
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Radu Claudiu Fierascu | -- | 3971 | 2022-09-20 10:53:14 | | | |
| 2 | Peter Tang | Meta information modification | 3971 | 2022-09-20 11:27:28 | | | | |
| 3 | Radu Claudiu Fierascu | + 13 word(s) | 3984 | 2022-09-22 11:39:30 | | | | |
| 4 | Peter Tang | Meta information modification | 3984 | 2022-09-23 02:50:03 | | |
Video Upload Options
Fragaria genus (Rosaceae), commonly known as strawberry, represents one of the most important food plants all over the world, with a double global production compared with all other fruit berries combined. Usually appreciated because of their specific flavor, the strawberries also possess biological properties, including antioxidant, antimicrobial, or anti-inflammatory effects.
1. Introduction
2. Composition of Fragaria L. Genus
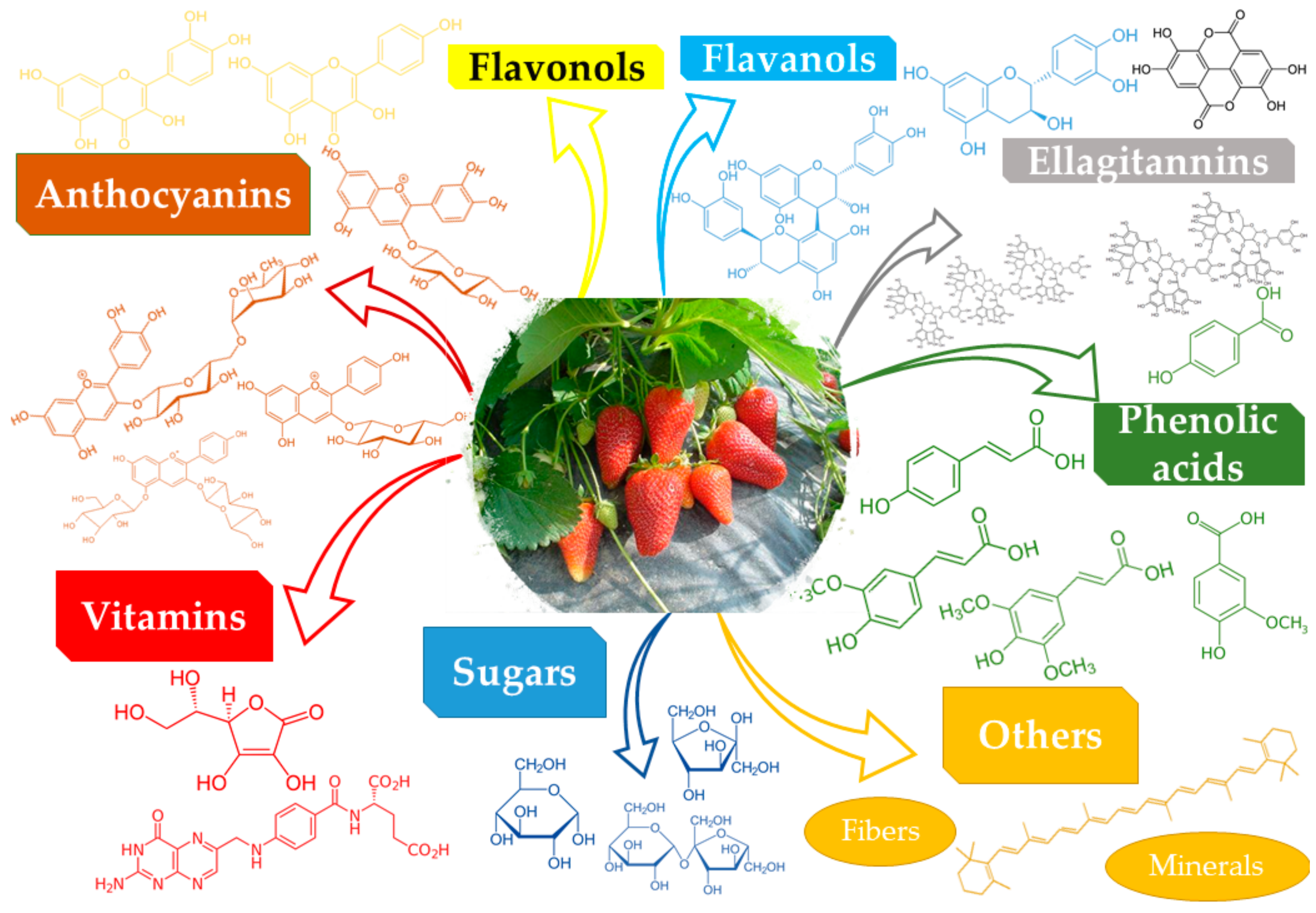
|
Class |
Compound |
Ref. |
|---|---|---|
|
Anthocyanins |
Pelargonidin 3-glucoside, cyanidin 3-glucoside, cyanidin 3-rutinoside, pelargonidin 3-galactoside, pelargonidin 3-rutinoside, pelargonidin 3-arabinoside, pelargonidin 3-malylglucoside |
|
|
Flavonols |
Quercetin, kaempferol, fisetin, their glucuronides, and glycosides |
|
|
Flavanols |
Catechin, proanthocyanidin B1, proanthocyanidin trimer, proanthocyanidin B3 |
[3] |
|
Ellagitannins |
Sanguiin H-6, ellagitannin, ellagic acid, lambertianin C, galloylbis-hexahydroxydiphenoyl-glucose |
[3] |
|
Phenolic acids |
4-coumaric acid, p-hydroxybenzoic acid, ferulic acid, vanillic acid, sinapic acid |
[7] |
|
Vitamins |
Vitamin C, vitamin B9 |
[6] |
|
Minerals |
Mn, K, Mg, P, Ca |
[3] |
|
Others |
Sugars (glucose, fructose, and sucrose), fibers |
[3] |
|
Species |
Plant Part, Other Variables |
Identified Compounds and Main Findings |
Identification Method |
Ref. |
|---|---|---|---|---|
|
F. chiloensis |
Ripe fruits |
Anthocyanins (cyanidin 3-O-glucoside, pelargonidin 3-O-glucoside cyanidin-malonyl-glucoside and pelargonidin-malonyl-glucoside); procyanidins, ellagitannins, ellagic acid and flavonol derivatives |
HPLC-DAD, LC-ESI-MS |
[9] |
|
F. chiloensis |
Leaves |
Procyanidins, ellagitannins, ellagic acid and flavonol derivatives |
HPLC-DAD, LC-ESI-MS |
[9] |
|
F. chiloensis |
Rhizomes |
Procyanidins, ellagitannins, ellagic acid and flavonol derivatives |
HPLC-DAD, LC-ESI-MS |
[9] |
|
Fragaria × ananassa |
Fruits |
Anthocyanins (pelargonidin-3-glucoside, pelargonidin-3-rutinoside, cyanidin-3-rutinoside, pelargonidin-3,5-diglucoside, pelargonidin-3-(6-acetyl)-glucoside, 5-carboxypyranopelargonidin-3-glucoside, delphinidin-3-glucoside, peonidin-3-glucoside, cyanidin-3-galactoside), p-hydroxybenzoic acid, (+)-catechin, ellagic acid, p-coumaric acid, quercetin glucoside |
LC-MS/MS, HPLC-UV/Vis |
[10] |
|
Fragaria × ananassa |
Fruits, cultivar and seasonal variations |
Vitamin C, β-carotene, total phenolics, total anthocyanins; genotype influence is stronger than the environmental influence |
Colorimetric |
[11] |
|
Fragaria × ananassa |
Fruits, different cultivars on different ripeness stage |
Total vitamin C, total phenolics, total anthocyanins, total ellagic acid/pelargonidin-3-glucoside and cyanidin-3-glucoside; higher amounts in pink fruits compared with fully ripped fruits |
Colorimetric/HPLC-DAD |
[12] |
|
Fragaria × ananassa |
Fruits, different farming methods |
Total phenolics/pelargonidin-3-glucoside and cyanidin-3-glucoside, vitamin C, higher in organic farming fruits |
Colorimetric/HPLC-DAD |
[13] |
|
Fragaria × ananassa |
Fruits, different cultivars (27) and ripening stages |
Phenolic compounds (multiple classes, including anthocyanins, flavanols and ellagitannins); composition dependent on cultivar, cinnamic acid conjugates and anthocyanins levels increased with the ripening stage |
HPLC-DAD-MS |
[14] |
|
Fragaria × ananassa, F. vesca |
Fruits |
Quercetin and isorhamnetin glycosides (higher levels in wild strawberry) |
HPLC-DAD, LC-ESI-MS |
[15] |
|
Fragaria × ananassa, F. vesca |
Fruits, different cultivars |
Volatile esters (including ethyl acetate, hexyl acetate, methyl butanoate, ethyl butanoate, hexyl butanoate, methyl hexanoate, ethyl hexanoate, hexyl hexanoate); higher levels in cultivated strawberries. |
GC-MS |
[16] |
|
F. vesca |
Fruits, two different cultivars |
Anthocyanins (cyanidin 3-O-glucoside, pelargonidin 3-O-glucoside, peonidin 3-O-glucoside, cyanidin 3-O-malonylglucoside, pelargonidin 3-O-malonylglucoside, peonidin 3-O-malonylglucoside), dihydroflavonol and flavonols (taxifolin 3-O-arabinoside, kaempferol 3-O-glucoside, quercetin 3-O-glucoside, quercetin-acetylhexoside, kaempferol 3-O-acetylhexosides), flavan-3-ols and proanthocyanidins (catechin, B type proanthocyanidin dimers, trimers, and tetramers), ellagic acid and derivatives (glycosylated, methyl pentoside, methylellagic acid methyl pentoside, ellagitannins), other compounds (benzoic acid, ferulic acid hexose derivative, citric acid, furaneol glucoside) |
HPLC-DAD |
[17] |
|
Fragaria × ananassa, F. vesca |
Fruits |
Anthocyanins (cyanidin, pelargonidin), cyanidin glycosides (cyanidin 3-glucoside, cyanidin 3-arabinoside, cyanidin 3-sambubioside, delphinidin 3-galactoside, delphinidin 3-glucoside, delphinidin 3-malonylglucoside); higher levels of cyanidin glycosides in wild species |
HPLC-DAD |
[18] |
|
F. vesca |
Leaves |
Ellagitannins (sanguiin H-2 isomer, sanguiin H-10 isomer, sanguiin H-6/agrimoniin/lambertianin A isomer, castalagin/vescalagin isomer, sanguiin H-10 isomer, sanguiin H-2 isomer, casuarictin/potentillin isomer) |
LC-PDA-ESI-MS |
[19] |
|
Fragaria × ananassa |
Fruits, different cultivars and production years |
Vitamin C, anthocyanins (pelargonidin 3-glucoside, cyanidin 3-glucoside, pelargonidin 3-rutinoside), ellagic acid; strongly dependent on the cultivar and production year |
HPLC-UV/Vis |
[20] |
|
Fragaria × ananassa |
Fruits, at different ripening stage |
Vitamin C, pelargonidin-3-rutinoside, ellagic acid, cyanidin-3-glucoside, quercetin (red fruits), neochlorogenic, pelargonidin-3-glucoside, pelargonidin-3-rutinoside, epicatechin, quercetin-3-β-d-glucoside, ellagic acid (green fruits) |
LC-ESI-TOF |
[21] |
|
Fragaria × ananassa |
Calyx (red and green) |
Quercetin-3-β-d-glucoside, ellagic acid, kaempferol-3-O-glucoside, vitamin C (red), catechin, quercetin-3-β-d-glucoside, ellagic acid (green) |
LC-ESI-TOF |
[21] |
|
Fragaria × ananassa |
Flower |
Catechin, quercetin-3-β-d-glucoside, ellagic acid, kaempferol-3-O-glucoside, vitamin C |
LC-ESI-TOF |
[21] |
|
Fragaria × ananassa |
Leaf |
Procyanidin dimer and trimer, catechin, quercetin-3-β-d-glucoside, vitamin C, ellagic acid |
LC-ESI-TOF |
[21] |
|
Fragaria × ananassa |
Stolon |
Neochlorogenic, procyanidin dimer, catechin, quercetin-3-β-d-glucoside, ellagic acid, vitamin C, kaempferol-3-O-glucoside |
LC-ESI-TOF |
[21] |
|
Fragaria × ananassa |
Stem |
Procyanidin dimer, catechin, ferulic acid, quercetin-3-β-d-glucoside, ellagic acid |
LC-ESI-TOF |
[21] |
|
Fragaria × ananassa |
Crown |
Procyanidin dimer and trimer, catechin, propelargonidin dimer, ellagic acid |
LC-ESI-TOF |
[21] |
|
Fragaria × ananassa |
Root |
Procyanidin dimer and trimer, catechin, neochlorogenic, propelargonidin dimer |
LC-ESI-TOF |
[21] |
|
Fragaria × ananassa |
Fruits, different novel cultivars |
Phenolic acids (p-coumaric acid, ellagic acid, ferulic acid derivative, p-coumaric acid derivatives), monomeric flavanols ((+)-catechin), flavonols (quercetin 3-O-glucoside, fisetin, quercetin 3-O-glucoside derivative), anthocyanins (cyanidin 3-glucoside, cyanidin 3-rutinoside, cyanidin pentoside, pelargonidin 3-galactoside, pelargonidin 3,5-diglucoside, pelargonidin 3-glucoside, pelargonidin 3-rutinoside, cyanidin 3-Oacetylglucoside, cyanidin hexoside, pelargonidin 3-O-monoglucuronide, pelargonidin derivatives) |
HPLC-DAD, LC-ESI-QTOF |
[22] |
|
Fragaria × ananassa |
Fruits, grown on different altitudes, on consecutive years |
Hydroxybenzoic acid, p-coumaric acid, other hydroxycinnamic acids, (+)-catechin, (−)-epicatechin, procyanidins, flavonols, anthocyanins (cyanidin 3-glucoside, pelargonidin 3-glucoside, pelargonidin derivative); higher levels recorded at lower altitudes. |
HPLC-DAD |
[23] |
|
Fragaria × ananassa |
Fruits |
Kaempferol 3-(6-methylglucuronide), quercetin 3-(6-methylglucuronide), isorhamnetin 3-(6-methylglucuronide), trichocarpin, 2-p-hydroxybenzoyl-2,4,6-tri hydroxyphenylacetate, 2-p-hydroxyphene thyl-6-caffeoylglucoside, zingerone 4-glucoside, b-hydroxypropiovanillone 3-glucoside, (+)-isolariciresinol 90-glucoside, (−)-isolariciresinol 90-glucoside, aviculin, (−)-secoisolariciresinol 4-glucoside, cupressoside A, cedrusin, icariside E4, dihydrodehydrodiconiferyl alcohol 90-glucoside, massonianoside A, urolignoside, (−)-pinoresinol 4-glucoside, 2,3”-epoxy-4-(butan-2-one-3-yl)-5,7,40-trihydroxy flavane 3-glucoside, kaempferol 3-(6-butylglucuronide), benzyl 2-glucosyl-6-rhamnosylbenzoate |
1H NMR, 13C NMR, HMBC, HPLC-UV/Vis, LC-MS/MS, HR-ESI-MS, |
[24] |
|
F. vesca |
Fruits, wild and cultivated, from different geographical areas |
39 phenolic compounds (including cyanidin 3-O-glucoside, delphinidin-3-O-glucoside, pelargonidin-3-O-glucoside, pelargonidin-3-O-rutinoside, (+) catechin, (−) epicatechin, procyanidin B1 and B2, isoquercetin, gallic acid, p-coumaric acid, phloridzin); composition dependent on the geographical area |
LC-ESI-Orbitrap-MS, LC-ESI-QTrap-MS, LC-ESI-QTrap-MS/MS |
[25] |
|
Fragaria × ananassa |
Fruits, different cultivars |
Cyanidin 3-O-glucoside, pelargonidin-3-O-glucoside, pelargonidin-O-rutinoside, total anthocyanins content, dependent on the cultivar |
UPLC-PDA-ESI-MS, HPLC-DAD |
[26] |
|
F. vesca |
Fruits |
Volatile composition—one hundred compounds (including esters, aldehydes, ketones, alcohols, terpenoids, furans and lactones). |
GS-MS |
[27] |
|
F. vesca |
Leaves |
27 metabolites (organic acids, flavonoids, catechin and its oligomers, ellagitannins), including quinic acid, chelidonic acid, quercetin derivatives, catechin and procyanidins, phloridzin, pedunculagin, methyl ellagic acid glucuronide. |
LC-ESI-Orbitrap-MS |
[28] |
|
Fragaria × ananassa, F. vesca |
White-fruited mutants, different genotypes |
Anthocyanins, flavonols, flavan-3-ols, hydroxycinnamic acids, and ellagic acid—derived compounds, dependent on genotype |
LC-ESI-MS/MS |
[29] |
|
F. chiloensis |
Fruits |
Anthocyanins (cyanidin-3-O-glucoside, pelargonidin hexoside, cyanidin manlonyl hexoside, pelargonidin-malonyl hexoside), ellagitannins (ellagic acid hexoside, pentoside, rhamnoside), proanthocyanidin dimers, epicatechin, flavonols (quercetin pentoside, glucuronide) |
HPLC-DAD, LC-ESI-MS |
[30] |
|
Fragaria × ananassa |
Fruits, different cultivars |
Anthocyanins, flavonoids, cinnamic acid derivatives, tannins and related compounds, triterpenoids; concentration dependent on the cultivar |
UPLC-ESI-QTOF-MS/MS, HPLC-DAD |
[31] |
where:13C NMR—Carbon-13 nuclear magnetic resonance; GC-MS—gas chromatography–mass spectrometry; 1H NMR—proton nuclear magnetic resonance; HMBC —heteronuclear multiple bond correlation; HPLC-DAD—high-performance liquid chromatography with diode array detector; HPLC-UV/Vis—high-performance liquid chromatography equipped with UV/vis detector; HR-ESI-MS—high-resolution electrospray ionization mass spectrometry analysis; LC-ESI-MS(/MS)—liquid chromatography electrospray ionization (tandem) mass spectrometry analysis; LC-ESI-Orbitrap-MS—liquid chromatography electrospray ionization Orbitrap mass spectrometry; LC-ESI-QTrap-MS(/MS)—liquid chromatography electrospray ionization quadrupole ion trap mass spectrometry; LC–ESI–(Q)TOF—liquid chromatography electrospray ionization with (quadrupole) time-of-flight; LC-MS/MS—liquid chromatography–tandem mass spectrometry; LC-PDA-ESI-MS—liquid chromatography equipped with photodiode array detector coupled to mass spectrometry using the electrospray ionization interface; UPLC-ESI-QTOF-MS/MS—ultra-performance liquid chromatography equipped quadrupole time of flight coupled to tandem mass spectrometry using the electrospray ionization interface; UPLC-PDA-ESI-MS—ultra-performance liquid chromatography equipped with photodiode array detector coupled to mass spectrometry using the electrospray ionization interface.
3. Biological Activities of Fragaria Genus
3.1. Antioxidant Properties
|
Species |
Extraction Method |
Antioxidant Assay |
Antioxidant Potential |
Responsible Compounds |
Ref. |
|---|---|---|---|---|---|
|
Fragaria × ananassa, Camarosa var. fruits |
Anthocyanins isolated using CCC |
ORAC, FRAP |
ORAC: 2.7–24.46 mmol Trolox/g; FRAP: 2.75–12.5 mmol Fe2+/g (depending on the fraction) |
Anthocyanins |
[10] |
|
Fragaria chiloensis spp. chiloensis form chiloensis fruits |
Methanol: formic acid (99:1 v/v) extraction |
DPPH, SAS |
DPPH assay: IC50 = 38.7 mg/L; SAS: 79.3%) |
Aglycone and glycosylated ellagic acid and flavonoids |
[9] |
|
Fragaria chiloensis spp. chiloensis form chiloensis leaves |
Methanol: formic acid (99:1 v/v) extraction |
DPPH, SAS |
DPPH assay: IC50 = 49.4 mg/L; SAS: 67.60% |
Aglycone and glycosylated ellagic acid and flavonoids |
[9] |
|
Fragaria chiloensis spp. chiloensis form chiloensis rhizomes |
Methanol: formic acid (99:1 v/v) extraction |
DPPH, SAS |
DPPH assay: IC50 = 64.8 mg/L; SAS: 55% |
Aglycone and glycosylated ellagic acid and flavonoids |
[9] |
|
Fragaria x ananassa Osogrande var. frozen fruits |
Acetone (80%) extraction |
DPPH, FRAP |
DPPH: 11.91–12.83 μmol BHT eq./g FW; best results for ripe fruits FRAP: 27.37–36.75 μmol FS eq./g FW; best results for green fruits |
Total phenolic content, vitamin C |
[12] |
|
Fragaria x ananassa Camino Real var. frozen fruits |
Acetone (80%) extraction |
DPPH, FRAP |
DPPH: 9.75–12.01 μmol BHT eq./g FW, FRAP: 24.13–28.49 μmol FS eq./g FW (best results for pink fruits) |
Total phenolic content, vitamin C |
[12] |
|
F. vesca leaves |
Methanol, ultrasounds extraction |
DPPH, FRAP |
DPPH: IC50 = 13.46 mg/L; FRAP: 0.878 mmol Fe2+/g DW |
Total phenols, total tannins |
[37] |
|
F. vesca roots, wild-growing |
Hydromethanolic extraction, infusion, decoction |
DPPH, FRAP, β-Carotene bleaching inhibition, TBARS |
IC50, mg/L: DPPH—50.03/50.56/50.62; FRAP—40.98/44.78/49.23; β-C bleaching—116.26/44.88/66.10; TBARS—35.76/4.76/6.14 |
Total phenolics, total flavan-3-ols, total dihydroflavonols, |
[38] |
|
F. vesca roots, commercial |
Hydromethanolic extraction, infusion, decoction |
DPPH, FRAP, β-Carotene bleaching inhibition, TBARS |
IC50, mg/L: DPPH—68.89/255.81/51.32; FRAP—327.75/78.99/67.92; β-C bleaching—68.34/23.44/114.67; TBARS—6.69/24.25/10.62 |
Total phenolics, total flavan-3-ols, total dihydroflavonols, |
[38] |
|
Fragaria × ananassa var. Amaou, fruits, at different ripening stage |
Ethanol or water room temperature extraction |
Modified ABTS assay |
Ethanol: 150.5/151.9; water: 227.2/189.4 (red/green fruits) μmol TE/100 g FW |
Total phenolic content |
[21] |
|
Fragaria × ananassa var. Amaou calyx (red and green) |
Ethanol or water room temperature extraction |
Modified ABTS assay |
Ethanol: 241.1/1239.9; water: 1716.6/577.7 μmol TE/100 g FW (red/green calyx) |
Total phenolic content |
[21] |
|
Fragaria × ananassa var. Amaou flower |
Ethanol or water room temperature extraction |
Modified ABTS assay |
4234.4/387.5 μmol TE/100 g FW (ethanol/water) |
Total phenolic content |
[21] |
|
Fragaria × ananassa var. Amaou leaves |
Ethanol or water room temperature extraction |
Modified ABTS assay |
2401.7/241.1 μmol TE/100 g FW (ethanol/water) |
Total phenolic content |
[21] |
|
Fragaria × ananassa var. Amaou stolon |
Ethanol or water room temperature extraction |
Modified ABTS assay |
1089.4/1856.7 μmol TE/100 g FW (ethanol/water) |
Total phenolic content |
[21] |
|
Fragaria × ananassa var. Amaou stem |
Ethanol or water room temperature extraction |
Modified ABTS assay |
1338.6/1123.1 μmol TE/100 g FW (ethanol/water) |
Total phenolic content |
[21] |
|
Fragaria × ananassa var. Amaou crown |
Ethanol or water room temperature extraction |
Modified ABTS assay |
6213.3/128.7 μmol TE/100 g FW (ethanol/water) |
Total phenolic content |
[21] |
|
Fragaria × ananassa var. Amaou root |
Ethanol or water room temperature extraction |
Modified ABTS assay |
253.1/69.2 μmol TE/100 g FW (ethanol/water) |
Total phenolic content |
[21] |
|
F. vesca vegetative parts (leaves and stems), wild-growing |
Hydromethanolic and aqueous extracts; wild-growing infusion microencapsulated in alginate and incorporated in k-carrageenan gelatine |
DPPH, FRAP, β-Carotene bleaching inhibition, TBARS |
IC50, mg/L: DPPH—123.67/86.17/109.10; FRAP—81.40/62.36/77.28; β-C bleaching—56.71/12.34/13.40; TBARS—12.63/3.12/5.03 (hydromethanolic/infusion/decoction); Final formulation (mg/mL)—DPPH—2.74; FRAP = 1.23 |
Total phenolics, total flavan-3-ols, total dihydroflavonols, |
[39] |
|
F. vesca vegetative parts (leaves and stems), commercial |
Hydromethanolic and aqueous extracts |
DPPH, FRAP, β-Carotene bleaching inhibition, TBARS |
IC50, mg/L: DPPH—139.33/121.94/118.89; FRAP—324.49/91.88/88.20; β-C bleaching—388.90/76.41/69.98; TBARS—24.36/23.07/17.52 (hydromethanolic/infusion/decoction). |
Total phenolics, total flavan-3-ols, total dihydroflavonols, |
[39] |
|
Fragaria x ananassa cv. Falandi fruit |
22 compounds isolated from ethanolic extracts |
ABTS, DPPH, FRAP |
Best results (IC50): ABTS—4.42 μM kaempferol 3-(6-methylglucuronide); DPPH—32.12 μM quercetin 3-(6-methylglucuronide); FRAP—0.05 mmol/g—urolignoside. |
Individual compounds |
[24] |
|
Fragaria x ananassa cv. Albion, Aromas, Camarosa, Camino Real, Monte Rey, Portola, and San Andreas fruits |
Ultrasonic extraction with acidified methanol |
DPPH |
IC50 (mg/mL) ranging from 76.73 (Camarosa)—100 (Camino Real) |
Total anthocyanin content |
[26] |
|
F. vesca leaves native to Italy |
Ultrasonic extraction with ethanol: water solvent (70:30, v/v) |
TEAC |
0.34–0.35 mg/mL Trolox eq., compared with quercetin (0.40) |
Condensed tannins and flavonoid derivatives |
[28] |
|
Fragaria x ananassa cv. Tochiotome leaves |
Supercritical CO2 extraction with different entrainers |
DPPH |
0.07 (simple supercritical extraction)—5.82 μmol BHT/g sample (with ethanol, dried at 40 °C) |
Phenolic compounds |
[40] |
|
Fragaria × ananassa fruits (90 cultivars) |
Ultrasonic aqueous methanol (70%) acidified with 1.5% formic acid, at room temperature |
DPPH, ABTS |
Average values (μmol Trolox/100 g):765.06 (DPPH), 1637.96 (ABTS) |
Tannin-based compounds. |
[31] |
where: ABTS—2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) assay; BHT—butylated hydroxytoluene; DPPH—reduction of 2,2-diphenyl-1-picrylhydrazyl; DW—dry weight; eq.—equivalents; FRAP—ferric reducing ability of plasma; FS—ferrous sulphate; FW—fresh weight; IC50—half maximal inhibitory concentration; ORAC—oxygen radical absorbance capacity; SAS—superoxide anion assay; TBARS—thiobarbituric acid reactive substances assay; TEAC—Trolox equivalent antioxidant capacity.
3.2. Anti-Inflammatory Properties
3.3. Other Potential Applications
|
Action |
Plant |
Extraction Method |
Assay |
Results |
Responsible Compounds |
Ref. |
|---|---|---|---|---|---|---|
|
Anti-inflammatory on inflammatory bowel disease |
Fragaria vesca leaves |
Eth. extraction |
MPO activity; GSH, SOD and CAT levels |
Prevention of increase in colon weight and disease activity index, decrease in macroscopic and microscopic lesion score; significant improvement of MPO, CAT and SOD levels at 500 mg/kg 5 days oral treatment |
Phenolic acids, flavonoids |
[51] |
|
Anti-inflammatory |
Fragaria vesca leaves |
Eth. extraction at room temperature, infusion |
Nitric oxide production, western blot analysis (expression of pro-inflammatory proteins in lipopolysaccharide-triggered macrophages); nitric oxide scavenger activity |
Inhibition of nitrite production on pre-treated cells (at 80 and 160 mg/L—31%/40%); 23% inhibition in culture media, at 160 mg/L |
Phenolic content |
[46] |
|
Anti-inflammatory |
Fragaria x ananassa, var. Alba fruits |
Meth. extraction at room temperature, infusion |
Determination of ROS intracellular levels, apoptosis detection, antioxidant enzyme activities, immunoblotting analysis, determination of mitochondrial respiration and extracellular acidification rate in cells |
Reduction of intracellular ROS levels (significant at 100 mg/L), decreased apoptotic rate (significant at 50 and 100 mg/L); Increased ARE-antioxidant enzymes expression, reduced NO and inflammatory cytokines production (at 50 and 100 mg/L) to control levels |
Vitamin C, anthocyanins, flavonoids |
[52] |
|
Anti-inflammatory, hepatoprotective |
Fragaria chiloensisssp. Chiloensis fruits |
Aq. extracts |
Histological analyses, determination of transaminases, cytokines, F2-isoprostanes, and glutathione assays |
maintained hepatocellular membrane, structural integrity, attenuated hepatic oxidative stress, and inhibited inflammatory response in LPS-induced liver injury; downregulation of cytokines (TNFa, IL-1β, and IL-6) |
Phenolic content |
[53] |
|
Anti-inflammatory |
Fragaria x ananassa var. Camarosa fruits |
Ultrasonic-assisted, acidified meth. extraction, separation |
In vivo: quantification of the leukocyte content, exudate concentration, MPO and ADA activities, nitric oxide products, TNF-α and IL-6 levels; in vitro: MTT assay, measurement of nitric oxide products, TNF-α and IL-6 levels, western blot analysis |
Inhibition of the carrageenan-induced leukocyte influx to the pleural cavity; reduction of myeloperoxidase activity, exudate concentration, NO levels. |
Phenolic compounds, anthocyanins (particularly pelargonidin-3-O-glucoside) |
[54] |
|
Anti-inflammatory, wound healing |
Fragaria x ananassa var. San Andreas fruits |
Ultrasound-assisted extraction, acidified meth.: aq. (80:20); separation of different fractions |
MTT assay, ROS, NO levels, effects on inflammatory markers and on skin fibroblast migration |
ROS reduction, suppression of IL-1β, IL-6 and iNOS gene expressions; enhanced skin fibroblast migration |
Polyphenolic compounds, especially anthocyanins |
[55] |
|
Anti-microbial |
Fragaria vesca leaves and roots |
Centrifugation extraction with meth.: aq. (80:20) |
Disc diffusion assay |
6–9 mm inhibition zones for leaves, 5–9 mm for roots (depending on S. aureus strain) |
Phenolic compounds |
[47] |
|
Anti-microbial |
Fragaria vesca leaves |
Hydroalcoholic extraction, separation |
Disc diffusion assay |
Good inhibition potential at 25 mg/mL, better effect for the ellagitannin-enriched fraction |
Ellagitannins |
[48] |
|
Anti-allergenic |
Fragaria x ananassa var. Minomusume fruits |
Methanol fraction of fruits juice (obtained by squeezing) |
Antigen-stimulated degranulation in RBL-2H3 cells |
degranulation suppression (95–60% inhibition for linocinnamarin, cinnamic acid, chrysin, kaempferol, trans-tiliroside) |
Best results - phenylpropanoid glycoside |
[49] |
|
Anti-diabetic |
Fragaria x ananassa var. Falandi fruits |
Compounds isolated from eth. extracts |
α-glucosidase inhibitory activity |
IC50 values better than the positive control (acarbose) for nine compounds (537.43 to 25.39 μM) |
Individual compounds |
[24] |
|
Anti-obesity, anti-allergy, skin-lightening |
Fragaria ×ananassa var. Amaou, entire plant (red fruit, green fruit, red calyx, green calyx, flower, leaf, stolon, stolon leaf, stem, crown and root) |
Eth. or aq. room temperature extraction |
Anti-lipase assay, adipocyte differentiation inhibition assay, melanogenesis inhibition assay, β-hexosaminidase inhibition assay, tyrosinase inhibition assay |
Crown, stolon leaf and flowers extracts exhibited the highest effects |
Total phenolic content |
[21] |
|
Antihyperuricemic |
Fragaria x ananassa cv. Tochiotome leaves |
Supercritical CO2 extraction with different entrainers |
Uric acid production in AML12 hepatocytes |
Reduction of uric acid at 100 mg/mL (96 mmol/2 h/mg protein), compared with the control (16,096 mmol/2 h/mg protein) |
Kaempferol, quercetin |
[40] |
|
Cytotoxic, anti-proliferative |
Fragaria x ananassa fruits |
Meth. extraction |
Ex vivo: cell viability assay; in vivo: developing tumor size determination |
Cytotoxic on cancer cells, blocked the proliferation of tumor cells |
Phenolic compounds |
[56] |
|
Antineoplastic |
Fragaria x ananassa var. Pajaro fruits |
Acidified hydro-eth. extraction |
Transglutaminase assay and polyamine detection, immunoblot analysis |
reduction of cell proliferation, lowering of the intracellular levels of polyamine, enhancement of tissue transglutaminase activity |
Anthocyanins |
[57] |
|
Cytotoxic |
Fragaria vesca L. leaves |
Hydroalcoholic extract at room temperature, ellagitannins-enriched fraction |
Effects on HepG2 cells—cell viability assessment, cell proliferation, cell cycle and cell death analysis, Western blot analysis, proteasome chymotrypsin-like activity |
Inhibition of HepG2 cell viability IC50 = 690 mg/L (extract)/113 mg/L (fraction); fraction induced necrosis and apoptosis, influenced the cellular proteolytic mechanisms |
Ellagitannins |
[19] |
|
Chemopreventive |
Lyophilized Fragaria x ananassa fruits |
Ultrasound-assisted extraction with acidified acetone |
Histological studies, Western blot analysis, PGE2 measurement, and nitrate/nitrite colorimetric assay |
Decreased tumor incidence, decreased levels of TNF-α, IL-1β, IL-6, COX-2 and iNOS, inhibition of the phosphorylation of PI3K, Akt, ERK, and NFκB |
anthocyanins, ellagitannin/ellagic acid/ellagic acid derivatives flavonols |
[58] |
|
Cytotoxic |
Fragaria x ananassa leaves |
Hydroalcoholic extracts (meth., eth., isopropanol) from in vitro cell suspension |
Cell proliferation, cell viability |
Under 50% viable cells for colorectal adenocarcinoma and colon adenocarcinoma upon treatment with extracts containing 0.29 mM ethoxy-dihydrofuro-furan |
Polyphenols |
[59] |
where: ADA—adenosine-deaminase; Akt—Protein Kinase B; aq.—water (aqueous); CAT—catalase; COX-2—cyclooxygenase-2 enzyme; ERK—extracellular signal-regulated kinase; eth—ethanol; GSH—glutathione; HepG2—human liver cancer cell line; IC50—half maximal inhibitory concentration; IL-1β—Interleukin 1 beta cytokine protein; IL-6—interleukin 6; iNOS—inducible nitric oxide synthase; meth.—methanol; MPO—myeloperoxidase; MTT—3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NFκB—nuclear factor kappa-light-chain-enhancer of activated B cells; NO—nitric oxide; PGE2—Prostaglandin E2; PI3K—phosphatidylinositol 3-kinase; RBL—rat basophilic leukemia cells; ROS—reactive oxygen species; SOD—superoxide dismutase; TNF-α—tumor necrosis factor alpha;.
References
- Liston, A.; Cronn, R.; Ashman, T.L. Fragaria: A genus with deep historical roots and ripe for evolutionary and ecological insights. Am. J. Bot. 2014, 101, 1686–1699.
- Awad, M.A.; De Jager, A. Influences of air and controlled atmosphere storage on the concentration of potentially healthful phenolics in apples and other fruits. Postharv. Biol. Technol. 2003, 27, 53–58.
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19.
- Morales-Quintana, L.; Ramos, P. Chilean strawberry (Fragaria chiloensis): An integrative and comprehensive review. Food Res. Int. 2019, 119, 769–776.
- Jimenez-Garcia, S.N.; Guevara-Gonzalez, R.G.; Miranda-Lopez, R.; Feregrino-Perez, A.A.; Torres-Pacheco, I.; Vazquez-Cruz, M.A. Functional properties and quality characteristics of bioactive compounds in berries: Biochemistry, biotechnology, and genomics. Food Res. Int. 2013, 54, 1195–1207.
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144.
- Vuong, Q.V.; Hirun, S.; Phillips, P.A.; Chuen, T.L.; Bowyer, M.C.; Goldsmith, C.D.; Scarlett, C.J. Fruit-derived phenolic compounds and pancreatic cancer: Perspectives from Australian native fruits. J. Ethnopharmacol. 2014, 152, 227–242.
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A dietary antioxidant for health promotion. Antioxid. Redox Signal. 2013, 2, 151–162.
- Simirgiotis, M.J.; Schmeda-Hirschmann, G. Determination of phenolic composition and antioxidant activity in fruits, rhizomes and leaves of the white strawberry (Fragaria chiloensis spp. Chiloensis form chiloensis) using HPLC-DAD–ESI-MS and free radical quenching techniques. J. Food Compos. Anal. 2010, 23, 545–553.
- Cerezo, A.B.; Cuevas, E.; Winterhalter, P.; Garcia-Parrilla, M.C.; Troncoso, A.M. Isolation, identification, and antioxidant activity of anthocyanin compounds in Camarosa strawberry. Food Chem. 2010, 123, 574–582.
- Singh, A.; Singh, B.K.; Deka, B.C.; Sanwal, S.K.; Patel, R.K.; Verma, M.R. The genetic variability, inheritance and inter-relationships of ascorbic acid, β-carotene, phenol and anthocyanin content in strawberry (Fragaria×ananassa Duch.). Sci. Horticult. 2011, 129, 86–90.
- Pineli, L.L.O.; Moretti, C.L.; dos Santos, M.S.; Campos, A.B.; Brasileiro, A.V.; Cordova, A.C.; Chiarello, M.D. Antioxidants and other chemical and physical characteristics of two strawberry cultivars at different ripeness stages. J. Food Compos. Anal. 2011, 24, 11–16.
- Crecente-Campo, J.; Nunes-Damaceno, M.; Romero-Rodrıguez, M.A.; Vazquez-Oderiz, M.L. Color, anthocyanin pigment, ascorbic acid and total phenolic compound determination in organic versus conventional strawberries (Fragaria x ananassa Duch, cv Selva). J. Food Compos. Anal. 2012, 28, 23–30.
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria x ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97.
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC-MSn identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012, 135, 2138–2146.
- Dong, J.; Zhang, Y.; Tang, X.; Jin, W.; Han, Z. Differences in volatile ester composition between Fragaria×ananassa and F. vesca and implications for strawberry aroma patterns. Sci. Horticult. 2013, 150, 47–53.
- Sun, J.; Liu, X.; Yang, T.; Slovin, J.; Chen, P. Profiling polyphenols of two diploid strawberry (Fragaria vesca) inbred lines using UHPLC-HRMSn. Food Chem. 2014, 146, 289–298.
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. LWT Food Sci. Technol. 2015, 60, 509–517.
- Liberal, J.; Costa, G.; Carmo, A.; Vitorino, R.; Marques, C.; Domingues, M.R.; Domingues, P.; Goncalves, A.C.; Alves, R.; Sarmento-Ribeiro, A.B.; et al. Chemical characterization and cytotoxic potential of an ellagitannin-enriched fraction from Fragaria vesca leaves. Arab. J. Chem. 2015.
- Kim, S.K.; Kim, D.S.; Kim, D.Y.; Chun, C. Variation of bioactive compounds content of 14 oriental strawberry cultivars. Food Chem. 2015, 184, 196–202.
- Zhu, Q.; Nakagawa, T.; Kishikawa, A.; Ohnuki, K.; Shimizu, K. In vitro bioactivities and phytochemical profile of various parts of the strawberry (Fragaria × ananassa var. Amaou). J. Funct. Food 2015, 13, 38–49.
- Fernández-Lara, R.; Gordillo, B.; Rodríguez-Pulido, F.J.; González-Miret, M.L.; del Villar-Martínez, A.A.; Dávila-Ortiz, G.; Heredia, F.J. Assessment of the differences in the phenolic composition and color characteristics of new strawberry (Fragaria x ananassa Duch.) cultivars by HPLC-MS and Imaging Tristimulus Colorimetry. Food Res. Int. 2015, 76, 645–653.
- Guerrero-Chavez, G.; Scampicchio, M.; Andreotti, C. Influence of the site altitude on strawberry phenolic composition and quality. Sci. Horticult. 2015, 192, 21–28.
- Yang, D.; Xie, H.; Jiang, Y.; Wei, X. Phenolics from strawberry cv. Falandi and their antioxidant and α-glucosidase inhibitory activities. Food Chem. 2016, 194, 857–863.
- D’Urso, G.; Maldini, M.; Pintore, G.; d’Aquino, L.; Montoro, P.; Pizza, C. Characterisation of Fragaria vesca fruit from Italy following a metabolomics approach through integrated mass spectrometry techniques. LWT Food Sci. Technol. 2016, 74, 387–395.
- Chaves, V.C.; Calvete, E.; Reginatto, F.H. Quality properties and antioxidant activity of seven strawberry (Fragaria x ananassa Duch) cultivars. Sci. Horticult. 2017, 225, 293–298.
- Urrutia, M.; Rambla, J.L.; Alexiou, K.G.; Granell, A.; Monfort, A. Genetic analysis of the wild strawberry (Fragaria vesca) volatile composition. Plant Physiol. Biochem. 2017, 121, 99–117.
- D’Urso, G.; Pizza, C.; Piacente, S.; Montoro, P. Combination of LC–MS based metabolomics and antioxidant activity for evaluation of bioactive compounds in Fragaria vesca leaves from Italy. J. Pharmaceut. Biomed. Anal. 2018, 150, 233–240.
- Roy, S.; Wu, B.; Liu, W.; Archbold, D.D. Comparative analyses of polyphenolic composition of Fragaria spp. Color mutants. Plant Physiol. Biochem. 2018, 125, 255–261.
- Chamorro, M.F.; Reiner, G.; Theoduloz, C.; Ladio, A.; Schmeda-Hirschmann, G.; Gómez-Alonso, S.; Jiménez-Aspee, F. Polyphenol composition and (bio)activity of berberis species and wild strawberry from the Argentinean Patagonia. Molecules 2019, 24, 3331.
- Nowicka, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Fecka, I. Comparison of polyphenol content and antioxidant capacity of strawberry fruit from 90 cultivars of Fragaria × ananassa Duch. Food Chem. 2019, 270, 32–46.
- Wichtl, M. Herbal drugs and phytopharmaceuticals. In A Handbook of Practice on a Scientific Basis; Brinckmann, J.A., Lindenmaier, M.P., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 220–221.
- Saric-Kundalic, B.; Dobes, C.; Klatte-Asselmeyer, V.; Saukel, J. Ethnobotanical study on medicinal use of wild and cultivated plants in middle, south and west Bosnia and Herzegovina. J. Ethnopharmacol. 2010, 131, 33–55.
- Zhu, F. Anthocyanins in cereals: Composition and health effects. Food Res. Int. 2018, 109, 232–249.
- Sinopoli, A.; Calogero, G.; Bartolotta, A. Computational aspects of anthocyanidins and anthocyanins: A review. Food Chem. 2019, 297, 124898.
- Braga, A.R.C.; Murador, D.C.; de Souza Mesquita, L.M.; Rosso, V.V. Bioavailability of anthocyanins: Gaps in knowledge, challenges and future research. J. Food Compos. Anal. 2018, 68, 31–40.
- Zugic, A.; Ðordevic, S.; Arsic, I.; Markovic, G.; Zivkovic, J.; Jovanovic, S.; Tadi, V. Antioxidant activity and phenolic compounds in 10 selected herbs from Vrujci Spa, Serbia. Ind. Crop Prod. 2014, 52, 519–527.
- Dias, M.I.; Barros, L.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic profile and antioxidant properties of commercial and wild Fragaria vesca L. roots: A comparison between hydromethanolic and aqueous extracts. Ind. Crop Prod. 2015, 63, 125–132.
- Dias, M.I.; Barros, L.; Fernandes, I.P.; Ruphuy, G.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Barreiro, M.F.; Ferreira, I.C.F.R. A bioactive formulation based on Fragaria vesca L. vegetative parts: Chemical characterization and application in κ-carrageenan gelatin. J. Funct. Food. 2015, 16, 243–255.
- Sato, T.; Ikeya, Y.; Adachi, S.I.; Yagasaki, K.; Nihei, K.I.; Itoh, N. Extraction of strawberry leaves with supercritical carbon dioxide and entrainers: Antioxidant capacity, total phenolic content, and inhibitory effect on uric acid production of the extract. Food Bioprod. Process 2019, 117, 160–169.
- Giampieri, F.; Alvarez-Suarez, J.M.; Battino, M. Strawberry and human health: Effects beyond antioxidant activity. J. Agricult. Food Chem. 2014, 62, 3867–3876.
- Vendrame, S.; Klimis-Zacas, D.J. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor-κB and mitogen-activated protein kinase signaling cascades. Nutr. Rev. 2015, 73, 348–358.
- Li, S.; Wu, B.; Fu, W.; Reddivari, L. The anti-inflammatory effects of dietary anthocyanins against ulcerative colitis. Int. J. Mol. Sci. 2019, 20, 2588.
- Szymanowska, U.; Złotek, U.; Karaś, M.; Baraniak, B. Anti-inflammatory and antioxidative activity of anthocyanins from purple basil leaves induced by selected abiotic elicitors. Food Chem. 2015, 172, 71–77.
- Peng, Y.; Yan, Y.; Wan, P.; Chen, D.; Ding, Y.; Ran, L.; Mi, J.; Lu, L.; Zhang, Z.; Li, X.; et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic. Biol. Med. 2019, 136, 96–108.
- Liberal, J.; Francisco, V.; Costa, G.; Figueirinha, A.; Amaral, M.T.; Marques, C.; Girão, H.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Bioactivity of Fragaria vesca leaves through inflammation, proteasome and autophagy modulation. J. Ethnopharmacol. 2014, 158, 113–122.
- Gomes, F.; Martins, N.; Barros, L.; Rodrigues, M.E.; Oliveira, M.B.P.P.; Henriques, M.; Ferreira, I.C.F.R. Plant phenolic extracts as an effective strategy to control Staphylococcus aureus, the dairy industry pathogen. Ind. Crop. Prod. 2018, 112, 515–520.
- Cardoso, O.; Donato, M.M.; Luxo, C.; Almeida, N.; Liberal, J.; Figueirinha, A.; Batista, M.T. Anti-Helicobacter pylori potential of Agrimonia eupatoria L. and Fragaria vesca. J. Funct. Food. 2018, 44, 299–303.
- Ninomiya, M.; Itoh, T.; Ishikawa, S.; Saiki, M.; Narumiya, K.; Yasuda, M.; Koshikawa, K.; Nozawa, Y.; Koketsu, M. Phenolic constituents isolated from Fragaria ananassa Duch. Inhibit antigen-stimulated degranulation through direct inhibition of spleen tyrosine kinase activation. Bioorg. Med. Chem. 2010, 18, 5932–5937.
- Abdulazeez, S.S. Effects of freeze-dried Fragaria x ananassa powder on alloxan-induced diabetic complications in Wistar rats. J. Taibah Univ. Med. Sci. 2014, 9, 268–273.
- Kanodia, L.; Borgohain, M.; Das, S. Effect of fruit extract of Fragaria vesca L. on experimentally induced inflammatory bowel disease in albino rats. Indian. J. Pharmacol. 2011, 43, 18–21.
- Gasparrini, M.; Giampieri, F.; Forbes-Hernandez, T.Y.; Afrin, S.; Cianciosi, D.; Reboredo-Rodriguez, P.; Varela-Lopez, A.; Zhang, J.; Quiles, J.L.; Mezzetti, B.; et al. Strawberry extracts efficiently counteract inflammatory stress induced by the endotoxin lipopolysaccharide in Human Dermal Fibroblast. Food Chem. Toxicol. 2018, 114, 128–140.
- Molinett, S.; Nuñez, F.; Moya-León, M.A.; Zúñiga-Hernández, J. Chilean strawberry consumption protects against LPS-induced liver injury by anti-inflammatory and antioxidant capability in Sprague-Dawley rats. Evid.-Based Compl. Alt. Med. 2015, 2015, 320136.
- Duarte, L.J.; Chaves, V.C.; dos Santos Nascimento, M.V.P.; Calvete, E.; Li, M.; Ciraolo, E.; Ghigo, A.; Hirsch, E.; Simões, C.M.O.; Reginatto, F.H.; et al. Molecular mechanism of action of Pelargonidin-3-O-glucoside, the main anthocyanin responsible for the anti-inflammatory effect of strawberry fruits. Food Chem. 2018, 247, 56–65.
- Van de Velde, F.; Esposito, D.; Grace, M.H.; Pirovani, M.E.; Lila, M.A. Anti-inflammatory and wound healing properties of polyphenolic extracts from strawberry and blackberry fruits. Food Res. Int. 2019, 121, 453–462.
- Somasagara, R.R.; Hegde, M.; Chiruvella, K.K.; Musini, A.; Choudhary, B.; Raghavan, S.C. Extracts of strawberry fruits induce intrinsic pathway of apoptosis in breast cancer cells and inhibits tumor progression in mice. PLoS ONE 2012, 7, 47021.
- Forni, C.; Braglia, R.; Mulinacci, N.; Urbani, A.; Ronci, M.; Gismondi, A.; Tabolacci, C.; Provenzano, B.; Lentini, A.; Beninati, S. Antineoplastic activity of strawberry (Fragaria x ananassa Duch.) crude extracts on B16-F10 melanoma cells. Mol. Biosyst. 2014, 10, 1255–1263.
- Shi, N.; Clinton, S.K.; Liu, Z.; Wang, Y.; Riedl, K.M.; Schwartz, S.J.; Zhang, X.; Pan, Z.; Chen, T. Strawberry phytochemicals inhibit azoxymethane/dextran sodium sulfate-induced colorectal carcinogenesis in Crj: CD-1 mice. Nutrients 2015, 7, 1696–1715.
- Lucioli, S.; Pastorino, F.; Nota, P.; Ballan, G.; Frattarelli, A.; Fabbri, A.; Forni, C.; Caboni, E. Extracts from cell suspension cultures of strawberry (Fragaria x ananassa Duch): Cytotoxic effects on human cancer cells. Molecules 2019, 24, 1738.




