommended that in patients with a low or moderate pre-test probability for PE, D dimer values are also determined, while in patients with a high probability, sophisticated diagnostic methods are immediately implemented [4][20]. However, both decreased and elevated D dimer values can lead the doctor to misdiagnose cases. Elevated D-dimer values are also found in patients with inflammatory processes, who suffer from chronic renal failure, cancer, during pregnancy, injuries, and surgeries [21][22]. Patients with elevated D-dimer levels due to their low positive predictive value should undergo further diagnostic testing to confirm and/or rule out the diagnosis of PE. Given that normal D-dimer values increase with age, it is necessary to take the patient’s age into account when interpreting the results, which is achieved with age-adjusted D-dimer values [23]. Studies have shown that an age-adjusted D-dimer cutoff may be effective and safe in ruling out PE compared with conventional D-dimer (500 µg/L) [24][25]. Elevated values of the D-dimer are physiologically present in pregnancy (they increase in relation to the trimester of pregnancy) which can lead to a wrong diagnosis [26]. Clinical suspicion of the existence of PE and elevated D dimer values are often the reason for performing CT pulmonary angiography and/or ventilation–perfusion scanning, which exposes both the mother and the fetus to the harmful effects of radiation [27]. In the case of negative D-dimer values combined with a low clinical probability, PE can be ruled out without further diagnostic procedures [20]. A study that analyzed data from the national collaborative study (PIOPED II) showed that despite a low clinical probability for the presence of PE (according to the Wells score), patients had a CT-proven embolus in the main or lobar branches of the PA [13].
Despite advances in medicine, Alonso-Martínez et al. showed that misdiagnosis occurred in 50% (95% CI 44–55) of patients. A higher age, more days of delay and the absence of syncope or sudden-onset dyspnea were factors associated with misdiagnosis [28]. To reduce the number of missed diagnoses of PE, the YEARS rule can be used, which consists of three items: the clinical suspicion of PE, presence of clinical signs of DVT, and the presence of hemoptysis [4]. The Pulmonary Embolism Rule-out Criteria (PERC) score is also used, where it is possible to rule out PE in patients with a low clinical probability who, in addition, have fulfilled all eight criteria of the PERC rule. The impossibility of its widespread use is reflected in the fact that it can only be applied in clinical settings with a low (<5%) prevalence of PE [4][17]. Tome van der Hulle and colleagues showed in a prospective cohort study that D-dimer testing in combination with clinical pretest probability assessments using the YEARS criteria or the revised Geneva score can exclude pulmonary embolism [29].
In a systematic review by Kwok Chun S and associates (analysis of 18 studies), it was shown that the most common diagnoses with which PE is mixed are pneumonia, bronchitis, exacerbation of chronic obstructive pulmonary disease, heart failure and acute coronary syndrome [30].
The presence of individual signs and symptoms for PE has a low sensitivity and specificity, while combining them increases the probability that a patient suspected of PE really has this disease. The latest European recommendations for the diagnosis of PE suggest the use of the revised Geneva score and the Wells score. Regardless of which score is used, the expected percentage of patients with PE is about 10% in the low-probability category, 30% in the intermediate category, and 65% in the high-probability category [4].
Although changes in the electrocardiogram (ECG) in patients with PE are considered insufficiently specific and sensitive for establishing a diagnosis, they can point researchers to this disease. Most often, sinus tachycardia can be registered in the ECG (present in about 40% of patients); sign S1Q3T3 (Mc Ginn White sign—in about 10% of patients); complete or incomplete right bundle branch block (present in about 25%; in hemodynamically unstable patients up to 30%) [31][32]. The presence of right bundle branch block in the electrocardiogram in hemodynamically stable patients is often associated with RV dysfunction compared to patients without it (15% vs. 5%, p < 0.001) [33]. Right bundle branch block in patients with PE is associated with a poor prognosis [34]. Ermis et al. also found an association between presence of right axis deviation and the severity of PE with this finding in 3% of low-risk PE, 15% of intermediate-risk PE and 28% of high-risk PE cases (p = 0.009) [35]. P pulmonale occurs in the ECG in up to 19% with acute PE. ST segment depression in leads V1–V6 is present in about 26% of PE patients. The presence of inversion T waves in anterior leads has been reported with variable frequency from 16% to 68% [32]. The presence of atrial fibrillation in patients with PE was observed in 15% to 21% of patients [36]. The Qr configuration in V1 (Weber and Phillips sign) is specific for PE but has a low prevalence (11–19%) [37]. This sign is a predictor of RV dysfunction.
Echocardiography is a non-invasive method with a huge role in the diagnosis and clinical assessment of patients with PE. Enlargement of RV, hypokinesia of the free wall of RV and interventricular septal flattening were found in 27.4%, 26.6%, and 18.4% of patients, respectively [38]. An enlarged RV with akinesia of the basal segment of the free wall (McConnell’s sign) can be seen in approximately 20% of patients with PE [38]. The presence of this sign has 77% sensitivity and 94% specificity for the diagnosis of acute PE [39]. Casazza et al. demonstrated that McConnell’s sign can also be seen in cases of RV infarction and thus cannot be considered pathognomonic for acute PE [40][41]. The only sure sign for the existence of PE with this method is the visualization of thrombus masses in the right heart cavities, which occurs in 4–18% of patients with acute PE [42]. Transesophageal echocardiography, when it comes to central PE, has a sensitivity of 90–95% and a specificity of 100%. Thromboembolus is very difficult to visualize in the middle part of the left pulmonary artery because the interposition of the left main bronchus interferes with the ultrasound beam.
Thanks to the results of The Prospective Investigation On Pulmonary Embolism Diagnosis (PIOPED) II study, CT pulmonary angiography (CTPA) has become the method of choice for the diagnosis of PE (sensitivity of 83% and specificity of 96%) [13].
2. Risk Stratification in Patients with PE
Risk stratification in patients with acute PE is necessary to determine the initial therapeutic approach. Determining the correct initial therapeutic regimen is very important because the risk of early mortality in normotensive patients is still high and amounts to 2–8%, while in patients presenting with cardiogenic shock it is up to 30%, and in the case of the need for cardiopulmonary resuscitation, the risk of early mortality increases up to 65% [43]. Initial stratification is based on clinical symptoms and signs: hemodynamic status at initial presentation, PESI score (Pulmonary Embolism Severity Index) and simplified PESI score (sPESI), and the presence of RV dysfunction based on transthoracic echocardiography results and biomarker values (Troponin and BNP-a) [4][44]. Cardiac troponin, a marker of myocardial damage, was shown to be a significant predictor of early mortality in patients with PE, and the presence of positive troponin even in the case of low risk, as assessed by the PESI score, indicates higher early mortality [45]. RV pressure overload due to PE is associated with increased myocardial distension causing the release of BNP and N-terminal pro BNP (NT pro BNP). Natriuretic peptide levels reflect the severity of RV dysfunction in acute PE [46].
All patients with PE can be classified into three risk categories: low, intermediate and high. Patients who are hypotensive (systolic blood pressure less than 90 mmHg) or in shock (systolic blood pressure less than 80 mmHg) on admission belong to the category of patients with high-risk PE (about 5% of PE cases) [4][47]. Such patients should be treated with thrombolytic therapy and/or embolectomy (Figure 1).
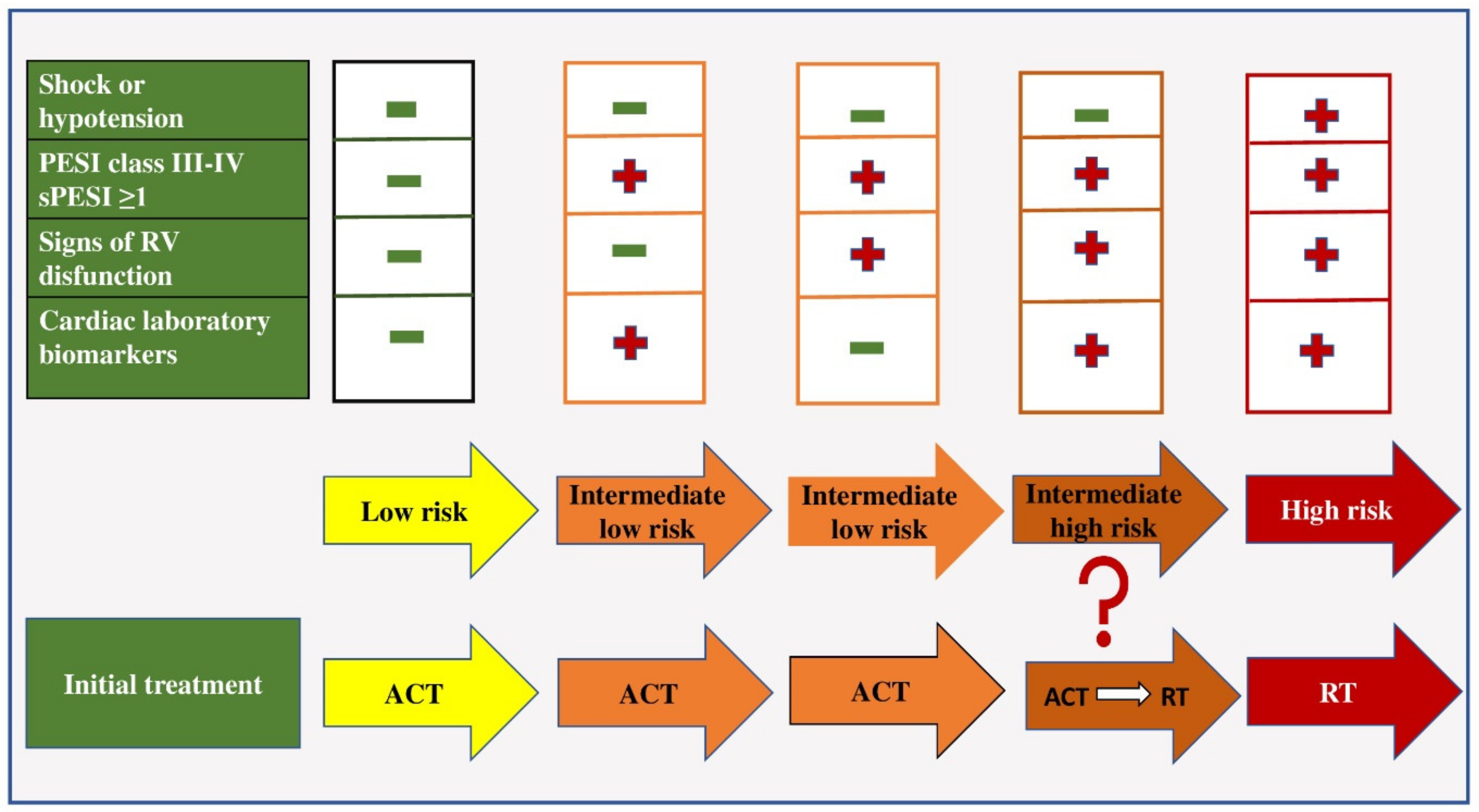
Figure 1. Early mortality risk and initial treatment in patients with acute pulmonary embolism. Legend: ACT—anticoagulant therapy; RT-reperfusion therapy; PESI—The Pulmonary Embolism Severity Index Score; sPESI—simplified Pulmonary Embolism Severity Index Score.
In the group of patients in whom hemodynamic instability is not present, further risk stratification is based on the assessment of prognostic criteria, namely clinical, visualization and laboratory indicators (mostly related to proving the presence of RV dysfunction). Patients with a low risk of early mortality are normotensive on admission, have a sPESI score of less than one, have no RV dysfunction on echocardiographic examination and have negative biomarker values and are treated with anticoagulant therapy [48].
Patients with an intermediate risk of early mortality are hemodynamically stable on admission and have an sPESI score greater than one. If they have positive values or biomarkers or signs of RV dysfunction proven by echocardiography, they belong to the group of patients with intermediate–low risk and they should be treated with anticoagulant therapy [4]. If these patients have positive biomarker values and the presence of RV dysfunction, they belong to the intermediate–high risk group and are initially treated with anticoagulant therapy, and in case of hemodynamic decompensation, with thrombolytic therapy [49].
It is also important to note that the risk of pulmonary embolism is a dynamic category, so the initially set therapeutic decision can be changed depending on the patient’s clinical condition. Choosing the right initial treatment for PE patients not only affects their survival, but also reduces the frequency of post-thromboembolic pulmonary hypertension, which worsens the long-term prognosis of these patients and reduces their quality of life.
References
- Heit, J.A.; Spencer, F.A.; White, R.H. The epidemiology of venous thromboembolism. J. Thromb. Thrombolysis 2016, 41, 3–14.
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Buller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.; Kakkar, A.; Konstantinides, S.V.; McCumber, M.; et al. ISTH Steering Committee for World Thrombosis Day. Thrombosis: A major contributor to global disease burden. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2363–2371.
- Mazzolai, L.; Aboyans, V.; Ageno, W.; Agnelli, G.; Alatri, A.; Bauersachs, R.; Brekelmans, M.P.A.; Büller, H.R.; Elias, A.; Farge, D.; et al. Diagnosis and management of acute deep vein thrombosis: A joint consensus document from the European Society of Cardiology working groups of aorta and peripheral vascular diseases and pulmonary circulation and right ventricular function. Eur. Heart J. 2018, 39, 4208–4218.
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603.
- Tsuyuki, H.; Yamamoto, N.; Unno, N.; Inuzuka, K.; Sano, M.; Katahashi, K.; Yata, T.; Kayama, T.; Yamanaka, Y.; Endo, Y.; et al. Characteristics and Prognostic Factors of Venous Thromboembolism in Cancer Patients. Ann. Vasc. Dis. 2022, 15, 107–112.
- Gussoni, G.; Frasson, S.; La Regina, M.; Di Micco, P.; Monreal, M. RIETE Investigators. Three-month mortality rate and clinical predictors in patients with venous thromboembolism and cancer. Findings from the RIETE registry. Thromb. Res. 2013, 131, 24–30.
- Gialeraki, A.; Valsami, S.; Pittaras, T.; Panayiotakopoulos, G.; Politou, M. Oral Contraceptives and HRT Risk of Thrombosis. Clin. Appl. Thromb. Hemost. 2018, 24, 217–225.
- Kukla, P.; McIntyre, W.F.; Koracevic, G.; Kutlesic-Kurtovic, D.; Fijorek, K.; Atanaskovic, V.; Krupa, E.; Mirek-Bryniarska, E.; Jastrzębski, M.; Bryniarski, L.; et al. Relation of atrial fibrillation and right-sided cardiac thrombus to outcomes in patients with acute pulmonary embolism. Am. J. Cardiol. 2015, 115, 825–830.
- Righini, M.; Robert-Ebadi, H.; Le Gal, G. Diagnosis of acute pulmonary embolism. J. Thromb. Haemost. 2017, 15, 1251–1261.
- Morrone, D.; Morrone, V. Acute Pulmonary Embolism: Focus on the Clinical Picture. Korean Circ. J. 2018, 48, 365–381.
- Nilsson, L.T.; Andersson, T.; Larsen, F.; Lang, I.M.; Liv, P.; Söderberg, S. Dyspnea after pulmonary embolism: A nation-wide population-based case-control study. Pulm. Circ. 2021, 11, 20458940211046831.
- Pollack, C.V.; Schreiber, D.; Goldhaber, S.Z.; Slattery, D.; Fanikos, J.; O’Neil, B.J.; Thompson, J.R.; Hiestand, B.; Briese, B.A.; Pendleton, R.C.; et al. Clinical characteristics, management, and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department: Initial report of EMPEROR (Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry). J. Am. Coll. Cardiol. 2011, 57, 700–706.
- Stein, P.D.; Beemath, A.; Matta, F.; Weg, J.G.; Yusen, R.D.; Hales, C.A.; Hull, R.D.; Leeper, K.V.; Sostman, H.D., Jr.; Tapson, V.F.; et al. Clinical characteristics of patients with acute pulmonary embolism: Data from PIOPED II. Am. J. Med. 2007, 120, 871–879.
- Stein, P.D.; Hull, R.D.; Kayali, F.; Ghali, W.A.; Alshab, A.K.; Olson, R.E. Venous thromboembolism according to age: The impact of an aging population. Arch. Intern. Med. 2004, 164, 2260–2265.
- Ji, Q.Y.; Wang, M.F.; Su, C.M.; Yang, Q.F.; Feng, L.F.; Zhao, L.Y.; Fang, S.Y.; Zhao, F.H.; Li, W.M. Clinical symptoms and related risk factors in pulmonary embolism patients and cluster analysis based on these symptoms. Sci. Rep. 2017, 7, 14887.
- Kucher, N.; Rossi, E.; De Rosa, M.; Goldhaber, S.Z. Massive pulmonary embolism. Circulation 2006, 113, 577–582.
- Khan, F.; Tritschler, T.; Kahn, S.R.; Rodger, M.A. Venous thromboembolism. Lancet. 2021, 398, 64–77.
- Tarbox, A.K.; Swaroop, M. Pulmonary embolism. Int. J. Crit. Illn. Inj. Sci. 2013, 3, 69–72.
- Righini, M.; Robert-Ebadi, H. Diagnosis of acute Pulmonary Embolism. Hamostaseologie 2018, 38, 11–21.
- Kearon, C.; de Wit, K.; Parpia, S.; Schulman, S.; Afilalo, M.; Hirsch, A.; Spencer, F.A.; Sharma, S.; D’Aragon, F.; Deshaies, J.F.; et al. PEGeD Study Investigators. Diagnosis of Pulmonary Embolism with d-Dimer Adjusted to Clinical Probability. N. Engl. J. Med. 2019, 381, 2125–2134.
- Ziegler, T.; Murzik, M.; Schau, A.; Winkler, C.; Fünfstück, R. Interpretation erhöhter D-Dimerkonzentration . Hamostaseologie 2004, 24, 144–146.
- Shafiee, M.A.; Hosseini, S.F.; Mortazavi, M.; Emami, A.; Mojtahed Zadeh, M.; Moradi, S.; Shaker, P. Anticoagulation therapy in COVID-19 patients with chronic kidney disease. J. Res. Med. Sci. 2021, 26, 63.
- Righini, M.; Goehring, C.; Bounameaux, H.; Perrier, A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am. J. Med. 2000, 109, 357–361.
- Righini, M.; Van Es, J.; Den Exter, P.L.; Roy, P.M.; Verschuren, F.; Ghuysen, A.; Rutschmann, O.T.; Sanchez, O.; Jaffrelot, M.; Trinh-Duc, A.; et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: The ADJUST-PE study. JAMA 2014, 311, 1117–1124.
- Van Es, N.; Kraaijpoel, N.; Klok, F.A.; Huisman, M.V.; Den Exter, P.L.; Mos, I.C.; Galipienzo, J.; Büller, H.R.; Bossuyt, P.M. The original and simplified Wells rules and age-adjusted D-dimer testing to rule out pulmonary embolism: An individual patient data meta-analysis. J. Thromb. Haemost. 2017, 15, 678–684.
- Righini, M.; Robert-Ebadi, H.; Elias, A.; Sanchez, O.; Le Moigne, E.; Schmidt, J.; Le Gall, C.; Cornuz, J.; Aujesky, D.; Roy, P.M.; et al. CT-PE-Pregnancy Group. Diagnosis of Pulmonary Embolism During Pregnancy: A Multicenter Prospective Management Outcome Study. Ann. Intern. Med. 2018, 169, 766–773.
- Tromeur, C.; van der Pol, L.M.; Le Roux, P.Y.; Ende-Verhaar, Y.; Salaun, P.Y.; Leroyer, C.; Couturaud, F.; Kroft, L.J.M.; Huisman, M.V.; Klok, F.A. Computed tomography pulmonary angiography versus ventilation-perfusion lung scanning for diagnosing pulmonary embolism during pregnancy: A systematic review and meta-analysis. Haematologica 2019, 104, 176–188.
- Alonso-Martínez, J.L.; Sánchez, F.J.; Echezarreta, M.A. Delay and misdiagnosis in sub-massive and non-massive acute pulmonary embolism. Eur. J. Intern. Med. 2010, 21, 278–282.
- Van der Hulle, T.; Cheung, W.Y.; Kooij, S.; Beenen, L.F.M.; van Bemmel, T.; van Es, J.; Faber, L.M.; Hazelaar, G.M.; Heringhaus, C.; Hofstee, H.; et al. YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): A prospective, multicentre, cohort study. Lancet 2017, 390, 289–297.
- Kwok, C.S.; Wong, C.W.; Lovatt, S.; Myint, P.K.; Loke, Y.K. Misdiagnosis of pulmonary embolism and missed pulmonary embolism: A systematic review of the literature. Health Sci. Rev. 2022, 3, 100022.
- Su, X.F.; Fan, N.; Yang, X.M.; Song, J.M.; Peng, Q.H.; Liu, X. A Novel Electrocardiography Model for the Diagnosis of Acute Pulmonary Embolism. Front. Cardiovasc. Med. 2022, 9, 825561.
- Digby, G.C.; Kukla, P.; Zhan, Z.Q.; Pastore, C.A.; Piotrowicz, R.; Schapachnik, E.; Zareba, W.; Bayés de Luna, A.; Pruszczyk, P.; Baranchuk, A.M. The value of electrocardiographic abnormalities in the prognosis of pulmonary embolism: A consensus paper. Ann. Noninvasive Electrocardiol. 2015, 20, 207–223.
- Vanni, S.; Polidori, G.; Vergara, R.; Pepe, G.; Nazerian, P.; Moroni, F.; Garbelli, E.; Daviddi, F.; Grifoni, S. Prognostic value of ECG among patients with acute pulmonary embolism and normal blood pressure. Am. J. Med. 2009, 122, 257–264.
- Novicic, N.; Dzudovic, B.; Subotic, B.; Shalinger-Martinovic, S.; Obradovic, S. Electrocardiography changes and their significance during treatment of patients with intermediate-high and high-risk pulmonary embolism. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 271–278.
- Ermıs, N.; Ermıs, H.; Sen, N.; Kepez, A.; Cuglan, B. QT dispersion in patients with pulmonary embolism. Wien. Klin. Wochenschr. 2010, 122, 691–697.
- Ptaszynska-Kopczynska, K.; Kiluk, I.; Sobkowicz, B. Atrial Fibrillation in Patients with Acute Pulmonary Embolism: Clinical Significance and Impact on Prognosis. Biomed Res. Int. 2019, 2019, 7846291.
- Kucher, N.; Walpoth, N.; Wustmann, K.; Noveanu, M.; Gertsch, M. QR in V1--an ECG sign associated with right ventricular strain and adverse clinical outcome in pulmonary embolism. Eur. Heart J. 2003, 24, 1113–1119.
- Kurnicka, K.; Lichodziejewska, B.; Goliszek, S.; Dzikowska-Diduch, O.; Zdończyk, O.; Kozłowska, M.; Kostrubiec, M.; Ciurzyński, M.; Palczewski, P.; Grudzka, K.; et al. Echocardiographic Pattern of Acute Pulmonary Embolism: Analysis of 511 Consecutive Patients. J. Am. Soc. Echocardiogr. 2016, 29, 907–913.
- McConnell, M.V.; Solomon, S.D.; Rayan, M.E.; Lee, R.T.; Come, P.C.; Goldhaber, S.Z.; Lee, R.T. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am. J. Cardiol. 1996, 78, 469–473.
- Casazza, F.; Bongarzoni, A.; Capozi, A.; Agostoni, O. Regional right ventricular dysfunction in acute pulmonary embolism and right ventricular infarction. Eur. J. Echocardiogr. 2005, 6, 11–14.
- Shafiq, Q.; Assaly, R.; Kanjwal, Y. McConnell Sign in a Patient with Massive Acute Pulmonary Embolism. Case Rep. Cardiol. 2011, 2011, 201097.
- Naeem, K. Floating thrombus in the right heart associated with pulmonary embolism: The role of echocardiography. Pak. J. Med. Sci. 2015, 31, 233–235.
- Bĕlohlávek, J.; Dytrych, V.; Linhart, A. Pulmonary embolism, part I: Epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp. Clin. Cardiol. 2013, 18, 129–138.
- Elias, A.; Mallett, S.; Daoud-Elias, M.; Poggi, J.N.; Clarke, M. Prognostic models in acute pulmonary embolism: A systematic review and meta-analysis. BMJ 2016, 6, e010324.
- Duffett, L.; Castellucci, L.A.; Forgie, M.A. Pulmonary embolism: Update on management and controversies. BMJ 2020, 370, m2177.
- Mirambeaux, R.; León, F.; Bikdeli, B.; Morillo, R.; Barrios, D.; Mercedes, E.; Moores, L.; Tapson, V.; Yusen, R.D.; Jiménez, D. Intermediate-High Risk Pulmonary Embolism. TH Open 2019, 3, e356–e363.
- Yamamoto, T. Management of patients with high-risk pulmonary embolism: A narrative review. J. Intensive Care. 2018, 6, 16.
- Bledsoe, J.R.; Woller, S.C.; Stevens, S.M.; Aston, V.; Patten, R.; Allen, T.; Horne, B.D.; Dong, L.; Lloyd, J.; Snow, G.; et al. Management of Low-Risk Pulmonary Embolism Patients Without Hospitalization: The Low-Risk Pulmonary Embolism Prospective Management Study. Chest 2018, 154, 249–256.
- Piazza, G. Advanced Management of Intermediate- and High-Risk Pulmonary Embolism: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 76, 2117–2127.
More
Information
Subjects:
Medicine, General & Internal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
873
Revisions:
2 times
(View History)
Update Date:
14 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





