Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sharon Bruoha | -- | 1560 | 2022-09-21 10:27:44 | | | |
| 2 | Rita Xu | Meta information modification | 1560 | 2022-09-21 10:38:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bruoha, S.; Yosefy, C.; Taha, L.; Dvir, D.; Shuvy, M.; Jubeh, R.; Carasso, S.; Glikson, M.; Asher, E. Cardiogenic Shock Complicating Acute Myocardial Infarction. Encyclopedia. Available online: https://encyclopedia.pub/entry/27426 (accessed on 08 February 2026).
Bruoha S, Yosefy C, Taha L, Dvir D, Shuvy M, Jubeh R, et al. Cardiogenic Shock Complicating Acute Myocardial Infarction. Encyclopedia. Available at: https://encyclopedia.pub/entry/27426. Accessed February 08, 2026.
Bruoha, Sharon, Chaim Yosefy, Louay Taha, Danny Dvir, Mony Shuvy, Rami Jubeh, Shemy Carasso, Michael Glikson, Elad Asher. "Cardiogenic Shock Complicating Acute Myocardial Infarction" Encyclopedia, https://encyclopedia.pub/entry/27426 (accessed February 08, 2026).
Bruoha, S., Yosefy, C., Taha, L., Dvir, D., Shuvy, M., Jubeh, R., Carasso, S., Glikson, M., & Asher, E. (2022, September 21). Cardiogenic Shock Complicating Acute Myocardial Infarction. In Encyclopedia. https://encyclopedia.pub/entry/27426
Bruoha, Sharon, et al. "Cardiogenic Shock Complicating Acute Myocardial Infarction." Encyclopedia. Web. 21 September, 2022.
Copy Citation
Cardiogenic shock complicating acute myocardial infarction is a complex clinical condition associated with dismal prognosis. Routine early target vessel revascularization remains the most effective treatment to substantially improve outcomes, but mortality remains high. Temporary circulatory support devices have emerged with the aim to enhance cardiac unloading and improve end-organ perfusion.
cardiogenic shock
acute myocardial infarction
mechanical circulatory support
1. Introduction
Cardiogenic shock (CS) is the leading cause of death in acute myocardial infarction (AMI), and it is characterized by tissue hypoperfusion and hypoxia related to low cardiac output [1]. It is often associated with rapid hemodynamic deterioration, unresponsiveness to intensive supportive measures, and high mortality rate [2].
Nationwide databases examining temporal trends in CS have shown inconsistent data regarding the incidence of CS. While some studies demonstrate an increase in the overall incidence of CS in recent years [3], others report a decrease [4][5].
CS complicates approximately 5–10% of AMI’s with a higher incidence in ST elevation myocardial infarction (STEMI) and is more frequently seen among women and patients > 75 years old [3][6].
The clinical and hemodynamic heterogenicity of CS with only few randomized clinical trials evaluating the various therapeutic approaches and recommendations lead to uncertainties as to the best treatment strategies. Thus, management of CS is often challenging and requires early diagnosis and institution of high-quality interdisciplinary care [7]. When treated conservatively, CS carries ~70–80% risk of mortality [8]. In contrast, early reperfusion has been associated with improvements in survival [9]. However, for more than two decades, in-hospital and 1-year mortality remain unchanged and unacceptably high with a reported rate of 40–50% [10].
Supportive pharmacologic and device-based therapies are also frequently utilized with little evidence of benefit [11]. Hence, several mechanical circulatory support (MCS) devices have emerged as a treatment option for CS. Nevertheless, data regarding this MCS devices in CS are still debatable and ambiguous [12].
2. Mechanical Circulatory Support
The main goals of temporary MCS devices are to improve cardiac output by reducing intracardiac filling pressures; reduce left ventricular LV volumes, wall stress, and myocardial oxygen consumption; and ameliorate coronary perfusion to improve tissue perfusion.
MCS devices are designed to provide either a temporary, short-term cardiac output support or a long-term assistance to the left and/or right ventricle. Short-term percutaneous platforms are widely used in the setting of CS, in particular in patients refractory to medical therapy, either alone or in combination. Temporary devices may serve as a bridge to recovery or until further decisions in management are made (bridge to decision), such as the need for long-term support, heart transplantation, or destination therapy. Short-term MCS is increasingly used as a bridge to decision in patients with refractory cardiogenic shock [13]. In a meta-analysis evaluating support duration and clinical outcome of a bridge to decision strategy using multiple temporary MCS devices in CS due to various etiologies, including AMI patients, the mean duration (range) of support duration was 1.6–25 days, the mean (range) rates of conversion to durable VAD was 3–30%, and the mean (range) discharge proportion was 45–66% [13]. Assessment of the utility (and futility) of invasive therapy is complex and often requires shared decision making of the multidisciplinary team caring for the patient, with the patient and family taking into consideration patient wishes and objective clinical information.
Recent studies have shown that standardized approach to CS, including early target vessel revascularization along with early use of MCS along with close monitoring of hemodynamic parameters and markers of target organ perfusion, may improve outcomes [14][15]. However, there is lack of evidence regarding patient selection and the use of a specific device criteria. Thus, MCS candidacy should be evaluated by a multidisciplinary team with expertise in management of cardiac support devices. Currently, the use of MCS in CS has a general class IIa–III level of recommendation depending on the specific MCS device [11].
Options for acute MCS (Figure 1) include the intra-aortic balloon pump (IABP), percutaneous ventricular assist devices (VAD) (Impella, TandemHeart), and veno-arterial extracorporeal membrane oxygenation (VA-ECMO) [16]. The characteristics of the various devices are summarized in Table 2 [10][14][16][17].
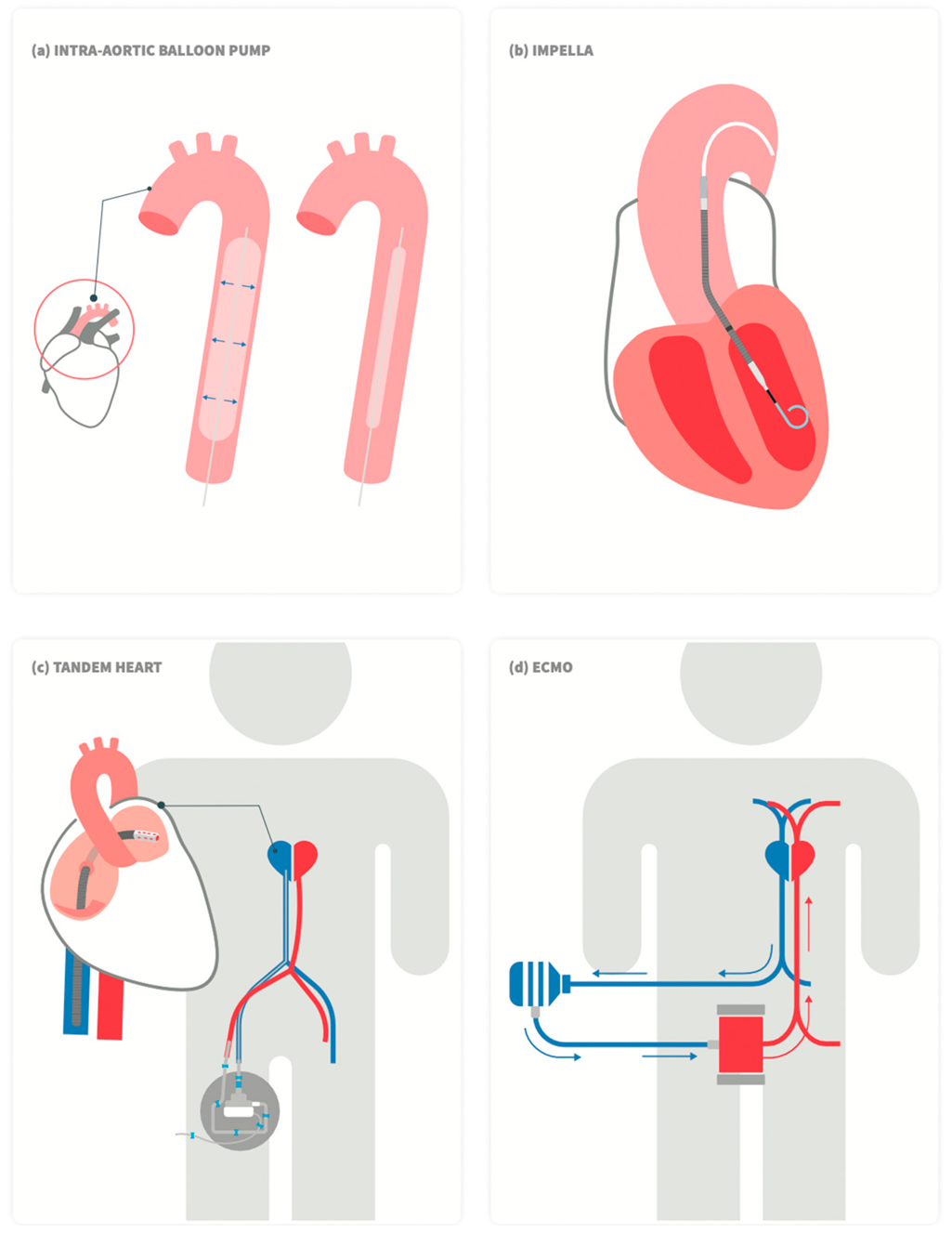
Figure 1. Schematic drawing of commercially available left ventricular percutaneous mechanical support devices. (a) Intra-aortic balloon pump, (b) Impella, (c)TandemHeart, and (d) Veno-arterial (VA) extracorporeal membrane oxygenation (ECMO).
3. Intra-Aortic Balloon Pump (IABP)
Intra-aortic balloon pump (IABP) counter-pulsation is one of the earliest types of short term MCS. It consists of a flexible 30–50 cc helium-filled balloon catheter (7–8F), inserted percutaneously via the femoral artery, connected to a mobile console that times periodic balloon inflation and deflation according to the cardiac cycle. When inflated in diastole (immediately after the closure of the aortic valve), diastolic and mean arterial pressure rise, thus theoretically improving coronary flow and myocardial oxygenation. On the other hand, when rapidly deflated just prior to blood ejection from the LV, it provides immediate systolic blood pressure attenuation and consequently afterload reduction, leading to an increase in stroke volume. Overall, myocardial oxygen demand is reduced [18].
IABP has been investigated in multiple clinical scenarios, including high-risk PCI and CS in the setting of AMI [19][20][21]. One of the most important trials investigating IABP in the setting of CS was the IABP-SHOCK II trial, which randomized 600 patients with CS complicating AMI to routine use of IABP vs. no IABP in addition to early revascularization along with the accepted available medical therapy [22]. At 30 days, no difference in mortality or any secondary endpoint (serum lactate levels, creatinine clearance, C-reactive protein levels, and severity of disease as assessed with the use of the Simplified Acute Physiology Score [SAPS] II) was evident. In addition, long-term mortality also did not differ between the IABP and the control group [23]. Lack of clinical benefit has also been reported in metanalyses [24] and registries [25].
Accordingly, the routine use of IBAP was given a class III indication for CS complicating AMI in the STEMI European guidelines [26]. Moreover, the timing for initiation the use of IABP therapy (before vs. post primary PCI) does not appear to impact short-term and long-term survival in patients with CS complicating AMI undergoing primary PCI [27].
In summary, there is no convincing evidence to support routine use of IABP in post-MI CS patients. Consequently, the overall use of IBAP in the management of ischemic CS is consistently decreasing, with the exception of CS due to severe mitral regurgitation, where the use of IABP is still rated as IIa indication [3][6][26].
4. Impella
Impella (Abiomed Inc., Danvers, MA, USA) is a temporary VAD frequently included in the management of patients with post-AMI CS and as a support measure in PCI for high-risk patients [21][28]. The device requires a large bore access (12–14F) and is introduced retrogradely, via the femoral artery, under fluoroscopic guidance, across the aortic valve. It consists of a pump motor that delivers forward blood flow from the LV into the aorta in a non-pulsatile, continuous fashion. The Impella 2.5 and Impella CP allow for a sustained peak flow of 2.5 L/min and 4.3 L/min, respectively. The Impella 5.0 and Impella 5.5 with SmartAssist require a surgical access to the femoral/subclavian arteries and provide up to 5 L/min and > 6 L/min of blood flow, respectively. By unloading the ventricle, the Impella reduces intracardiac pressures and myocardial oxygen consumption. Coronary blood flow is, theoretically, increased by means of increased blood pressure and reduced LV end diastolic pressure [29]. The new Impella ECP (Expandable CP) (9F) can provide peak flow of > 3.5 L/min [10].
The safety and feasibility of the Impella 2.5 and CP devices have been reported in large registries [30]. Impella was also evaluated in comparison to IBAP in the setting of CS. The ISAR-SHOCK (Efficacy Study of LV Assist Device to Treat Patients With Cardiogenic Shock) trial showed that the use of Impella provided more hemodynamic support than IABP, but there was no difference in the mortality rate between the two devices [31]. The IMPRESS in Severe Shock (IMPella versus IABP Reduces mortality in STEMI patients treated with primary PCI in Severe cardiogenic Shock) study randomized 48 patients with CS complicating AMI to Impella CP vs. IABP. However, there was, again, no significant difference in 30-day and 6-months mortality rates (~50% at 6 months for both groups) [32]. In addition, no mortality difference between groups was observed on long-term 5-year follow up [33]. Nevertheless, in one large cohort of 15,259 consecutive patients with post-MI CS treated with Impella, pre-PCI Impella placement was associated with improved survival as compared with post PCI [34]. Other than the rather disappointing evidence of benefit, the use of Impella has been linked to a greater risk of vascular complications, major bleeding, and stroke compared with the use IABP [10][29][35] (Schrage, 2019, Impella Support for Acute Myocardial Infarction Complicated by Cardiogenic Shock).
The Impella RP (right percutaneous), introduced via the femoral vein, supports the RV. It is utilized to maintain blood flow from the inferior vena cava into the pulmonary artery with peak flow rate > 4 L/min. The RECOVER RIGHT (The Use of Impella RP Support System in Patients With Right Heart Failure) study was the first to suggest the feasibility and safety of the RV support device in selected patients with RV failure [36]. Nevertheless, data regarding its benefit are still scarce. Interestingly, despite the absence of good clinical data to support the use of Impella in CS complicating AMI, studies have reported a substantial and consistent use of VADs in recent years [37].
References
- Thiele, H.; Ohman, E.M.; De Waha-Thiele, S.; Zeymer, U.; Desch, S. Management of cardiogenic shock complicating myocardial infarction: An update 2019. Eur. Heart J. 2019, 40, 2671–2683.
- Osman, M.; Syed, M.; Patibandla, S.; Sulaiman, S.; Kheiri, B.; Shah, M.K.; Bianco, C.; Balla, S.; Patel, B. Fifteen-Year Trends in Incidence of Cardiogenic Shock Hospitalization and In-Hospital Mortality in the United States. J. Am. Heart Assoc. 2021, 10, e021061.
- Kolte, D.; Khera, S.; Aronow, W.S.; Mujib, M.; Palaniswamy, C.; Sule, S.; Jain, D.; Gotsis, W.; Ahmed, A.; Frishman, W.H.; et al. Trends in Incidence, Management, and Outcomes of Cardiogenic Shock Complicating ST-Elevation Myocardial Infarction in the United States. J. Am. Heart Assoc. 2014, 3, e000590.
- Aissaoui, N.; Puymirat, E.; Delmas, C.; Ortuno, S.; Durand, E.; Bataille, V.; Drouet, E.; Bonello, L.; Bonnefoy-Cudraz, E.; Lesmeles, G.; et al. Trends in cardiogenic shock complicating acute myocardial infarction. Eur. J. Heart Fail. 2020, 22, 664–672.
- Jeger, R.; Radovanovic, D.; Hunziker, P.R.; Pfisterer, M.E.; Stauffer, J.-C.; Erne, P.; Urban, P. For the AMIS Plus Registry Investigators Ten-year trends in the incidence and treatment of cardiogenic shock. Ann. Intern. Med. 2008, 149, 618–626.
- Van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation 2017, 136, e232–e268.
- Henry, T.D.; Tomey, M.I.; Tamis-Holland, J.E.; Thiele, H.; Rao, S.V.; Menon, V.; Klein, D.G.; Naka, Y.; Piña, I.L.; Kapur, N.K.; et al. Invasive Management of Acute Myocardial Infarction Complicated by Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e815–e829.
- Goldberg, R.J.; Gore, J.M.; Alpert, J.S.; Osganian, V.; de Groot, J.; Bade, J.; Chen, Z.; Frid, D.; Dalen, J.E. Cardiogenic shock after acute myocardial infarction. Incidence and mortality from a community-wide perspective, 1975 to 1988. N. Engl. J. Med. 1991, 325, 1117–1122.
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Sanborn, T.A.; White, H.D.; Talley, J.D.; Christopher, E.B.; Jacobs, A.K.; Slater, J.N.; Col, J.; et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N. Engl. J. Med. 1999, 341, 625–634.
- Thiele, H.; de Waha-Thiele, S.; Freund, A.; Zeymer, U.; Desch, S.; Fitzgerald, S. Management of cardiogenic shock. EuroIntervention 2021, 17, 451–465.
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726.
- Samsky, M.D.; Morrow, D.A.; Proudfoot, A.G.; Hochman, J.S.; Thiele, H.; Rao, S.V. Cardiogenic Shock After Acute Myocardial Infarction: A Review. JAMA 2021, 326, 1840–1850.
- den Uil, C.A.; Akin, S.; Jewbali, L.S.; dos Reis Miranda, D.; Brugts, J.J.; Constantinescu, A.A.; Kappetein, A.P.; Caliskan, K. Short-term mechanical circulatory support as a bridge to durable left ventricular assist device implantation in refractory cardiogenic shock: A systematic review and meta-analysis. Eur. J. Cardio-Thorac. Surg. 2017, 52, 14–25.
- Tehrani, B.N.; Truesdell, A.G.; Psotka, M.A.; Rosner, C.; Singh, R.; Sinha, S.S.; Damluji, A.A.; Batchelor, W.B. A Standardized and Comprehensive Approach to the Management of Cardiogenic Shock. JACC Heart Fail. 2020, 8, 879–891.
- Ba, M.R.F.; Kapur, N.K.; Patel, K.; Salam, M.A.; Schreiber, T.; Kaki, A.; Hanson, I.; Almany, S.; Timmis, S.; Dixon, S.; et al. Improved Outcomes Associated with the use of Shock Protocols: Updates from the National Cardiogenic Shock Initiative. Catheter. Cardiovasc. Interv. 2019, 93, 1173–1183.
- Telukuntla, K.S.; Estep, J.D. Acute Mechanical Circulatory Support for Cardiogenic Shock. Methodist DeBakey Cardiovasc. J. 2020, 16, 27–35.
- Eckman, P.M.; Katz, J.N.; El Banayosy, A.; Bohula, E.A.; Sun, B.; van Diepen, S. Veno-Arterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock: An Introduction for the Busy Clinician. Circulation 2019, 140, 2019–2037.
- Kimman, J.R.; Van Mieghem, N.M.; Endeman, H.; Brugts, J.J.; Constantinescu, A.A.; Manintveld, O.C.; Dubois, E.A.; Uil, C.A.D. Mechanical Support in Early Cardiogenic Shock: What Is the Role of Intra-aortic Balloon Counterpulsation? Curr. Heart Fail. Rep. 2020, 17, 247–260.
- Patterson, T.; Perera, D.; Redwood, S.R. Intra-Aortic Balloon Pump for High-Risk Percutaneous Coronary Intervention. Circ. Cardiovasc. Interv. 2014, 7, 712–720.
- Thiele, H.; Sick, P.; Boudriot, E.; Diederich, K.-W.; Hambrecht, R.; Niebauer, J.; Schuler, G. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur. Heart J. 2005, 26, 1276–1283.
- Dangas, G.D.; Kini, A.S.; Sharma, S.K.; Henriques, J.P.; Claessen, B.; Dixon, S.R.; Massaro, J.M.; Palacios, I.; Popma, J.J.; Ohman, E.M.; et al. Impact of Hemodynamic Support With Impella 2.5 Versus Intra-Aortic Balloon Pump on Prognostically Important Clinical Outcomes in Patients Undergoing High-Risk Percutaneous Coronary Intervention (from the PROTECT II Randomized Trial). Am. J. Cardiol. 2013, 113, 222–228.
- Thiele, H.; Zeymer, U.; Neumann, F.-J.; Ferenc, M.; Olbrich, H.-G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. N. Engl. J. Med. 2012, 367, 1287–1296.
- Thiele, H.; Zeymer, U.; Thelemann, N.; Neumann, F.-J.; Hausleiter, J.; Abdel-Wahab, M.; Meyer-Saraei, R.; Fuernau, G.; Eitel, I.; Hambrecht, R.; et al. Intraaortic Balloon Pump in Cardiogenic Shock Complicating Acute Myocardial Infarction: Long-Term 6-Year Outcome of the Randomized IABP-SHOCK II Trial. Circulation 2019, 139, 395–403.
- Ahmad, Y.; Sen, S.; Shun-Shin, M.J.; Ouyang, J.; Finegold, J.A.; Al-Lamee, R.K.; Davies, J.E.R.; Cole, G.D.; Francis, D.P. Intra-aortic Balloon Pump Therapy for Acute Myocardial Infarction. JAMA Intern. Med. 2015, 175, 931–939.
- Prondzinsky, R.; Unverzagt, S.; Russ, M.; Lemm, H.; Swyter, M.; Wegener, N.; Buerke, U.; Raaz, U.; Ebelt, H.; Schlitt, A.; et al. Hemodynamic Effects of Intra-aortic Balloon Counterpulsation in Patients With Acute Myocardial Infarction Complicated by Cardiogenic Shock. Shock 2012, 37, 378–384.
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177.
- Cui, K.; Lyu, S.; Liu, H.; Song, X.; Yuan, F.; Xu, F.; Zhang, M.; Zhang, M.; Wang, W.; Zhang, D.; et al. Timing of initiation of intra-aortic balloon pump in patients with acute myocardial infarction complicated by cardiogenic shock: A meta-analysis. Clin. Cardiol. 2019, 42, 1126–1134.
- Chhabra, L.; Chaudhry, H.; Chhabra, R.; Kayani, W.T. Impella device use in high-risk PCI. EuroIntervention 2019, 15, 731.
- Schrage, B.; Ibrahim, K.; Loehn, T.; Werner, N.; Sinning, J.-M.; Pappalardo, F.; Pieri, M.; Skurk, C.; Lauten, A.; Landmesser, U.; et al. Impella Support for Acute Myocardial Infarction Complicated by Cardiogenic Shock. Circulation 2019, 139, 1249–1258.
- Vetrovec, G.W.; Anderson, M.; Schreiber, T.; Popma, J.; Lombardi, W.; Maini, B.; Møller, J.E.; Schäfer, A.; Dixon, S.R.; Hall, S.; et al. The cVAD registry for percutaneous temporary hemodynamic support: A prospective registry of Impella mechanical circulatory support use in high-risk PCI, cardiogenic shock, and decompensated heart failure. Am. Heart J. 2018, 199, 115–121.
- Seyfarth, M.; Sibbing, D.; Bauer, I.; Fröhlich, G.; Bott-Flügel, L.; Byrne, R.; Dirschinger, J.; Kastrati, A.; Schömig, A. A Randomized Clinical Trial to Evaluate the Safety and Efficacy of a Percutaneous Left Ventricular Assist Device Versus Intra-Aortic Balloon Pumping for Treatment of Cardiogenic Shock Caused by Myocardial Infarction. J. Am. Coll. Cardiol. 2008, 52, 1584–1588.
- Ouweneel, D.M.; Eriksen, E.; Sjauw, K.D.; van Dongen, I.M.; Hirsch, A.; Packer, E.J.; Vis, M.M.; Wykrzykowska, J.J.; Koch, K.T.; Baan, J.; et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 69, 278–287.
- Karami, M.; Eriksen, E.; Ouweneel, D.M.; Claessen, B.E.; Vis, M.M.; Baan, J.; Beijk, M.; Packer, E.J.S.; Sjauw, K.D.; Engstrom, A.; et al. Long-term 5-year outcome of the randomized IMPRESS in severe shock trial: Percutaneous mechanical circulatory support vs. intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 1009–1015.
- O’Neill, W.W.; Grines, C.; Schreiber, T.; Moses, J.; Maini, B.; Dixon, S.R.; Ohman, E.M. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am. Heart J. 2018, 202, 33–38.
- Dhruva, S.S.; Ross, J.; Mortazavi, B.J.; Hurley, N.C.; Krumholz, H.M.; Curtis, J.P.; Berkowitz, A.; Masoudi, F.A.; Messenger, J.C.; Parzynski, C.S.; et al. Association of Use of an Intravascular Microaxial Left Ventricular Assist Device vs Intra-aortic Balloon Pump With In-Hospital Mortality and Major Bleeding Among Patients With Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA 2020, 323, 734.
- Anderson, M.B.; Goldstein, J.; Milano, C.; Morris, L.D.; Kormos, R.L.; Bhama, J.; Kapur, N.K.; Bansal, A.; Garcia, J.; Baker, J.N.; et al. Benefits of a novel percutaneous ventricular assist device for right heart failure: The prospective RECOVER RIGHT study of the Impella RP device. J. Heart Lung Transplant. 2015, 34, 1549–1560.
- Amin, A.P.; Spertus, J.A.; Curtis, J.P.; Desai, N.; Masoudi, F.A.; Bach, R.G.; McNeely, C.; Al-Badarin, F.; House, J.A.; Kulkarni, H.; et al. The Evolving Landscape of Impella Use in the United States Among Patients Undergoing Percutaneous Coronary Intervention With Mechanical Circulatory Support. Circulation 2020, 141, 273–284.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Entry Collection:
Hypertension and Cardiovascular Diseases
Revisions:
2 times
(View History)
Update Date:
21 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




