The high separation efficiencies, relative low costs, small footprint, and ease of operation associated with integrated photocatalytic-membrane (IPM) technologies are gaining an all-inclusive attention. Conversely, photocatalysis and membrane technologies face some degree of setbacks, which limit their worldwide application in wastewater settings for the treatment of emerging contaminants.
1. Introduction
In a growing economy, clean and drinkable water, free from toxic chemicals, carcinogenic substances and harmful microorganisms are essential for human health and sustainability
[1][2][1,2]. However, the fast population growth associated with urbanization, irrigated agriculture and industrialization have driven the environmental concern of wastewater and demand for fresh water globally
[3][4][3,4]. This opioid epidemic, be it due to insufficient accessibility to clean water, poor water quality or waterborne diseases, has seriously threatened human lives in many underdeveloped and developing countries
[4][5][6][4,5,6]. The result has been an upsurge of stringent environmental standards to provide clean water with high quality and sustainable waste management facilities
[4][5][6][7][4,5,6,7]. In addition, conventional technologies (coagulation, flocculation, biological) are limited when it comes to complete decontamination of water containing emerging contaminants (hormones, persistent organic pollutants, antibiotics, pharmaceuticals, heavy metals, nano plastics, etc.)
[6][8][9][10][6,8,9,10], and to avoid secondary pollution problems an advanced treatment technology is required. It is therefore valuable to explore the development of advanced wastewater treatment technologies to effectively purify water in a more ecofriendly and economical routine.
In recent years, advances in nanoengineering and nanotechnology to develop nanosorbents, nanocatalysts, bioactive nanoparticles, and filtration systems coupled with nanoparticles have been seen to be very promising in wastewater treatment settings
[1][9][11][12][13][14][15][1,9,11,12,13,14,15]. Most of the nanotechnology-based materials can exhibit distinctive physical and chemical properties at a nanoscale (1–100 nm) as compared to their bulk complements
[9][12][9,12]. Intensive research devoted to understanding the fundamental mechanism of nano-based materials has revealed that semiconductor photocatalysts, such as TiO
2, possess high surface-to-volume ratios, chemical stability, hydrophilicity and high photo-reactivity with antireflection and self-cleaning abilities
[13][16][17][13,16,17]. Semiconductor photocatalysis is driven by energy sources (UV light, ultrasonic, heat or sunlight) to activate metal oxides (TiO
2, ZnO, and Fenton reagent) in order to generate radical species (OH−, H+) in the occurrence of wet or air oxidation states like ozone or hydrogen peroxide
[10][18][19][20][21][10,18,19,20,21].
Heterogeneous photocatalysts and semiconductors assisted with UV light for photodegradation of organic pollutants in water and wastewater settings have been reported by many researchers
[9][11][16][22][23][9,11,16,22,23]. These include BiVO
4, TiO
2 Fe–ZnIn
2S
4, WO
3, BiOBr, BiFeO
3, Fe
2O
3, CuS, and ZnO
[11][17][24][11,17,24]. Among these photocatalysts and nanoparticles, titanium dioxide (TiO
2), being cheap, commercially available, nontoxic and chemically stable, has been the most extensively researched in water settings
[25][26][27][25,26,27]. Aside from water and wastewater treatment applications, the TiO
2 photocatalyst has been widely used in air purification and other biological applications
[25][28][29][30][31][32][33][25,28,29,30,31,32,33]. Notwithstanding, there are several setbacks associated with TiO
2 industrial applications at a large scale via the advanced oxidation process (AOP)
[11][18][25][34][35][11,18,25,34,35]. Some of these include high cost of energy resources and chemicals as oxidants and their potential to handle large amounts of wastewater with high organic strengths
[11][34][35][11,34,35]. The separation, recovery, and re-use of TiO
2 nanoparticles is also a major constraint to its industrial applications
[34][36][37][34,36,37].
In general, most chemicals or nanoparticles used in conventional systems end up converting contaminants from one form to another
[11][15][11,15]. For instance, generation of biosolids or sludge becomes the new pollutant, which requires further treatment before discharge. Additionally, in the application of TiO
2 photocatalysis, the low rate of electrostatic interaction to oxygen and high rate of electron-hole recombination significantly slows its adsorption of organic contaminants onto its surface
[11][38][11,38]. Yet, there are still some research gaps associated with TiO
2 nanoparticle applications, which include low quantum efficiency due to inefficient visible light harvesting, acceptable photoreactor design, recovery, re-use, scale-up, etc.
[25]. Thus, in conjunction with unaddressed concerns of emerging contaminants
[1], the search for ecofriendly techniques capable of degrading pollutants rather than simply converting them from one form to another becomes essential. Consequentially, many researchers have pursued to develop new heterogeneous photocatalysts with a suitable crystal structure, high specific surface area, and easy separation and re-use capabilities
[18][26][30][39][18,26,30,39]. This includes synthesizing metal/TiO
2 nanocomposites as an efficient way to improve TiO
2 photocatalytic efficiency by enhancing its electron-hole separation
[18][30][40][18,30,40]. In addition, the embedding of Fe
3O
4 has been proven to be very useful in order to provide TiO
2 with magnetic separation ability
[41][42][43][41,42,43].
2. Advanced Oxidation Process (AOP)
Advanced oxidation processes (AOPs) have attracted a great deal of attention of stakeholders in the water and wastewater treatment sector. Photocatalytic degradation technology, being one of the AOPs, involves the use of a semiconductor photocatalyst and photogeneration of highly oxidative hydroxyl radicals
[22][34][22,34]. In addition, the chemical reactions in AOPs caused by the absorption or desorption of photocatalysts remains unchanged in both photocatalysis and photoelectrochemical splitting of water using titania
[19][22][44][19,22,49]. Furthermore, AOPs differ from conventional chemical and biological wastewater treatment systems as their produced hydroxyl radicals are potent oxidants used to degrade toxic and recalcitrant contaminants into simple and harmless inorganic molecules without creating a secondary excess
[45][46][47][46,50,51]. Additionally, AOPs are very robust so as to enhance precipitations and elimination of heavy metals as metal hydroxides, and subsequently, based on the irradiation period, can lead to total mineralization
[19][34][48][19,34,52]. AOPs are classified into two categories, namely homogeneous and heterogeneous systems, that can be carried out with or without UV radiation.
Table 1 presents some of the reported AOPs commonly used in wastewater treatment settings. The thermal Fenton reaction process is a homogeneous process that involves Fe
2+ reacting with H
2O
2. The aforementioned phase (Fe
2+/Fe
3+, H
2O
2, UV-Vis) is commonly termed as a photo-Fenton reaction
[19]. Heterogeneous photocatalysis involves a photoinduced reaction accelerated by the presence of TiO
2 photocatalysts
[49][50][53,54]. This process stands out among the heterogeneous AOPs because UV irradiation of TiO
2 creates hydroxyl radicals. This facilitates the oxidation and more efficient mineralization of organic material present in the effluent when it is photocatalyzed
[51][55]. In contrast with other AOPs and biological treatments, TiO
2 photocatalysis offers significant advantages
[19][45][19,46]. Thus, the TiO
2/UV system can handle contaminants in both the gas and solution phases. Furthermore, TiO
2 is inexpensive, practically insoluble in water, and biologically and chemically inert
[19][22][45][19,22,46].
Table 1. Types of advanced oxidation processes [19][45]. Types of advanced oxidation processes; adapted from [19,46].
|
Chemical Process
|
Photochemical Process
|
(adapted from [49,54]).
|
Crystalline Forms
|
Anatase
|
Rutile
|
Brookite
|
|
Wet air oxidation
|
|
Crystalline structure |
Photo-Fenton reaction
|
|
|
Tetragonal
|
Tetragonal
|
Rhombohedral
|
Supercritical water oxidation
|
UV/ultrasound system
|
|
Lattice constants (nm)
|
a = b = 0.3733
c = 0.9370
|
Ultrasound/H2O2 system
|
UV/O3/H2O2 system
|
|
| a = b = 0.4584 |
c = 0.2953
|
Ultrasound/Sonolysis
|
UV/H2O2 system
|
|
| a = 0.5436; b = 0.9166 |
| c = 0.5135
|
Fenton reaction
|
UV/O3 system
|
|
Density (g.cm−3)
|
3.83
|
4.24
|
4.17
|
|
Bravais lattice
|
Simple, body-centered
|
Simple, body-centered
|
Simple
|
Ozonation in alkaline |
|
Melting point (°C)
|
Turning into rutile
|
1870
|
Turning into rutile
|
|
|
Boiling point (°C)
|
UV photolysis
|
|
| 2927
|
-
|
|
O3/H2O2 system
|
UV/O2/TiO2 system
|
|
Electron—Fenton reaction
|
UV/H2O2/TiO2 system
|
2.1. TiO
2
Photocatalyst
TiO
2 is the most widely used photocatalyst because of its strong physical and chemical stability, insolubility in water, resistance to acids, inertness to most chemicals and long-term photostability
[25][40][52][25,40,47]. TiO
2 exhibits three main crystal structures such as rutile (stable), anatase (metastable), and brookite structures (
Table 2).
Table 2.
Chemo-physical properties of TiO- |
|
|
|
|
Band gap (eV) |
|
|
|
| 3.2
|
3.0
|
-
|
|
Refractive index (ng)
|
2.5688
|
2.9467
|
2.809
|
|
Standard heat capacity (cp)
|
55.52
|
55.60
|
-
|
|
Dielectric constant
|
55
|
110–117
|
78
|
The tetragonal structure consists of both rutile and anatase, while brookite has a rhombohedral structure
[44][46][53][48,49,50]. In addition to their shape and nanoscale or size, the unique physicochemical properties of TiO
2 crystals are a result of their intrinsic electronic structure and crystal structures
[17][25][47][17,25,51]. Anatase has been found to be more active than rutile, but both forms of TiO
2 have been reported to be photoactive. Additionally, a mixture of anatase and rutile for photocatalysis has been found to exhibit synergy due to the photoinduced interfacial electrons of anatase that can be transformed into rutile
[27][39][27,39].
Rutile and anatase, with 3.0 eV and 3.2 eV band gaps, respectively, are the most common polymorphs of TiO
2 [44][46][49,50]. In
Figure 1, the large TiO
2 energy band means that higher UV light energy is required to excite electrons to create hydroxyl radicals, which is the key to the photodegradation of contaminants in the presence of oxygen
[20][27][48][20,27,52].
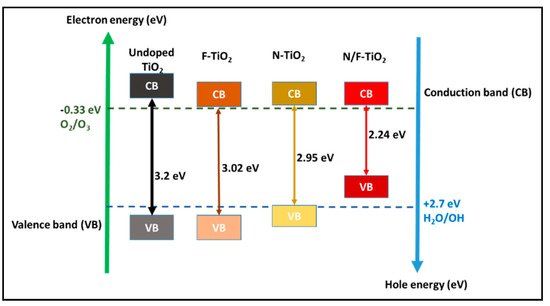
Figure 1.
Schematic diagram of TiO
2 with different valence and conduction band levels [44][49]. with different valence and conduction band levels; adapted from [49,53].
The crystalline phase of anatase is desired because of its maximum efficiency under UV irradiation
[20]. The photogenerated electrons form superoxide radicals in the rutile conduction band and the holes in the anatase valence band play a significant role in oxidation reactions. In addition, the 3.2 eV anatase band gap (
Figure 1) corresponding to the light wavelength of 388 nm means that only UV light will activate TiO
2. This limits the use of sunlight and greener energy for the activation of TiO
2, as only around 5% of sunlight is formed by UV radiation. Thus, during the photo activation step, TiO
2 undergoes a rapid recombination of electrons and holes, which then reduces photoactivity. Hence, by doping the TiO
2 with inert supports, the electron–hole recombination and wide band gap can be addressed while increasing the TiO
2 nanoparticle size
[20][25][20,25].
Furthermore, in photocatalytic activities, TiO
2 with a valence band (VB) and conduction band (CB) levels of +2.9 and −0.33 eV can result in a band gap energy of 3.2 eV. As shown in
Figure 1, the TiO
2 VB and CB levels are more positive and negative than the distinctive redox potential of O
2/H
2O (1.23 eV). The presence of Ti
3+ defects beneath the CB surface causes the band gap reduction in F-doped TiO
2 (3.02 eV), while in N-doped TiO
2 (2.95 eV) the mid-band states are created as the N species fill voids as impurities above the VB. However, there is a doping-induced effect and a transition in the VB tail; co-doping N and F into TiO
2 results in the greatest band gap reduction, from 3.2 eV to 2.24 eV (
Figure 1).
2.2. Operational Parameters in Photocatalysis
The photocatalytic system’s oxidation rates and semiconductor catalyst performance coupled with a photoreactor are all highly dependent on several operational parameters that regulate the kinetics
[18][26][54][18,26,69]. Outstanding studies of the different parameters affecting the photocatalysis process, including pH, catalyst loading, amount of oxygen, contaminant loading, light intensity and duration of light irradiation, preparation method of the catalyst, its calcination temperature, and amount and type of dopant, have been carried out and reported in the literature
[18][25][26][47][49][55][56][18,25,26,51,53,70,71]. In comparison to immobilized films, photocatalysts mostly take the form of suspended powder (slurries), giving higher efficiency
[25]. However, separating photocatalysts from treated water in this form is difficult, implying that a recovery step for photocatalyst re-use is required
[57][58][59][72,73,74]. Photocatalysts based on TiO
2 with a large surface area have a high photocatalytic efficiency, so nano-sized TiO
2 particles have been used in most studies
[25][59][25,74].
3. Integrated Photocatalytic Membrane (IPM) Reactors
Membrane separation has become one of the most improved technologies for water treatment in recent decades due to its small carbon footprint, high separation efficiency, and ease of operation
[2][39][56][2,39,71]. Membrane fouling is caused by the formation of a cake layer on the membrane surface and results in pore blocking in conventional membrane processes
[60][81]. As a result, water flux is reduced significantly, and energy consumption and treatment costs are increased. Furthermore, membrane filtration can only concentrate contaminants into a high concentration retentate, which requires additional treatment prior to discharge
[39][60][61][39,81,82]. Therefore, advancements in membrane science and technology are useful in developing a cost-effective membrane process. In essence, membrane technology has progressed to the point where there is a plethora of membranes made for specific pollutants as well as membrane configurations and technologies tailored for specific industries (milk production, beer production, desalination, solvent separation) and material regeneration
[2][4][18][25][62][2,4,18,25,83].
Moreover, membranes are classified according to their pore sizes as microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and reverse osmosis (RO), which determine their selectivity
[25][61][62][25,82,83]. Nanoporous membranes (0.1–1 nm) are made up of a thin film through which molecules are transported via solution diffusion. The driving forces across such membrane transport include pressure, concentration, or potential gradient
[2][25][63][2,25,61]. Reverse osmosis (RO), NF and most recently forward osmosis (FO) are processes that use nonporous membranes. Microfiltration (MF) and ultrafiltration (UF) are two pressure-driven processes that use microporous membranes. MF membranes can separate particles with diameters ranging from 0.1 to 10 µm, while UF membranes can remove particles with diameters ranging from 1 to 100 nm
[2][64][2,84].
Generally, to meet strict wastewater discharge standards, a conventional activated sludge process is used to biologically treat wastewater for organic/nutrient removal, accompanied by an MF/UF process to produce high-quality permeate water (i.e., removing particles and bacteria/viruses, etc.) Furthermore, membrane filtration can only trap contaminants in a high concentration retentate, which requires additional post-treatment prior to discharge. In contrast, photocatalytic membrane processes may degrade contaminants in feed solutions by producing oxygen-reactive radicals in the presence of UV light, preventing the formation of a cake layer on the membrane surface
[3][65][3,45]. As a result, pore blocking is reduced, pollutant concentrations in the filtrate are reduced, and permeate quality is improved
[3][65][3,45]. This section delves into the different types of membranes used as supports as well as related photocatalytic membrane types, preparation, attributes and applications of TiO
2 photocatalytic membranes in removing contaminants in wastewater settings.