| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Emmanuel Kweinor Tetteh | -- | 2127 | 2022-09-20 16:51:58 | | | |
| 2 | Peter Tang | -6 word(s) | 2121 | 2022-09-21 03:20:30 | | |
Video Upload Options
The high separation efficiencies, relative low costs, small footprint, and ease of operation associated with integrated photocatalytic-membrane (IPM) technologies are gaining an all-inclusive attention. Conversely, photocatalysis and membrane technologies face some degree of setbacks, which limit their worldwide application in wastewater settings for the treatment of emerging contaminants.
1. Introduction
2. Advanced Oxidation Process (AOP)
|
Chemical Process |
Photochemical Process |
|---|---|
|
Wet air oxidation |
Photo-Fenton reaction |
|
Supercritical water oxidation |
UV/ultrasound system |
|
Ultrasound/H2O2 system |
UV/O3/H2O2 system |
|
Ultrasound/Sonolysis |
UV/H2O2 system |
|
Fenton reaction |
UV/O3 system |
|
Ozonation in alkaline |
UV photolysis |
|
O3/H2O2 system |
UV/O2/TiO2 system |
|
Electron—Fenton reaction |
UV/H2O2/TiO2 system |
2.1. TiO2 Photocatalyst
|
Crystalline Forms |
Anatase |
Rutile |
Brookite |
|---|---|---|---|
|
Crystalline structure |
Tetragonal |
Tetragonal |
Rhombohedral |
|
Lattice constants (nm) |
a = b = 0.3733 c = 0.9370 |
a = b = 0.4584 c = 0.2953 |
a = 0.5436; b = 0.9166 c = 0.5135 |
|
Density (g.cm−3) |
3.83 |
4.24 |
4.17 |
|
Bravais lattice |
Simple, body-centered |
Simple, body-centered |
Simple |
|
Melting point (°C) |
Turning into rutile |
1870 |
Turning into rutile |
|
Boiling point (°C) |
2927 |
- |
- |
|
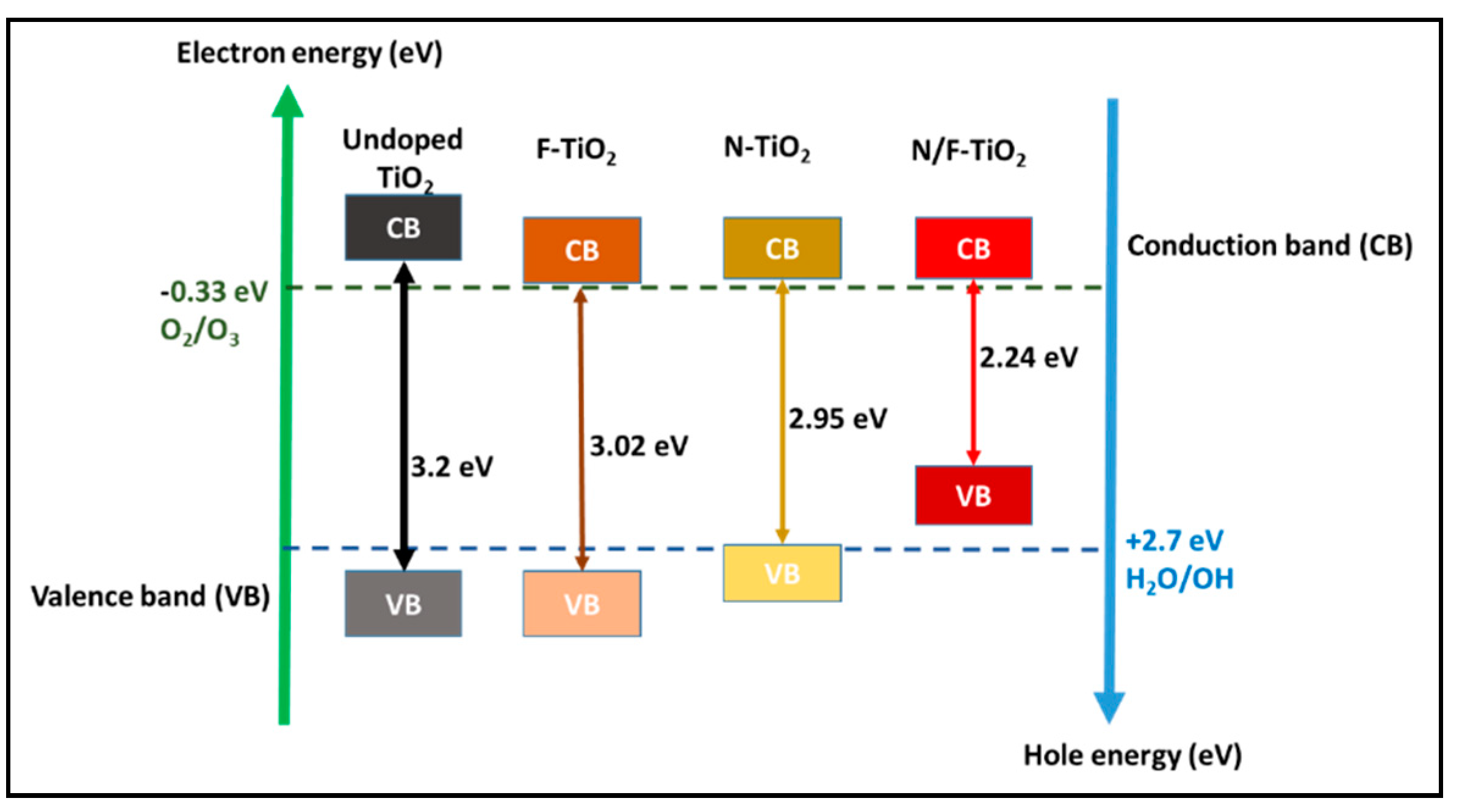
Band gap (eV) |
3.2 |
3.0 |
- |
|
Refractive index (ng) |
2.5688 |
2.9467 |
2.809 |
|
Standard heat capacity (cp) |
55.52 |
55.60 |
- |
|
Dielectric constant |
55 |
110–117 |
78 |
2.2. Operational Parameters in Photocatalysis
3. Integrated Photocatalytic Membrane (IPM) Reactors
References
- Tetteh, E.K.; Amankwa, M.O.; Armah, E.K.; Rathilal, S. Fate of covid-19 occurrences in wastewater systems: Emerging detection and treatment technologies—A review. Water 2020, 12, 2680.
- Zhang, J.; Tong, H.; Pei, W.; Liu, W.; Shi, F.; Li, Y.; Huo, Y. Integrated photocatalysis-adsorption-membrane separation in rotating reactor for synergistic removal of RhB. Chemosphere 2021, 270.
- Hube, S.; Eskafi, M.; Hrafnkelsdóttir, K.F.; Bjarnadóttir, B.; Bjarnadóttir, M.Á.; Axelsdóttir, S.; Wu, B. Direct membrane filtration for wastewater treatment and resource recovery: A review. Sci. Total Environ. 2020, 710.
- Schutte, C.F.; Focke, W. Evaluation of Nanotechnology for Application in Water and Wastewater Treatment and Related Aspects in South Africa; Water Research Commission: Pretoria, South Africa, 2007; p. 28.
- Chollom, M.N.; Rathilal, S.; Swalaha, F.M.; Bakare, B.F.; Tetteh, E.K. Removal of antibiotics during the anaerobic digestion of slaughterhouse wastewater. Int. J. Sustain. Dev. Plan. 2020, 15.
- Anvari, A.; Amoli-Diva, M.; Sadighi-Bonabi, R. Concurrent photocatalytic degradation and filtration with bi-plasmonic TiO2 for wastewater treatment. Micro Nano Lett. 2021, 16.
- Ezugbe, E.O.; Tetteh, E.K.; Rathilal, S.; Asante-Sackey, D.; Amo-Duodu, G. Desalination of municipal wastewater using forward osmosis. Membranes 2021, 11, 119.
- Chollom, M.N.; Rathilal, S.; Swalaha, F.M.; Bakare, B.F.; Tetteh, E.K. Anaerobic treatment of slaughterhouse wastewater: Evaluating operating conditions. WIT Trans. Ecol. Environ. 2019, 239.
- della Rocca, D.G.; Peralta, R.M.; Peralta, R.A.; Moreira, R.d.P.M. Recent development on Ag2MoO4-based advanced oxidation processes: A review. React. Kinet. Mech. Catal. 2021, 132.
- Ahmed, S.N.; Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29.
- Qiu, X.; Zhang, Y.; Zhu, Y.; Long, C.; Su, L.; Liu, S.; Tang, Z. Applications of Nanomaterials in Asymmetric Photocatalysis: Recent Progress, Challenges, and Opportunities. Adv. Mater. 2021, 33.
- Naddeo, V.; Balakrishnan, M.; Choo, K.-H. Frontiers in Water-Energy-Nexus—Nature-Based Solutions, Advanced Technologies and Best Practices for Environmental Sustainability: Proceedings of the 2nd WaterEnergyNEXUS Conference; Springer Nature: Salemo, Italy, 2019.
- Wang, Z.; Wang, L.; Yao, L.; Pei, M.; Zhang, G. Membrane separation coupled with photocatalysis for water supply and wastewater treatment. Adv. Mater. Res. 2013, 671–674, 2571–2574.
- Molinari, R.; Argurio, P.; Szymański, K.; Darowna, D.; Mozia, S. Photocatalytic membrane reactors for wastewater treatment. In Current Trends and Future Developments on (Bio-) Membranes: Membrane Technology for Water and Wastewater Treatment Advances and Emerging Processes; Elsevier: Amsterdam, The Netherlands, 2020.
- Cai, Z.; Dwivedi, A.D.; Lee, W.N.; Zhao, X.; Liu, W.; Sillanpää, M.; Zhao, D.; Huang, C.H.; Fu, J. Application of nanotechnologies for removing pharmaceutically active compounds from water: Development and future trends. Environ. Sci. Nano 2018, 5.
- Li, Q.; Kong, H.; Jia, R.; Shao, J.; He, Y. Enhanced catalytic degradation of amoxicillin with TiO2-Fe3O4 composites: Via a submerged magnetic separation membrane photocatalytic reactor (SMSMPR). RSC Adv. 2019, 9.
- Lavorato, C.; Argurio, P.; Molinari, R. TiO2 and Pd/TiO2 as Photocatalysts for Hydrogenation of Ketones and Perspective of Membrane Application. Int. J. Adv. Res. Chem. Sci. 2019, 6, 33–41.
- Indonesia, G.B. Development of TiO2 Nanoparticles/Nanosolution for Photocatalytic Activity. Ph.D. Thesis, Universiti Sains Malaysia, Penang, Malaysia, 2015.
- Thiruvenkatachari, R.; Vigneswaran, S.; Moon, I.S. A review on UV/TiO 2 photocatalytic oxidation process. Korean J. Chem. Eng. 2008, 25, 64–72.
- Zheng, Z. Synthesis and Modifications of Metal Oxide Nanostructures and Their Applications. Ph.D. Thesis, Queensland University of Technology Brisbane Australia, Brisbane, Australia, 2009; p. 27.
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Recent progress in g-C3N4, TiO2 and ZnO based photocatalysts for dye degradation: Strategies to improve photocatalytic activity. Sci. Total Environ. 2021, 767.
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2015, 4, 582–598.
- Luo, H.; Yan, M.; Wu, Y.; Lin, X.; Yan, Y. Facile synthesis of PVDF photocatalytic membrane based on NCQDs/BiOBr/TiO2 heterojunction for effective removal of tetracycline. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2021, 265.
- Choi, H.; Sofranko, A.C.; Dionysiou, D.D. Nanocrystalline TiO2 photocatalytic membranes with a hierarchical mesoporous multilayer structure: Synthesis, characterization, and multifunction. Adv. Funct. Mater. 2006, 16.
- Gupta, S.M.; Tripathi, M. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657.
- Ghosh, S.; Das, A.P. Modified titanium oxide (TiO2) nanocomposites and its array of applications: A review. Toxicol. Environ. Chem. 2015, 97.
- Fua, J.; Wanga, X.; Mac, Z.; Wenmingd, H.; Lie, J.; Wanga, Z.; Wanga, L. Photocatalytic ultrafiltration membranes based on visible light responsive photocatalyst: A review. Desalin. Water Treat. 2019, 168.
- Sabzehmeidani, M.M.; Ghaedi, M.; Karimi, H. Photocatalytic activity based on electrospun nanofibers. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 32, pp. 625–672.
- Umar, M.; Aziz, H.A. Photocatalytic degradation of organic pollutants in water. Organic pollutants-monitoring, risk and treatment. Org. Pollut.-Monit. Risk Treat. 2013, 30, 196–197.
- Padmanabhan, N.T.; John, H. Titanium dioxide based self-cleaning smart surfaces: A short review. J. Environ. Chem. Eng. 2020, 8.
- Jhaveri, J.H.; Murthy, Z.V.P. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379.
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24.
- Brunetti, A.; Pomilla, F.R.; Marcì, G.; Garcia-Lopez, E.I.; Fontananova, E.; Palmisano, L.; Barbieri, G. CO2 reduction by C3N4-TiO2 Nafion photocatalytic membrane reactor as a promising environmental pathway to solar fuels. Appl. Catal. B Environ. 2019, 255.
- Tetteh, E.K.; Ezugbe, E.O.; Rathilal, S.; Asante-Sackey, D. Removal of COD and SO4 from oil refinery wastewater using a photo-catalytic system-comparing TiO2 and zeolite efficiencies. Water 2020, 12, 214.
- Lin, Z.; Li, J.; Shen, W.; Corriou, J.P.; Chen, X.; Xi, H. Different photocatalytic levels of organics in papermaking wastewater by flocculation-photocatalysis and SBR-photocatalysis: Degradation and GC–MS experiments, adsorption and photocatalysis simulations. Chem. Eng. J. 2021, 412.
- Salmerón, I.; Sharma, P.K.; Polo-López, M.I.; Tolosana, A.; McMichael, S.; Oller, I.; Byrne, J.A.; Fernández-Ibáñez, P. Electrochemically assisted photocatalysis for the simultaneous degradation of organic micro-contaminants and inactivation of microorganisms in water. Process Saf. Environ. Prot. 2021, 147.
- Dominguez, S.; Huebra, M.; Han, C.; Campo, P.; Nadagouda, M.N.; Rivero, M.J.; Ortiz, I.; Dionysiou, D.D. Magnetically recoverable TiO 2 -WO 3 photocatalyst to oxidize bisphenol A from model wastewater under simulated solar light. Environ. Sci. Pollut. Res. 2017, 24, 12589–12598.
- Otieno, O.V.; Csáki, E.; Kéri, O.; Simon, L.; Lukács, I.E.; Szécsényi, K.M.; Szilágyi, I.M. Synthesis of TiO2 nanofibers by electrospinning using water-soluble Ti-precursor. J. Therm. Anal. Calorim. 2020, 139.
- Chen, F.; Zhao, J. Preparation and photocatalytic properties of a novel kind of loaded photocatalyst of TiO2/SiO2/γ-Fe2O3. Catal. Letters 1999, 58.
- Faraldos, M.; Bahamonde, A. Environmental applications of titania-graphene photocatalysts. Catal. Today 2017, 285.
- Esfandiari, N.; Kashefi, M.; Afsharnezhad, S.; Mirjalili, M. Insight into enhanced visible light photocatalytic activity of Fe3O4–SiO2–TiO2 core-multishell nanoparticles on the elimination of Escherichia coli. Mater. Chem. Phys. 2020, 244.
- Li, Q.; Kong, H.; Li, P.; Shao, J.; He, Y. Photo-Fenton degradation of amoxicillin via magnetic TiO2-graphene oxide-Fe3O4 composite with a submerged magnetic separation membrane photocatalytic reactor (SMSMPR). J. Hazard. Mater. 2019, 373.
- Sun, T.; Liu, Y.; Shen, L.; Xu, Y.; Li, R.; Huang, L.; Lin, H. Magnetic field assisted arrangement of photocatalytic TiO2 particles on membrane surface to enhance membrane antifouling performance for water treatment. J. Colloid Interface Sci. 2020, 570.
- di Paola, A.; Bellardita, M.; Palmisano, L. Brookite the least known TiO2 photocatalyst. Catalysts 2013, 3, 36.
- Maroudas, A.; Pandis, P.K.; Chatzopoulou, A.; Davellas, L.R.; Sourkouni, G.; Argirusis, C. Synergetic decolorization of azo dyes using ultrasounds, photocatalysis and photo-fenton reaction. Ultrason. Sonochem. 2021, 71.
- Shayegan, Z.; Lee, C.S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334.
- Naldoni, A.; Altomare, M.; Zoppellaro, G.; Liu, N.; Kment, S.; Zbořil, R.; Schmuki, P. Photocatalysis with reduced TiO 2: From Black TiO 2 to cocatalyst-free hydrogen production. ACS Catal. 2019, 9, 345–364.
- Vaiano, V.; Sacco, O.; Sannino, D.; Stoller, M.; Ciambelli, P.; Chianese, A. Photocatalytic removal of phenol by ferromagnetic N-TiO2/SiO2/Fe3O4 nanoparticles in presence of visible light irradiation. Chem. Eng. Trans. 2016, 47, 235–240.
- Abdullah, H.; Khan, M.M.R.; Ong, H.R.; Yaakob, Z. Modified TiO2 photocatalyst for CO2 photocatalytic reduction: An overview. J. CO2 Util. 2017, 22.
- Bhanvase, B.A.; Shende, T.P.; Sonawane, S.H. A review on graphene–TiO2 and doped graphene–TiO2 nanocomposite photocatalyst for water and wastewater treatment. Environ. Technol. Rev. 2017, 6.
- Lee, S.Y.; Park, S.J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19.
- Ge, M.; Li, Q.; Cao, C.; Huang, J.; Li, S.; Zhang, S.; Chen, Z.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. One-dimensional TiO2 Nanotube Photocatalysts for Solar Water Splitting. Adv. Sci. 2017, 4.
- Kuvarega, A.T.; Mamba, B.B. Photocatalytic Membranes for Efficient Water Treatment. In Semiconductor Photocatalysis Materials, Mechanisms and Applications; IntechOpen: London, UK, 2016.
- Sakarkar, S.; Muthukumran, S.; Jegatheesan, V. Factors affecting the degradation of remazol turquoise blue (RTB) dye by titanium dioxide (TiO2) entrapped photocatalytic membrane. J. Environ. Manag. 2020, 272.
- Tetteh, E.K.; Rathilal, S. Kinetics and nanoparticle catalytic enhancement of biogas production from wastewater using a magnetized biochemical methane potential (Mbmp) system. Catalysts 2020, 10, 1200.
- Blanco, M.; Monteserín, C.; Angulo, A.; Pérez-Márquez, A.; Maudes, J.; Murillo, N.; Aranzabe, E.; Ruiz-Rubio, L.; Vilas, J.L. TiO2-doped electrospun nanofibrous membrane for photocatalytic water treatment. Polymers 2019, 11, 747.
- Ghernaout, D. Magnetic field generation in the water treatment perspectives: An overview. Int. J. Adv. Appl. Sci. 2017, 5, 193–203.
- Štefušová, K.; Václavíková, M.; Lovás, M.; Hredzák, S. Use of magnetic filtration in wastewater treatment. Acta Montan. Slovaca 2012, 17, 81–84.
- Yao, H.; Fan, M.; Wang, Y.; Luo, G.; Fei, W. Magnetic titanium dioxide-based nanomaterials: Synthesis, characteristics, and photocatalytic application in pollutant degradation. J. Mater. Chem. A 2015, 3, 17511–17524.
- Goei, R.; Lim, T.T. Ag-decorated TiO2 photocatalytic membrane with hierarchical architecture: Photocatalytic and anti-bacterial activities. Water Res. 2014, 59.
- Liu, X.; Wang, L.; Zhou, X.; He, X.; Zhou, M.; Jia, K.; Liu, X. Design of polymer composite-based porous membrane for in-situ photocatalytic degradation of adsorbed organic dyes. J. Phys. Chem. Solids 2021, 154.
- Jia, Y.; Cai, F.; Lu, S.; Yang, F.; Zhao, Y. Research on the preparation and application of through-hole TiO2 nanotube arrays membranes. Cailiao Daobao/Materials Rev. 2016, 30.
- Ma, L.; Chen, Y.; Zheng, J. An efficient, stable and reusable polymer/TiO2 photocatalytic membrane for aqueous pollution treatment. J. Mater. Sci. 2021.
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44.
- Nasrollahi, N.; Ghalamchi, L.; Vatanpour, V.; Khataee, A. Photocatalytic-membrane technology: A critical review for membrane fouling mitigation. J. Ind. Eng. Chem. 2021, 93.