1. Mechanisms of Action of Ivermectin
Ivermectin is a broad-spectrum drug with numerous effects on parasites, nematodes, arthropods, flavivirus, mycobacteria and mammals through a variety of mechanisms. The mechanism of action of Ivermectin as an anthelmintic agent at various stages in the life cycle works by binding to glutamate-gated chloride ion channels in the nerve cells and invertebrate muscles of microfilaria [
42,
43]. The union of IVM with the channels causes an increment in the cell membrane permeability for chloride ions, hyperpolarizing the membrane and interrupting the motility, feeding and reproduction, leading to the paralysis and death of the parasite. In addition to the glutamate-gated ion channels, ivermectin is also an agonist of the neurotransmitter gamma-aminobutyric acid (GABA)-activated channels. Since GABA channels in mammals are exclusively found in the central nervous system, and IVM does not readily cross the blood–brain barrier, it has a favorable safety profile in regard to the treatment doses used in humans [
6,
42,
43]. Ivermectin achieves adequate levels of availability when administered orally, and due to its high lipid solubility, IVM is widely distributed, with a volume of distribution of 46.8 L. The metabolism of IVM is hepatic, primarily effected by the CYP3A4, and it is removed through the feces and only 1% through urine [
2,
42,
44].
2. Possible Benefits of Ivermectin in SARS-CoV-2
2.1. Antiviral Activity
One of the most exciting effects of IVM is its possible role as an antiviral against COVID-19 [
45]. Certain reviews have emphasized the antiviral effect of IVM in vitro and in vivo against RNA and DNA viruses [
46]. An experiment in which cell cultures were treated with and without IVM (20 μmol/L) over 24 h identified increases in the gene expression of proteins participating in four antiviral pathways that were statistically significant, including the routes of infection of HCMV, HPV, EBV and HIV1. These results support the broad-spectrum antiviral activity of IVM [
47]. It is essential to highlight that the movement of proteins between the cytoplasm and the nucleus is mediated by the superfamily of proteins called importins, which are essential for cellular processes, such as differentiation and development, and are fundamental in the pathological states of viral diseases and oncogenesis [
48]. The specific viral proteins enter the nucleus of infected cells to perform essential functions as part of the viral replication cycle [
49]. An example is the interaction between the HIV-1 integrase protein and the importin α/β1 heterodimer, which is blocked by IVM, thus inhibiting the nuclear import of the integrase protein and, therefore, damaging the viral replication mechanisms [
48].
The broad-spectrum antiviral activity of IVM is related to the fact that RNA viruses, to transport viral proteins to the nucleus of the host cell, depend on the importin alpha-beta (IMPα/β1) heterodimer during the viral infection process. This importin is blocked by IVM. The transport of viral proteins through IMPα/β1 to the nucleus occurs in order to inhibit the antiviral response that is assembled by a portion of the host cells. This mechanism has been observed in viruses such as Zika, Dengue, HIV-1, yellow fever, Chikungunya and many more [
45,
48,
50]. Regarding the antiviral response of IVM to DNA viruses, it has been shown that, if the viral proteins necessary for viral replication require entry to the nucleus through IMPα/β1, then this can have an antiviral effect, as in the case of the pseudorabies virus and polyomavirus BK [
51].
In the case of SARS-CoV-2, it is known that there is no transport of viral proteins to the cell nucleus, as in the case of the infection mechanisms of other viruses. This is because the viral replication cycle takes place exclusively in the cytoplasm of infected cells. However, it is also known that, as part of the antiviral response, there is a communication that involves the transport of proteins related to the regulation of the antiviral responses of infected cells [
52,
53,
54] (
Figure 2). There is great controversy regarding antiviral activity in the case of SARS-CoV-2; thus, we believe that more studies are required to clarify the mechanism by which a molecule can be considered to have an antiviral capacity.
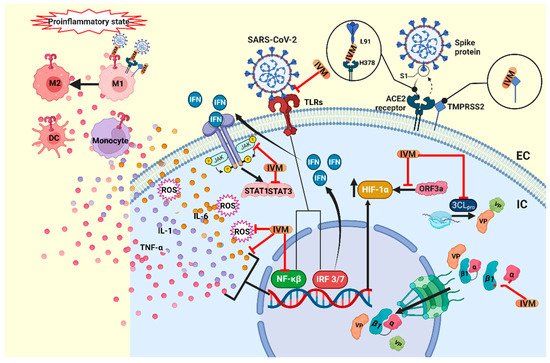
Figure 2. Proposed mechanism of action of ivermectin in COVID-19. IVM blocks the binding complex of the SARS-CoV-2 S protein and the ACE2 receptor, and additionally it blocks the TMPRSS2 protein, inhibiting viral entry into the host cell. IVM could also inhibit the TLR receptors and block NF-κβ, inhibiting the production of the cytokines TNF-α, IL-1 and IL-6 and ROS. TLR also activates IRF3 and IRF7, which initiate the production of type-I and -III IFNs. IFNs activate the JAK/STAT pathway, while IVM can lower the expression of JAK2 and the activity of STAT3. Moreover, in the cytosol, IVM blocks the 3CLpro, the main protease that participates in the viral replication, and blocks the importin complex α/β1 that transports the VP to the nucleus. Furthermore, IVM blocks the overexpression of HIF-1α, induced by the viral protein ORF3a. IVM has also been shown to mitigate the proinflammatory state, where the cytokine storm activates the participation of monocytes, dendritic cells and macrophages, and IVM also promotes the polarization of M2 macrophages over M1. IVM, ivermectin; IC, intracellular; EC, extracellular; VP, viral protein; TLRs, toll-like receptors; NF-κβ, nuclear factor-kappa beta; TNF-α, tumor necrosis factor-alpha; IL-1, interleukin-1, IL-6; interleukin-6; ROS, reactive oxygen species; IRF 3/7, interferon regulatory factors; DC, dendritic cells; M1, M1 macrophage; M2, M2 macrophage; 3CLpro, 3-chymotrypsin-like protease. Created with BioRender.com.
One of the first studies to suggest that IVM might have an effect against SARS-CoV-2 reported that IVM caused SARS-CoV-2 viral RNA to be reduced approximately 5000 fold within 48 h of its administration in infected cell cultures [
45]. Unfortunately, controversy arose after the publication of this study, after observations that the IVM concentration used in this study was 35 times higher than that approved by the FDA (Food and Drug Administration) for parasitic diseases, which raised doubts about the drug’s efficacy at the FDA-approved doses [
55]. It should be noted that the study questioning the dosage was based on an in silico analysis, meaning that the results obtained in an in vivo model could differ. Furthermore, the virus infects the alveolar epithelial cells [
55], and in the referred work, the African green monkey kidney cell line, Vero/hSLAM, was used, which does not express the ACE2 receptor, as expressed in the lung tissue.
What is currently known is that one of the mechanisms by which IVM could be effective against SARS-CoV-2 is its interference with the viral entry, since it was shown that IVM interacts with the SARS-CoV-2 spike protein and the ACE2 protein, binding to the spike protein at leucine 91 and the receptor ACE2 at histidine 378 [
56,
57]. Therefore, it is likely that high doses are not required in order to treat patients with COVID 19, as suggested by some authors. Similarly, Choudhury et al., through in silico studies, indicated that IVM could inhibit the formation of the spike-ACE2 complex formation, targeting the S2 subunit in the spike protein, as well as having a high binding affinity for TMPRSS2, interfering with viral entry. Though these findings are promising, further experimental studies are required to corroborate them [
58].
Through a computational analysis, in which 2447 drugs were analyzed to determine their capacity for interfering with the main protease (3CL pro), which is essential for the replication of SARS-CoV-2, IVM, Diosmin, and Selinexor were identified as candidates for use as anti-COVID-19 drugs through this mechanism of action [
59] .
Another option that has been proposed is to enhance the effect of IVM by combining it with other molecules that could enhance its effects. As seen on the
Clinicaltrials.gov platform, there are clinical trials of IVM in combination with different molecules.
Some preprints describe therapeutic benefits with safe doses of IVM. Thus, more attention should be paid and research devoted to IVM as a possible antiviral agent against SARS-CoV-2. A clinical trial reported that, after five days of IVM treatment, there was an earlier virological clearance (9.7 days) in the IVM-treated group than in the placebo group (12.7 days) [
60]. One study reported that IVM treatment did not affect the viral load of SARS-CoV-2 in the respiratory tracts of infected hamsters and attributed its beneficial effect to its anti-inflammatory effect, as described in the work [
61].
2.2. Immunomodulatory Effects
An interesting study showed that the standard dose of IVM (400 µg/kg) presented with an immunomodulatory activity through the cholinergic anti-inflammatory pathway, preventing clinical deterioration, reducing the olfactory deficit, and limiting the inflammation of the upper and lower respiratory tract in infected golden hamsters. IN the case of SARS-CoV-2, it was also observed that the IL-6/IL-10 ratio in the lung decreased dramatically [
61]. Macrophage polarization towards the M2 subpopulation was observed [
61,
68]. On the other hand, it was observed that the anti-inflammatory effect is influenced by sex, since the treatment led to a better response in women [
61]. It has been suggested that this positive allosteric effect of IVM is caused by the activation of neuronal α7 nicotinic acetylcholine receptors (α-7 nAChR) [
69] expressed in the subpopulation of the M2 macrophages [
70,
71]. Another study performed using a rat spinal cord injury model found that treatment with a combination of IVM and carbon nanotubes led to a decrease in the pro-inflammatory cytokines and oxidative stress modulated by the M1/M2 macrophage subpopulations [
72]. Over the past few years, there have been several reports on the anti-inflammatory effects of IVM [
73,
74], with reports indicating that, to achieve this effect in humans, 36 mg should be administered in a single dose with a standard weight of 70 kg [
75].
In in vitro and in vivo models, IVM has been observed to inhibit immune cell recruitment and to suppress mucus hypersecretion and cytokine liberation based on bronchoalveolar lavage in a mouse model of allergic asthma [
76]. Similarly, in a model sensitized by lipopolysaccharides (LPS), IVM inhibits the production of TNF-alpha, IL-1 and IL-6 [
77]. This effect is probably due to the suppression of pro-inflammatory factors, such as NF-κB and the MAP pathway kinase [
74]. Similarly, in an in vitro study, IVM was demonstrated to cause a significant reduction in TNF-α production, induced by TLR agonists, suggesting that IVM could block TLR activity [
58,
78]. In the pathogenesis of SARS-CoV-2, STAT1 activity is inhibited by the viral proteins NSP1 and ORF6, favoring the activation of STAT3 and enhancing the production of IL-6 [
36,
79]. IVM decreases the expression of JAK2 [
80] and the activity of STAT3 [
36,
80,
81], leading to a reduction in IL-6 production and inflammation. IVM has also been shown to modulate the immune activity in mast cells and macrophages [
3] and limit the production of nitric oxide and prostaglandin E2 [
82]. Furthermore, in animals infected with SARS-CoV-2, IVM treatment improves clinical outcomes and is associated with a reduction in the inflammatory state, though without impacting the viral load in the upper and lower respiratory tract [
61]. Additionally, the effect of IVM is being explored in the context of its participation in the pathogenesis of SARS-CoV-2 [
59,
83] (
Figure 2).
2.3. Antitumoral Mechanisms
The effect of IVM as an anti-tumor agent has been explored, and the concentrations necessary for achieving these effects in vivo are within the clinically approved dosages for the treatment of parasitosis [
84]. Some of the anti-tumor mechanisms attributed to IVM are the inhibition of the Akt/mTOR and WNT-TCF pathways [
85,
86], inhibition of MDR proteins, PAK1 helicase, DDX23 and the SIN3 domain [
87,
88], the activation of the P2X4/P2X7 [
89,
90], an increment in the chloride channel activity [
91], the downregulation of Nanog/Sox2/Oct4 genes [
92], and an antimitotic activity (through the damage of tubulin dynamics) [
93]. In the breast cancer cell lines MDA-MB-231, MDA-MB-468 and MCF-7, and the ovarian cancer cell line SKOV-3, IVM was demonstrated to have a more significant anti-tumor effect (the induction of the cell cycle arrest at the G
0-G
1 phase and reductions in the cell viability and tumor size) and a synergistic effect combined with docetaxel, cyclophosphamide and tamoxifen [
86]. In glioma cells, it was observed that it stimulated the activity of caspase-3 and -9, enhancing the expression of p53 and Bax, thus causing apoptosis and blocking the cell cycle in the G
0/G
1 phase [
94]. On the other hand, IVM increased TFE3-dependent autophagy via ROS signaling pathways in melanoma cells, inducing apoptosis [
95]. In porcine trophectoderm and uterine luminal epithelial cells, IVM has also been shown to cause apoptosis through the loss of calcium ion overload, the mitochondrial membrane potential, and the generation of reactive oxygen species [
96]. Furthermore, hypoxia, through hypoxia-inducible factors (HIF), plays an essential role in drug resistance [
97,
98], since, through HIF, cancer cells can resist the decrease in the oxygen concentration and even proliferate. In particular, HIF-1α is translocated to the nucleus by IMPα/β1, and IVM has been shown to block this mechanism [
99], making it a viable target for cancer treatments [
100]. Furthermore, Tian et al. found that the SARS-CoV-2 protein ORF3a elevates the production of HIF-1α, promoting an inflammatory state, and IVM could potentially mitigate the inflammatory response through the inhibition of HIF-1α [
101].
3. Ivermectin in COVID-19 Comorbidities
3.1. Nosocomial Pneumonia
Bacterial coinfections are common in respiratory viral infections [
122,
123], and patients with COVID-19 are no exception [
124]. Of the various studies that report on this situation, only a few representative studies are mentioned here. One study reported that, of 340 COVID-19 patients, 12% had secondary bacterial infections, and of these, 25.59% belonged to the species Klebsiella, 20.93% to methicillin-sensitive Staphylococcus aureus, 16.28% to Escherichia coli, 13.95% to methicillin-resistant Staphylococcus aureus, 11.63% to Enterobacter, 2.32% to Streptococcus pneumonia and 9.30% to Pseudomonas aeruginosa. Of the Enterobacteriaceae isolates, 74% were resistant to cotrimoxazole, 67% to piperacillin, 47.5% to ceftazidime and 42% to cefepime [
123].
It should be noted that atypical bacteria (Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumophila) may be masked by the presentation of COVID-19, as they have overlapping clinical and imaging features, and the timely identification of this co-infection could be vital in critically ill patients [
125]. Severely and critically ill patients are especially susceptible to co-infections. In one study, serum fungal antigens were observed more frequently in the critical group than in the severe group, and the positive frequency rate of serum fungal antigens increased with a prolonged stay in the intensive care unit (ICU) [
126]. These findings were replicated in seven ICUs in England, where an increase in the proportion of pathogens was correlated with the length of stay in the ICU, with the identification of mainly Gram-negative bacteria, particularly Klebsiella pneumonia and Escherichia coli. Patients with co-infections/co-colonization were more likely to die in the ICU than those without co-infections [
127].
Many factors can influence the development of co-infections in terms of the frequency and type of pathogens, including the level of development of the country or region—such is the case of COVID-associated Mucormycosis in India [
128]. Some studies even agree that antibiotic therapies targeting respiratory pathogens should be considered in severe cases [
129]. However, there developed a growing concern during the pandemic that the widespread use of empirical antibiotics could contribute to the rise of multidrug-resistant microorganisms, and antimicrobial administration programs are required to minimize and reduce this threat [
130]. Although, in theory, antibiotics do not directly affect SARS-CoV-2, viral respiratory infections often result in bacterial pneumonia. Some patients may die from bacterial coinfection rather than the viral infection itself; therefore, bacterial coinfections are considered critical risk factors for COVID-19 severity and mortality [
131]. Inversely, a predictor of rapid recovery from COVID-19 is the absence of bacterial coinfections [
132].
Some antibiotics are obtained from fermentation carried out by Gram-positive bacteria of the genus Streptomyces, as in the case of Streptomyces griseus, from which the well-known streptomycin is derived. Therefore, it is not surprising that the fermentation products of Streptomyces avermitilis have antibiotic properties. Strategies have been proposed that aim to search for new antimicrobials in order to combat multidrug resistance, and the repurposing of IVM as an antibiotic has potential. Among the avermectins group, IVM stands out for its antibacterial effects. In clinical isolates of multidrug-resistant Mycobacterium tuberculosis, IVM has shown bactericidal effects [
133]. Additionally, avermectins such as doramectin, IVM, moxidectin and selamectin inhibit the growth of strains of Mycobacterium Bovis BCG, Mycobacterium tuberculosis from H37Rv, CDC 1551, Erdman, and Mycobacterium smegmatis at concentrations ranging from 1 to 8 μg/mL [
133]. It has also been observed to have an antibacterial effect against Staphylococcus aureus at concentrations of 6.25 and 12.5 μg/mL [
134]. Macrolide antibiotics have a distinctive macrocyclic lactone ring, and their mechanism of action works through the inhibition of bacterial protein synthesis. However, they also have modulatory effects on the host defense responses and inflammatory responses [
135]. An example of this is the activation of the P2X4 receptors by IVM in macrophages, increasing the destruction of bacteria and protecting against sepsis [
136], which is most likely the most prominent antibacterial effect of IVM.
3.2. Wound Healing
Many COVID-19 patients show symptoms of acute lung injury that can eventually lead to pulmonary fibrosis [
137]. The treatment of inflammation with corticosteroids reduces inflammation and the likelihood of developing fibrosis [
138]. Regarding the effect of IVM on wound healing, a study reported that IVM cream, at low dosages (0.03–0.1%), induced wound healing, with minimal scarring, and decreased the macroscopic indices of wounds, such as exudation, the edge of oedema, hyperemia and granulation tissue deposits [
139]. Other works report a decrease in skin inflammation under certain conditions [
140,
141,
142,
143], which can help to avoid scar formation. It would be interesting to explore this mechanism of IVM directed against post-COVID-19 pulmonary fibrosis.
This entry is adapted from the peer-reviewed paper 10.3390/life12091384