Pulmonary thromboembolism is a very common cardiovascular disease, with a high mortality rate. This disease still represents a great challenge both in diagnosis and treatment. The heterogeneous clinical picture, often without pathognomonic signs and symptoms, represents a huge differential diagnostic problem even for experienced doctors. The decisions surrounding this therapeutic regimen also represent a major dilemma in the group of patients who are hemodynamically stable at initial presentation and have signs of right ventricular (RV) dysfunction proven by echocardiography and positive biomarker values (pulmonary embolism of intermediate–high risk).
- acute pulmonary embolism
- dilemmas
- therapeutic treatment
- recommendations
1. From Diagnostic Doubts to the Correct Diagnosis of Pulmonary Embolism
2. Risk Stratification in Patients with PE
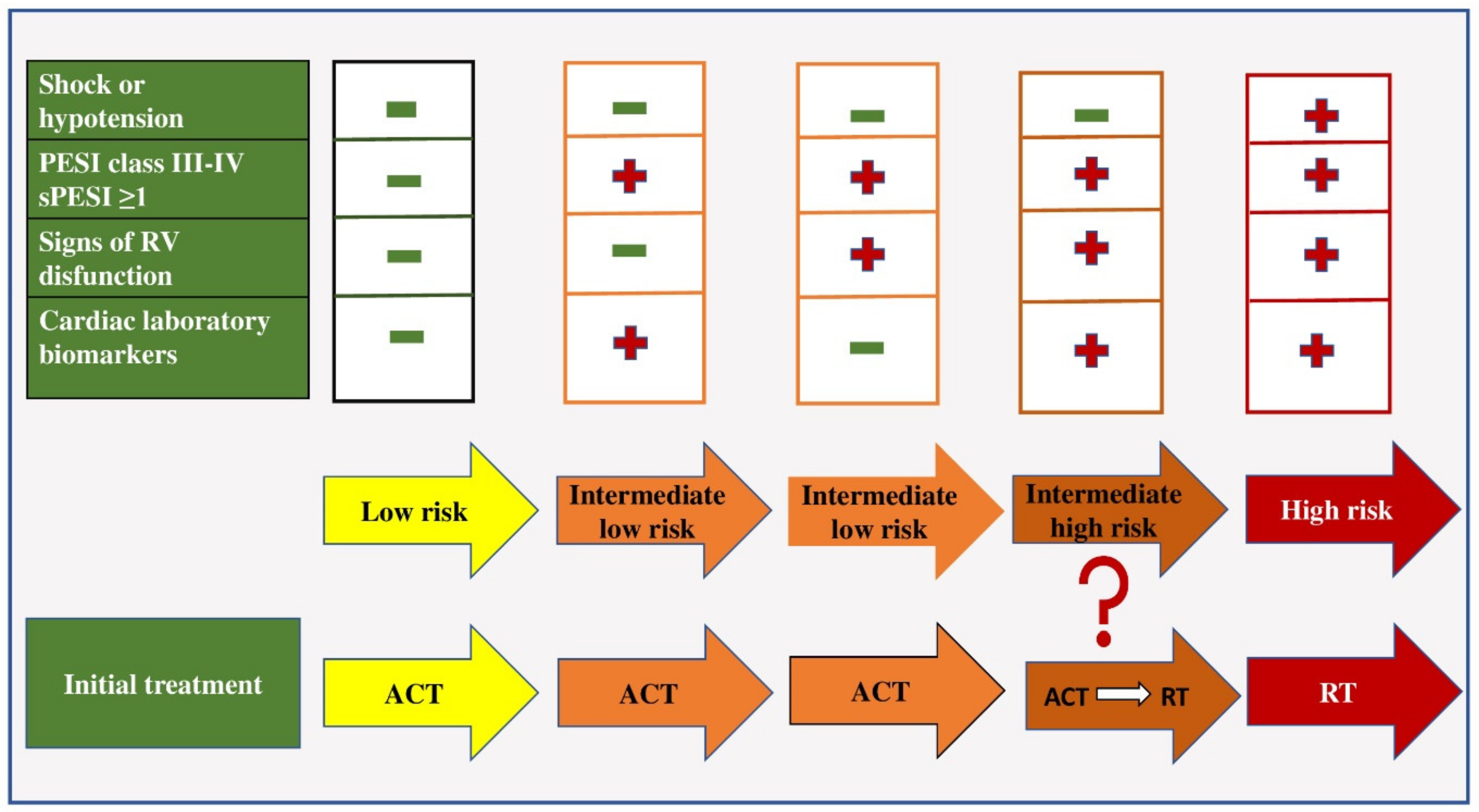
This entry is adapted from the peer-reviewed paper 10.3390/ph15091146
References
- Heit, J.A.; Spencer, F.A.; White, R.H. The epidemiology of venous thromboembolism. J. Thromb. Thrombolysis 2016, 41, 3–14.
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Buller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.; Kakkar, A.; Konstantinides, S.V.; McCumber, M.; et al. ISTH Steering Committee for World Thrombosis Day. Thrombosis: A major contributor to global disease burden. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2363–2371.
- Mazzolai, L.; Aboyans, V.; Ageno, W.; Agnelli, G.; Alatri, A.; Bauersachs, R.; Brekelmans, M.P.A.; Büller, H.R.; Elias, A.; Farge, D.; et al. Diagnosis and management of acute deep vein thrombosis: A joint consensus document from the European Society of Cardiology working groups of aorta and peripheral vascular diseases and pulmonary circulation and right ventricular function. Eur. Heart J. 2018, 39, 4208–4218.
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603.
- Tsuyuki, H.; Yamamoto, N.; Unno, N.; Inuzuka, K.; Sano, M.; Katahashi, K.; Yata, T.; Kayama, T.; Yamanaka, Y.; Endo, Y.; et al. Characteristics and Prognostic Factors of Venous Thromboembolism in Cancer Patients. Ann. Vasc. Dis. 2022, 15, 107–112.
- Gussoni, G.; Frasson, S.; La Regina, M.; Di Micco, P.; Monreal, M. RIETE Investigators. Three-month mortality rate and clinical predictors in patients with venous thromboembolism and cancer. Findings from the RIETE registry. Thromb. Res. 2013, 131, 24–30.
- Gialeraki, A.; Valsami, S.; Pittaras, T.; Panayiotakopoulos, G.; Politou, M. Oral Contraceptives and HRT Risk of Thrombosis. Clin. Appl. Thromb. Hemost. 2018, 24, 217–225.
- Kukla, P.; McIntyre, W.F.; Koracevic, G.; Kutlesic-Kurtovic, D.; Fijorek, K.; Atanaskovic, V.; Krupa, E.; Mirek-Bryniarska, E.; Jastrzębski, M.; Bryniarski, L.; et al. Relation of atrial fibrillation and right-sided cardiac thrombus to outcomes in patients with acute pulmonary embolism. Am. J. Cardiol. 2015, 115, 825–830.
- Righini, M.; Robert-Ebadi, H.; Le Gal, G. Diagnosis of acute pulmonary embolism. J. Thromb. Haemost. 2017, 15, 1251–1261.
- Morrone, D.; Morrone, V. Acute Pulmonary Embolism: Focus on the Clinical Picture. Korean Circ. J. 2018, 48, 365–381.
- Nilsson, L.T.; Andersson, T.; Larsen, F.; Lang, I.M.; Liv, P.; Söderberg, S. Dyspnea after pulmonary embolism: A nation-wide population-based case-control study. Pulm. Circ. 2021, 11, 20458940211046831.
- Pollack, C.V.; Schreiber, D.; Goldhaber, S.Z.; Slattery, D.; Fanikos, J.; O’Neil, B.J.; Thompson, J.R.; Hiestand, B.; Briese, B.A.; Pendleton, R.C.; et al. Clinical characteristics, management, and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department: Initial report of EMPEROR (Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry). J. Am. Coll. Cardiol. 2011, 57, 700–706.
- Stein, P.D.; Beemath, A.; Matta, F.; Weg, J.G.; Yusen, R.D.; Hales, C.A.; Hull, R.D.; Leeper, K.V.; Sostman, H.D., Jr.; Tapson, V.F.; et al. Clinical characteristics of patients with acute pulmonary embolism: Data from PIOPED II. Am. J. Med. 2007, 120, 871–879.
- Stein, P.D.; Hull, R.D.; Kayali, F.; Ghali, W.A.; Alshab, A.K.; Olson, R.E. Venous thromboembolism according to age: The impact of an aging population. Arch. Intern. Med. 2004, 164, 2260–2265.
- Ji, Q.Y.; Wang, M.F.; Su, C.M.; Yang, Q.F.; Feng, L.F.; Zhao, L.Y.; Fang, S.Y.; Zhao, F.H.; Li, W.M. Clinical symptoms and related risk factors in pulmonary embolism patients and cluster analysis based on these symptoms. Sci. Rep. 2017, 7, 14887.
- Kucher, N.; Rossi, E.; De Rosa, M.; Goldhaber, S.Z. Massive pulmonary embolism. Circulation 2006, 113, 577–582.
- Khan, F.; Tritschler, T.; Kahn, S.R.; Rodger, M.A. Venous thromboembolism. Lancet. 2021, 398, 64–77.
- Tarbox, A.K.; Swaroop, M. Pulmonary embolism. Int. J. Crit. Illn. Inj. Sci. 2013, 3, 69–72.
- Righini, M.; Robert-Ebadi, H. Diagnosis of acute Pulmonary Embolism. Hamostaseologie 2018, 38, 11–21.
- Kearon, C.; de Wit, K.; Parpia, S.; Schulman, S.; Afilalo, M.; Hirsch, A.; Spencer, F.A.; Sharma, S.; D’Aragon, F.; Deshaies, J.F.; et al. PEGeD Study Investigators. Diagnosis of Pulmonary Embolism with d-Dimer Adjusted to Clinical Probability. N. Engl. J. Med. 2019, 381, 2125–2134.
- Ziegler, T.; Murzik, M.; Schau, A.; Winkler, C.; Fünfstück, R. Interpretation erhöhter D-Dimerkonzentration . Hamostaseologie 2004, 24, 144–146.
- Shafiee, M.A.; Hosseini, S.F.; Mortazavi, M.; Emami, A.; Mojtahed Zadeh, M.; Moradi, S.; Shaker, P. Anticoagulation therapy in COVID-19 patients with chronic kidney disease. J. Res. Med. Sci. 2021, 26, 63.
- Righini, M.; Goehring, C.; Bounameaux, H.; Perrier, A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am. J. Med. 2000, 109, 357–361.
- Righini, M.; Van Es, J.; Den Exter, P.L.; Roy, P.M.; Verschuren, F.; Ghuysen, A.; Rutschmann, O.T.; Sanchez, O.; Jaffrelot, M.; Trinh-Duc, A.; et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: The ADJUST-PE study. JAMA 2014, 311, 1117–1124.
- Van Es, N.; Kraaijpoel, N.; Klok, F.A.; Huisman, M.V.; Den Exter, P.L.; Mos, I.C.; Galipienzo, J.; Büller, H.R.; Bossuyt, P.M. The original and simplified Wells rules and age-adjusted D-dimer testing to rule out pulmonary embolism: An individual patient data meta-analysis. J. Thromb. Haemost. 2017, 15, 678–684.
- Righini, M.; Robert-Ebadi, H.; Elias, A.; Sanchez, O.; Le Moigne, E.; Schmidt, J.; Le Gall, C.; Cornuz, J.; Aujesky, D.; Roy, P.M.; et al. CT-PE-Pregnancy Group. Diagnosis of Pulmonary Embolism During Pregnancy: A Multicenter Prospective Management Outcome Study. Ann. Intern. Med. 2018, 169, 766–773.
- Tromeur, C.; van der Pol, L.M.; Le Roux, P.Y.; Ende-Verhaar, Y.; Salaun, P.Y.; Leroyer, C.; Couturaud, F.; Kroft, L.J.M.; Huisman, M.V.; Klok, F.A. Computed tomography pulmonary angiography versus ventilation-perfusion lung scanning for diagnosing pulmonary embolism during pregnancy: A systematic review and meta-analysis. Haematologica 2019, 104, 176–188.
- Alonso-Martínez, J.L.; Sánchez, F.J.; Echezarreta, M.A. Delay and misdiagnosis in sub-massive and non-massive acute pulmonary embolism. Eur. J. Intern. Med. 2010, 21, 278–282.
- Van der Hulle, T.; Cheung, W.Y.; Kooij, S.; Beenen, L.F.M.; van Bemmel, T.; van Es, J.; Faber, L.M.; Hazelaar, G.M.; Heringhaus, C.; Hofstee, H.; et al. YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): A prospective, multicentre, cohort study. Lancet 2017, 390, 289–297.
- Kwok, C.S.; Wong, C.W.; Lovatt, S.; Myint, P.K.; Loke, Y.K. Misdiagnosis of pulmonary embolism and missed pulmonary embolism: A systematic review of the literature. Health Sci. Rev. 2022, 3, 100022.
- Su, X.F.; Fan, N.; Yang, X.M.; Song, J.M.; Peng, Q.H.; Liu, X. A Novel Electrocardiography Model for the Diagnosis of Acute Pulmonary Embolism. Front. Cardiovasc. Med. 2022, 9, 825561.
- Digby, G.C.; Kukla, P.; Zhan, Z.Q.; Pastore, C.A.; Piotrowicz, R.; Schapachnik, E.; Zareba, W.; Bayés de Luna, A.; Pruszczyk, P.; Baranchuk, A.M. The value of electrocardiographic abnormalities in the prognosis of pulmonary embolism: A consensus paper. Ann. Noninvasive Electrocardiol. 2015, 20, 207–223.
- Vanni, S.; Polidori, G.; Vergara, R.; Pepe, G.; Nazerian, P.; Moroni, F.; Garbelli, E.; Daviddi, F.; Grifoni, S. Prognostic value of ECG among patients with acute pulmonary embolism and normal blood pressure. Am. J. Med. 2009, 122, 257–264.
- Novicic, N.; Dzudovic, B.; Subotic, B.; Shalinger-Martinovic, S.; Obradovic, S. Electrocardiography changes and their significance during treatment of patients with intermediate-high and high-risk pulmonary embolism. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 271–278.
- Ermıs, N.; Ermıs, H.; Sen, N.; Kepez, A.; Cuglan, B. QT dispersion in patients with pulmonary embolism. Wien. Klin. Wochenschr. 2010, 122, 691–697.
- Ptaszynska-Kopczynska, K.; Kiluk, I.; Sobkowicz, B. Atrial Fibrillation in Patients with Acute Pulmonary Embolism: Clinical Significance and Impact on Prognosis. Biomed Res. Int. 2019, 2019, 7846291.
- Kucher, N.; Walpoth, N.; Wustmann, K.; Noveanu, M.; Gertsch, M. QR in V1--an ECG sign associated with right ventricular strain and adverse clinical outcome in pulmonary embolism. Eur. Heart J. 2003, 24, 1113–1119.
- Kurnicka, K.; Lichodziejewska, B.; Goliszek, S.; Dzikowska-Diduch, O.; Zdończyk, O.; Kozłowska, M.; Kostrubiec, M.; Ciurzyński, M.; Palczewski, P.; Grudzka, K.; et al. Echocardiographic Pattern of Acute Pulmonary Embolism: Analysis of 511 Consecutive Patients. J. Am. Soc. Echocardiogr. 2016, 29, 907–913.
- McConnell, M.V.; Solomon, S.D.; Rayan, M.E.; Lee, R.T.; Come, P.C.; Goldhaber, S.Z.; Lee, R.T. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am. J. Cardiol. 1996, 78, 469–473.
- Casazza, F.; Bongarzoni, A.; Capozi, A.; Agostoni, O. Regional right ventricular dysfunction in acute pulmonary embolism and right ventricular infarction. Eur. J. Echocardiogr. 2005, 6, 11–14.
- Shafiq, Q.; Assaly, R.; Kanjwal, Y. McConnell Sign in a Patient with Massive Acute Pulmonary Embolism. Case Rep. Cardiol. 2011, 2011, 201097.
- Naeem, K. Floating thrombus in the right heart associated with pulmonary embolism: The role of echocardiography. Pak. J. Med. Sci. 2015, 31, 233–235.
- Bĕlohlávek, J.; Dytrych, V.; Linhart, A. Pulmonary embolism, part I: Epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp. Clin. Cardiol. 2013, 18, 129–138.
- Elias, A.; Mallett, S.; Daoud-Elias, M.; Poggi, J.N.; Clarke, M. Prognostic models in acute pulmonary embolism: A systematic review and meta-analysis. BMJ 2016, 6, e010324.
- Duffett, L.; Castellucci, L.A.; Forgie, M.A. Pulmonary embolism: Update on management and controversies. BMJ 2020, 370, m2177.
- Mirambeaux, R.; León, F.; Bikdeli, B.; Morillo, R.; Barrios, D.; Mercedes, E.; Moores, L.; Tapson, V.; Yusen, R.D.; Jiménez, D. Intermediate-High Risk Pulmonary Embolism. TH Open 2019, 3, e356–e363.
- Yamamoto, T. Management of patients with high-risk pulmonary embolism: A narrative review. J. Intensive Care. 2018, 6, 16.
- Bledsoe, J.R.; Woller, S.C.; Stevens, S.M.; Aston, V.; Patten, R.; Allen, T.; Horne, B.D.; Dong, L.; Lloyd, J.; Snow, G.; et al. Management of Low-Risk Pulmonary Embolism Patients Without Hospitalization: The Low-Risk Pulmonary Embolism Prospective Management Study. Chest 2018, 154, 249–256.
- Piazza, G. Advanced Management of Intermediate- and High-Risk Pulmonary Embolism: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 76, 2117–2127.
