
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Arnoldo Aquino-Gálvez | -- | 3010 | 2022-09-21 21:00:39 | | | |
| 2 | Amina Yu | -2 word(s) | 3008 | 2022-09-22 03:56:21 | | |
Video Upload Options
Ivermectin (IVM) is a broad-spectrum antiparasitic agent, developed and funded by Merck & Co. in 1974 to control and eradicate onchocerciasis caused by the parasitic worm Onchocerca volvulus in West Africa, which in the 1980s infected approximately 340,000 people. At the time, Africa did not have the resources necessary to seek treatments for this condition. The avermectins, of which IVM is a member, were discovered by Professor Satoshi Ōmura as fermentation products of the bacterium Streptomyces avermitilis at the Kitasato Institute in Tokyo. For this discovery, he received the 2015 Nobel Prize in Physiology and Medicine, which he shared with William Campbell. IVM is used to treat onchocerciasis, lymphatic filariasis, strongyloidiasis and scabies, and, very recently, has been used to combat lice. The drug’s low cost, high efficacy, safety, and marked tropism for helminths, as well as the fact that it has almost no impact on human biochemistry, have led to the inclusion of IVM in the twentieth list of essential medicines and sixth list of vital medicines in children, a recommendation made by the expert committee of the World Health Organization (WHO) in 2019. The safety profile is attributed to its selective affinity for ion channels.
1. Mechanisms of Action of Ivermectin
2. Possible Benefits of Ivermectin in SARS-CoV-2
2.1. Antiviral Activity
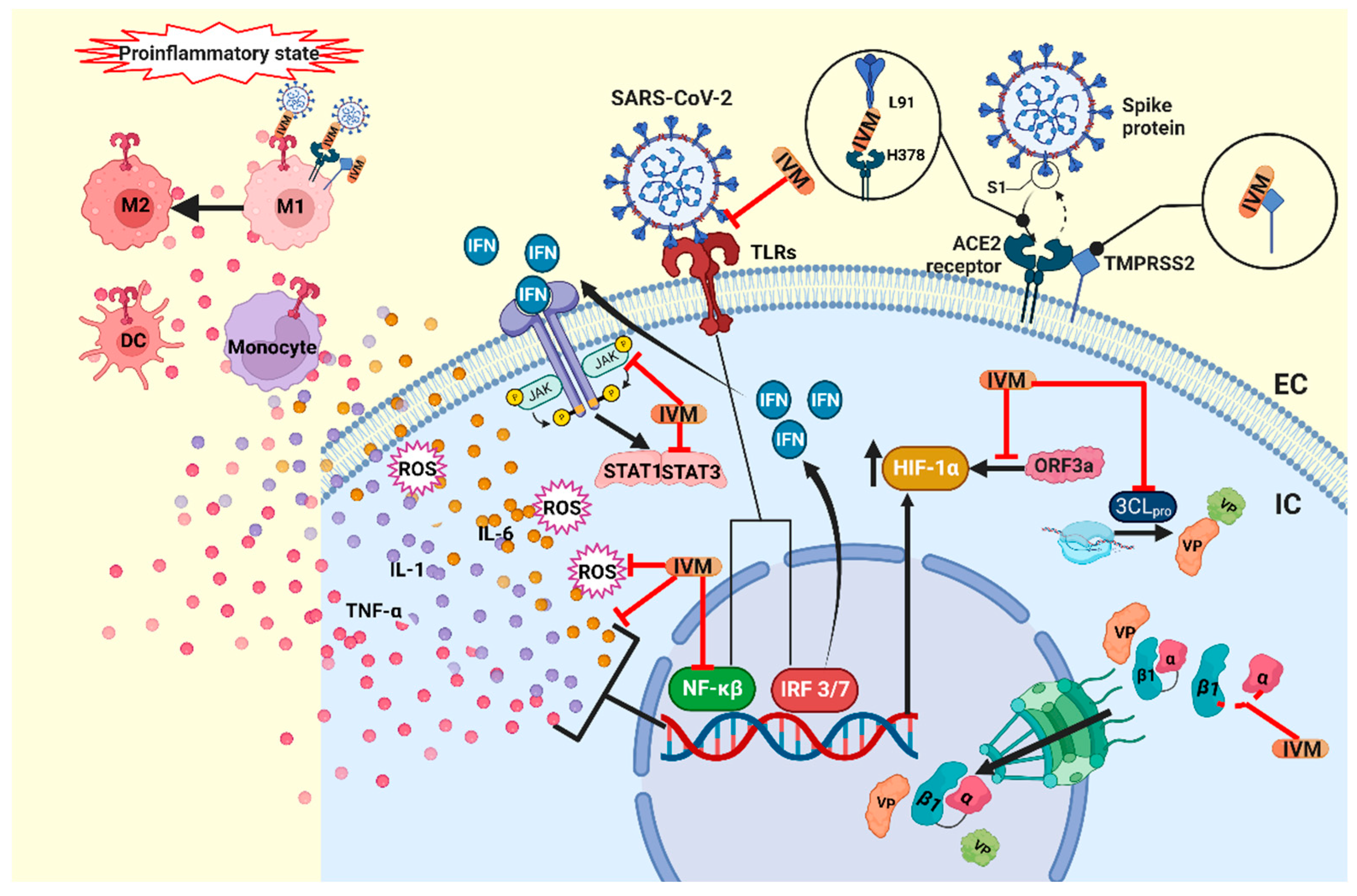
2.2. Immunomodulatory Effects
2.3. Antitumoral Mechanisms
3. Ivermectin in COVID-19 Comorbidities
3.1. Nosocomial Pneumonia
3.2. Wound Healing
References
- IVERMECTIN. Micromedex® (Electronic Version); IBM Watson Health: Greenwood Village, CO, USA; Available online: https://www.micromedexsolutions.com/ (accessed on 14 August 2022).
- González, P.; González, F.A.; Ueno, K. Ivermectin in human medicine, an overview of the current status of its clinical applications. Curr. Pharm. Biotechnol. 2012, 13, 1103–1109.
- Kircik, L.H.; Del Rosso, J.Q.; Layton, A.M.; Schauber, J. Over 25 Years of Clinical Experience with Ivermectin: An Overview of Safety for an Increasing Number of Indications. J. Drugs Dermatol. 2016, 15, 325–332.
- Canga, A.G.; Prieto, A.M.S.; Diez Liébana, M.J.; Martínez, N.F.; Sierra Vega, M.; García Vieitez, J.J. The pharmacokinetics and interac-tions of ivermectin in humans—A mini-review. AAPS J. 2008, 10, 42–46.
- Ashour, D.S. Ivermectin: From theory to clinical application. Int. J. Antimicrob. Agents 2019, 54, 134–142.
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 3–6.
- Heidary, F.; Gharebaghi, R. Ivermectin: A systematic review from antiviral effects to COVID-19 complementary regimen. J. Antibiot. 2020, 73, 593–602.
- Li, N.; Zhao, L.; Zhan, X. Quantitative proteomics reveals a broad-spectrum antiviral property of ivermectin, benefiting for COVID-19 treatment. J. Cell Physiol. 2021, 236, 2959–2975.
- Wagstaff, K.; Sivakumaran, H.; Heaton, S.; Harrich, D.; Jans, D. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 2012, 443, 851–856.
- Wagstaff, K.M.; Rawlinson, S.M.; Hearps, A.C.; Jans, D.A. An AlphaScreen®-based assay for high-throughput screening for spe-cific inhibitors of nuclear import. J. Biomol. Screen. 2011, 16, 192–200.
- Yang, S.N.Y.; Atkinson, S.C.; Wang, C.; Lee, A.; Bogoyevitch, M.A.; Borg, N.A.; Jans, D.A. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antivir. Res. 2020, 177, 104760.
- Lv, C.; Liu, W.; Wang, B.; Dang, R.; Qiu, L.; Ren, J.; Yan, C.; Yang, Z.; Wang, X. Ivermectin inhibits DNA polymerase UL42 of pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and vivo. Antivir. Res. 2018, 159, 55–62.
- Wolff, G.; Limpens, R.W.; Zevenhoven-Dobbe, J.C.; Laugks, U.; Zheng, S.; de Jong, A.W.; Koning, R.I.; Agard, D.A.; Grünewald, K.; Koster, A.J.; et al. A molecular pore spans the double membrane of the coronavirus replication organelle. Science 2020, 369, 1395–1398.
- Snijder, E.J.; Limpens, R.W.A.L.; de Wilde, A.H.; de Jong, A.W.M.; Zevenhoven-Dobbe, J.C.; Maier, H.J.; Faas, F.F.G.A.; Koster, A.J.; Bárcena, M. A unifying structural and functional model of the coronavirus replication organelle: Tracking down RNA synthesis. PLoS Biol. 2020, 18, e3000715.
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23.
- Schmith, V.D.; Zhou, J.; Lohmer, L.R. The Approved Dose of Ivermectin Alone is not the Ideal Dose for the Treatment of COVID-19. Clin. Pharmacol. Ther. 2020, 108, 762–765.
- Lehrer, S.; Rheinstein, P.H. Ivermectin docks to the SARS-CoV-2 spike receptor-binding domain attached to ACE2. In Vivo 2020, 34, 3023–3026.
- Elalfy, H.; Besheer, T.; El-Mesery, A.; El-Gilany, A.H.; Soliman, M.A.A.; Alhawarey, A.; Alegezy, M.; Elhadidy, T.; Hewidy, A.A.; Zaghloul, H.; et al. Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19. J. Med. Virol. 2021, 93, 3176–3183.
- Choudhury, A.; Das, N.C.; Patra, R.; Bhattacharya, M.; Ghosh, P.; Patra, B.C.; Mukherjee, S. Exploring the bind-ing efficacy of ivermectin against the key proteins of SARS-CoV-2 pathogenesis: An in silico approach. Future Virol. 2021, 16, 277–291.
- Yuce, M.; Cicek, E.; Inan, T.; Dag, A.B.; Kurkcuoglu, O.; Sungur, F.A. Repurposing of FDA-approved drugs against active site and potential allosteric drug-binding sites of COVID-19 main protease. Proteins Struct. Funct. Bioinform. 2021, 89, 1425–1441.
- Ahmed, S.; Karim, M.M.; Ross, A.G.; Hossain, M.S.; Clemens, J.D.; Sumiya, M.K.; Phru, C.S.; Rahman, M.; Zaman, K.; Somani, J.; et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int. J. Infect. Dis. 2021, 103, 214–216.
- de Melo, G.D.; Lazarini, F.; Larrous, F.; Feige, L.; Kornobis, E.; Levallois, S.; Marchio, A.; Kergoat, L.; Hardy, D.; Cokelaer, T.; et al. Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin. EMBO Mol. Med. 2021, 13, e14122.
- Laura CMiller, Y.S. Macrophage Polarization in Virus-Host Interactions. J. Clin. Cell Immunol. 2015, 6, 311.
- Miyazaki-Krause, R.; Buisson, B.; Bertrand, S.; Corringer, P.-J.; Galzi, J.-L.; Changeux, J.-P.; Bertrand, D. Ivermectin: A Positive Allosteric Effector of the α7 Neuronal Nicotinic Acetylcholine Receptor. Mol. Pharmacol. 1998, 53, 283–294.
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor α7 subunit is an essen-tial regulator of inflammation. Nature 2003, 421, 384–388.
- Galvis, G.; Lips, K.S.; Kummer, W. Expression of Nicotinic Acetylcholine Receptors on Murine Alveolar Macrophages. J. Mol. Neurosci. 2006, 30, 107–108.
- Rahbar, A.; Shakyba, S.; Ghaderi, M.; Kazemi, K.; Fagheh, A.F.; Farsinejad, P.; Khosravi, A.; Louyeh, P.A.; Mirzaeyian, E.; Chamanara, M.; et al. Ivermectin-functionalized multiwall carbon nanotube enhanced the locomotor activity and neuropathic pain by modulating M1/M2 macrophage and decrease oxidative stress in rat model of spinal cord injury. Heliyon 2021, 7, e07311.
- Ebbelaar, C.C.F.; Venema, A.W.; Van Dijk, M.R. Topical Ivermectin in the Treatment of Papulopustular Rosacea: A Systematic Review of Evidence and Clinical Guideline Recommendations. Dermatol. Ther. 2018, 8, 379–387.
- Ci, X.; Li, H.; Yu, Q.; Zhang, X.; Yu, L.; Chen, N.; Song, Y.; Deng, X. Avermectin exerts anti-inflammatory effect by downregulating the nuclear transcription factor kappa-B and mitogen-activated protein kinase activation pathway. Fundam. Clin. Pharmacol. 2009, 23, 449–455.
- Di Nicolantonio, J.J.; Barroso, J.; Mccarty, M. Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19. Open Heart 2020, 7, e001350.
- Zhang, X.; Li, J.; Chen, C.; Ci, X.; Yu, Q.; Zhang, X.; Deng, X. Protective effect of abamectin on acute lung injury induced by lipopoly-saccharide in mice. Fundam. Clin. Pharmacol. 2011, 25, 700–707.
- Zhang, X.; Song, Y.; Ci, X.; An, N.; Ju, Y.; Li, H.; Wang, X.; Han, C.; Cui, J.; Deng, X. Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice. Agents Actions 2008, 57, 524–529.
- Rynkiewicz, E.C.; Clerc, M.; Babayan, S.; Pedersen, A.B. Variation in Local and Systemic Pro-Inflammatory Immune Markers of Wild Wood Mice after Anthelmintic Treatment. Integr. Comp. Biol. 2019, 59, 1190–1202.
- Low, Z.Y.; Yip, A.J.W.; Lal, S.K. Repositioning Ivermectin for COVID-19 treatment: Molecular mechanisms of action against SARS-CoV-2 replication. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166294.
- Matsuyama, T.; Kubli, S.P.; Yoshinaga, S.K.; Pfeffer, K.; Mak, T.W. An aberrant STAT pathway is central to COVID-19. Cell Death Differ. 2020, 27, 3209–3225.
- Feng, Y.; Wang, J.; Cai, B.; Bai, X.; Zhu, Y. Ivermectin accelerates autophagic death of glioma cells by inhibiting glycolysis through blocking GLUT4 mediated JAK/STAT signaling pathway activation. Environ. Toxicol. 2021, 37, 754–764.
- Lu, H.; Zhou, L.; Zuo, H.; Le, W.; Hu, J.; Zhang, T.; Li, M.; Yuan, Y. Ivermectin synergizes sorafenib in hepatocellular carcinoma via targeting multiple oncogenic pathways. Pharmacol. Res. Perspect. 2022, 10, e00954.
- Batiha, G.E.-S.; Alqahtani, A.; Ilesanmi, O.; Saati, A.; El-Mleeh, A.; Hetta, H.; Beshbishy, A.M. Avermectin Derivatives, Pharmacokinetics, Therapeutic and Toxic Dosages, Mechanism of Action, and Their Biological Effects. Pharmaceuticals 2020, 13, 196.
- Zhang, X.; Song, Y.; Xiong, H.; Ci, X.; Li, H.; Yu, L.; Zhang, L.; Deng, X. Inhibitory effects of ivermectin on nitric oxide and prostaglandin E2 pro-duction in LPS-stimulated RAW 264.7 macrophages. Int. Immunopharmacol. 2009, 9, 354–359.
- Siemieniuk, R.A.C.; Bartoszko, J.J.; Ge, L.; Zeraatkar, D.; Izcovich, A.; Kum, E.; Pardo-Hernandez, H.; Rochwerg, B.; Lamontagne, F.; Han, M.A.; et al. Drug treatments for COVID-19: Living systematic review and network meta-analysis. BMJ 2020, 370, m2980.
- Juarez, M.; Schcolnik-Cabrera, A.; Dueñas-Gonzalez, A. The multitargeted drug ivermectin: From an antiparasitic agent to a repositioned cancer drug. Am. J. Cancer Res. 2018, 8, 317–331.
- Liu, Y.; Fang, S.; Sun, Q.; Liu, B. Anthelmintic drug ivermectin inhibits angiogenesis, growth and survival of glioblastoma through inducing mitochondrial dysfunction and oxidative stress. Biochem. Biophys. Res. Commun. 2016, 480, 415–421.
- Juarez, M.; Schcolnik-Cabrera, A.; Dominguez-Gomez, G.; Chavez-Blanco, A.; Diaz-Chavez, J.; Duenas-Gonzalez, A. Antitumor effects of ivermectin at clinically feasible concentrations support its clinical development as a repositioned cancer drug. Cancer Chemother. Pharmacol. 2020, 85, 1153–1163.
- Didier, A.; Loor, F. The abamectin derivative ivermectin is a potent P-glycoprotein inhibitor. Anti-Cancer Drugs 1996, 7, 745–751.
- Yin, J.; Park, G.; Lee, J.E.; Choi, E.Y.; Park, J.Y.; Kim, T.-H.; Park, N.; Jin, X.; Jung, J.-E.; Shin, D.; et al. DEAD-box RNA helicase DDX23 modulates glioma malignancy via elevating miR-21 biogenesis. Brain 2015, 138, 2553–2570.
- DraDraganov, D.; Gopalakrishna-Pillai, S.; Chen, Y.R.; Zuckerman, N.; Moeller, S.; Wang, C.; Ann, D.; Lee, P.P. Modulation of P2X4/P2X7/Pannexin-1 sensitivity to extracellular ATP via Ivermectin induces a non-apoptotic and inflammatory form of cancer cell death. Sci. Rep. 2015, 5, 16222.
- Nörenberg, W.; Sobottka, H.; Hempel, C.; Plötz, T.; Fischer, W.; Schmalzing, G.; Schaefer, M. Positive allosteric modulation by ivermectin of human but not murine P2X7 receptors. J. Cereb. Blood Flow Metab. 2012, 167, 48–66.
- Sharmeen, S.; Skrtic, M.; Sukhai, M.A.; Hurren, R.; Gronda, M.; Wang, X.; Fonseca, S.B.; Sun, H.; Wood, T.E.; Ward, R.; et al. The antiparasitic agent ivermectin induces chlo-ride-dependent membrane hyperpolarization and cell death in leukemia cells. Blood 2010, 116, 3593–3603.
- Dominguez-Gomez, G.; Chavez-Blanco, A.; Medina-Franco, J.L.; Saldivar-Gonzalez, F.; Flores-Torrontegui, Y.; Juarez, M.; Díaz-Chávez, J.; Gonzalez-Fierro, A.; Dueñas-González, A. Ivermectin as an inhibitor of cancer stem-like cells. Mol. Med. Rep. 2017, 17, 3397–3403.
- Ashraf, S.; Prichard, R. Ivermectin exhibits potent anti-mitotic activity. Vet. Parasitol. 2016, 226, 1–4.
- Song, D.; Liang, H.; Qu, B.; Li, Y.; Liu, J.; Zhang, Y.; Li, L.; Hu, L.; Zhang, X.; Gao, A. Ivermectin inhibits the growth of glioma cells by inducing cell cycle arrest and apoptosis in vitro and in vivo. J. Cell. Biochem. 2018, 120, 622–633.
- Deng, F.; Xu, Q.; Long, J.; Xie, H. Suppressing ROS-TFE3-dependent autophagy enhances ivermectin-induced apoptosis in hu-man melanoma cells. J. Cell Biochem. 2019, 120, 1702–1715.
- Lee, J.-Y.; Lim, W.; Ham, J.; Kim, J.; You, S.; Song, G. Ivermectin induces apoptosis of porcine trophectoderm and uterine luminal epithelial cells through loss of mitochondrial membrane potential, mitochondrial calcium ion overload, and reactive oxygen species generation. Pestic. Biochem. Physiol. 2019, 159, 144–153.
- Kilic, M.; Kasperczyk, H.; Fulda, S.; Debatin, K.-M. Role of hypoxia inducible factor-1 alpha in modulation of apoptosis resistance. Oncogene 2006, 26, 2027–2038.
- Rankin, E.B.; Giaccia, A.J. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008, 15, 678–685.
- Kosyna, F.K.; Nagel, M.; Kluxen, L.; Kraushaar, K.; Depping, R. The importin α/β-specific inhibitor Ivermectin affects HIF-dependent hypoxia response pathways. Biol. Chem. 2015, 396, 1357–1367.
- Vishnoi, K.; Viswakarma, N.; Rana, A.; Rana, B. Transcription Factors in Cancer Development and Therapy. Cancers 2020, 12, 2296.
- Tian, M.; Liu, W.; Li, X.; Zhao, P.; Shereen, M.A.; Zhu, C.; Huang, S.; Liu, S.; Yu, X.; Yue, M.; et al. HIF-1α promotes SARS-CoV-2 infection and aggravates inflammatory responses to COVID-19. Signal Transduct Target Ther. 2021, 6, 308.
- Gil, E.; Martyn, E.; Rokadiya, S.; Jain, S.; Chin, T.L. Bacterial Coinfection in COVID-19. Clin. Infect. Dis. 2021, 73, e843–e845.
- Mahmoudi, H. Bacterial co-infections and antibiotic resistance in patients with COVID-19. GMS Hyg. Infect. Control 2020, 15, 35.
- Rawson, T.M.; Moore, L.S.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and fungal co-infection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing Timothy. Clin Infect. Dis. 2020, 71, 2459–2468.
- Chaudhry, R.; Sreenath, K.; Batra, P.; Vinayaraj, E.V.; Rathor, N.; Saikiran, K.; Aravindan, A.; Singh, V.; Brijwal, M.; Soneja, M.; et al. Atypical bacterial co-infections among patients with COVID-19: A study from India. J. Med. Virol. 2022, 94, 303–309.
- Yang, S.; Hua, M.; Liu, X.; Du, C.; Pu, L.; Xiang, P.; Wang, L.; Liu, J. Bacterial and fungal co-infections among COVID-19 patients in intensive care unit. Microbes Infect. 2021, 23, 104806.
- Baskaran, V.; Lawrence, H.; Lansbury, L.E.; Webb, K.; Safavi, S.; Zainuddin, N.I.; Huq, T.; Eggleston, C.; Ellis, J.; Thakker, C.; et al. Co-infection in critically ill patients with COVID-19: An observational cohort study from England. J. Med. Microbiol. 2021, 70, 001350.
- Gambhir, R.S.; Aggarwal, A.; Bhardwaj, A.; Kaur, A.; Sohi, R.K.; Mehta, S. COVID-19 and mucormycosis (Black Fungus): An epidemic within the pandemic. Rocz. Państwowego Zakładu Hig. 2021, 72, 239–244.
- Elabbadi, A.; Turpin, M.; Gerotziafas, G.T.; Teulier, M.; Voiriot, G.; Fartoukh, M. Bacterial coinfection in critically ill COVID-19 pa-tients with severe pneumonia. Infection 2021, 49, 559–562.
- Chong, W.H.; Saha, B.K.; Ramani, A.; Chopra, A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection 2021, 49, 591–605.
- Mirzaei, R.; Goodarzi, P.; Asadi, M.; Soltani, A.; Aljanabi, H.A.A.; Jeda, A.S.; Dashtbin, S.; Jalalifar, S.; Mohammadzadeh, R.; Teimoori, A.; et al. Bacterial co-infections with SARS-CoV-2. IUBMB Life 2020, 72, 2097–2111.
- Ny, P.; Kelsom, C.; Chron, A.; Lou, M.; Nieberg, P.; Shriner, K.; Huse, H.; Wong-Beringer, A. Factors associated with prompt recovery among hospitalised patients with coronavirus disease 2019. Int. J. Clin. Pract. 2021, 75, e14818.
- Lim, L.E.; Vilchèze, C.; Ng, C.; Jacobs, W.R.; Ramón-García, S.; Thompson, C.J. Anthelmintic avermectins kill mycobacterium tu-berculosis, including multidrug-resistant clinical strains. Antimicrob. Agents Chemother. 2013, 57, 1040–1046.
- Ashraf, S.; Chaudhry, U.; Raza, A.; Ghosh, D.; Zhao, X. In vitro activity of ivermectin against Staphylococcus aureus clinical isolates. Antimicrob. Resist. Infect. Control 2018, 7, 7–12.
- Čulić, O.; Eraković, V.; Parnham, M.J. Anti-inflammatory effects of macrolide antibiotics. Eur. J. Pharmacol. 2001, 429, 209–229.
- Csóka, B.; Németh, Z.H.; Szabó, I.; Davies, D.L.; Varga, Z.V.; Pálóczi, J.; Falzoni, S.; Di Virgilio, F.; Muramatsu, R.; Yamashita, T.; et al. Macrophage P2X4 receptors augment bacterial killing and protect against sepsis. JCI Insight 2018, 3, e99431.
- Yim, J.; Lim, H.H.; Kwon, Y. COVID-19 and pulmonary fibrosis: Therapeutics in clinical trials, repurposing, and potential development. Arch. Pharmacal Res. 2021, 44, 499–513.
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Socie-ty/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748.
- Sia, D.K.; Mensah, K.B.; Opoku-Agyemang, T.; Folitse, R.D.; Darko, D.O. Mechanisms of ivermectin-induced wound healing. BMC Vet.-Res. 2020, 16, 397.
- Feaster, B.; Cline, A.; Feldman, S.R.; Taylor, S. Clinical effectiveness of novel rosacea therapies. Curr. Opin. Pharmacol. 2019, 46, 14–18.
- Charnowski, S.; Wollenberg, A.; Reinholz, M. Perioral Dermatitis Successfully Treated with Topical Ivermectin. Ann. Dermatol. 2019, 31, S27–S28.
- Ventre, E.; Rozières, A.; Lenief, V.; Albert, F.; Rossio, P.; Laoubi, L.; Dombrowicz, D.; Staels, B.; Ulmann, L.; Julia, V.; et al. Topical ivermectin improves allergic skin inflammation. Allergy 2017, 72, 1212–1221.
- Barańska-Rybak, W.; Kowalska-Olędzka, E. New indications for topical ivermectin 1% cream: A case series study. Adv. Dermatol. Allergol. 2019, 36, 58–62.




