
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | MICAELA GUIDOTTI TAKEUCHI | -- | 2227 | 2022-09-15 12:48:00 | | | |
| 2 | Lindsay Dong | Meta information modification | 2227 | 2022-09-19 02:56:41 | | | | |
| 3 | Lindsay Dong | -1 word(s) | 2226 | 2022-09-26 02:51:50 | | |
Video Upload Options
The use of essential oils (EO) loaded with nanoparticles is the most promising alternative to increase food quality and safety. Their association with different nanosystems allows novel developments in the micronutrition, health promotion, and pathogen control fields, preventing the aggravation of bacterial microevolution and combating antibiotic resistance. Benefits to the environment are also provided, as they are biodegradable and biocompatible.
1. Introduction
The EOs are plant-derived compounds with complex aromatic structures and high volatility. The volatilomes [1] are presented as the total fraction of strong-smelling molecules produced in specialized plant cells (oil cells, ducts, or glands) [2]. They are secondary plant metabolites, which play essential roles in the dynamics of plants with their habitat through protection against pathogens and attraction of polarizers [3]. They can be extracted from all parts of plants (from leaves to roots) by using different methodologies. The presence, yield, and chemotype of EOs are influenced by many climatic and plant nutritional factors, as well as stress conditions. For commercial EO production (where increases in the yield are desirable), breeding and selection programs are implemented as a measure to foster specific compositions [4]. The structural profiles of commercial EOs have been previously provided by gas chromatography and mass spectrometry analysis [5][6][7]. In general, their molecular structure reveals two or three major compounds, corresponding to more than 20% of the full molecule, usually responsible for their biological properties [8][9][10].
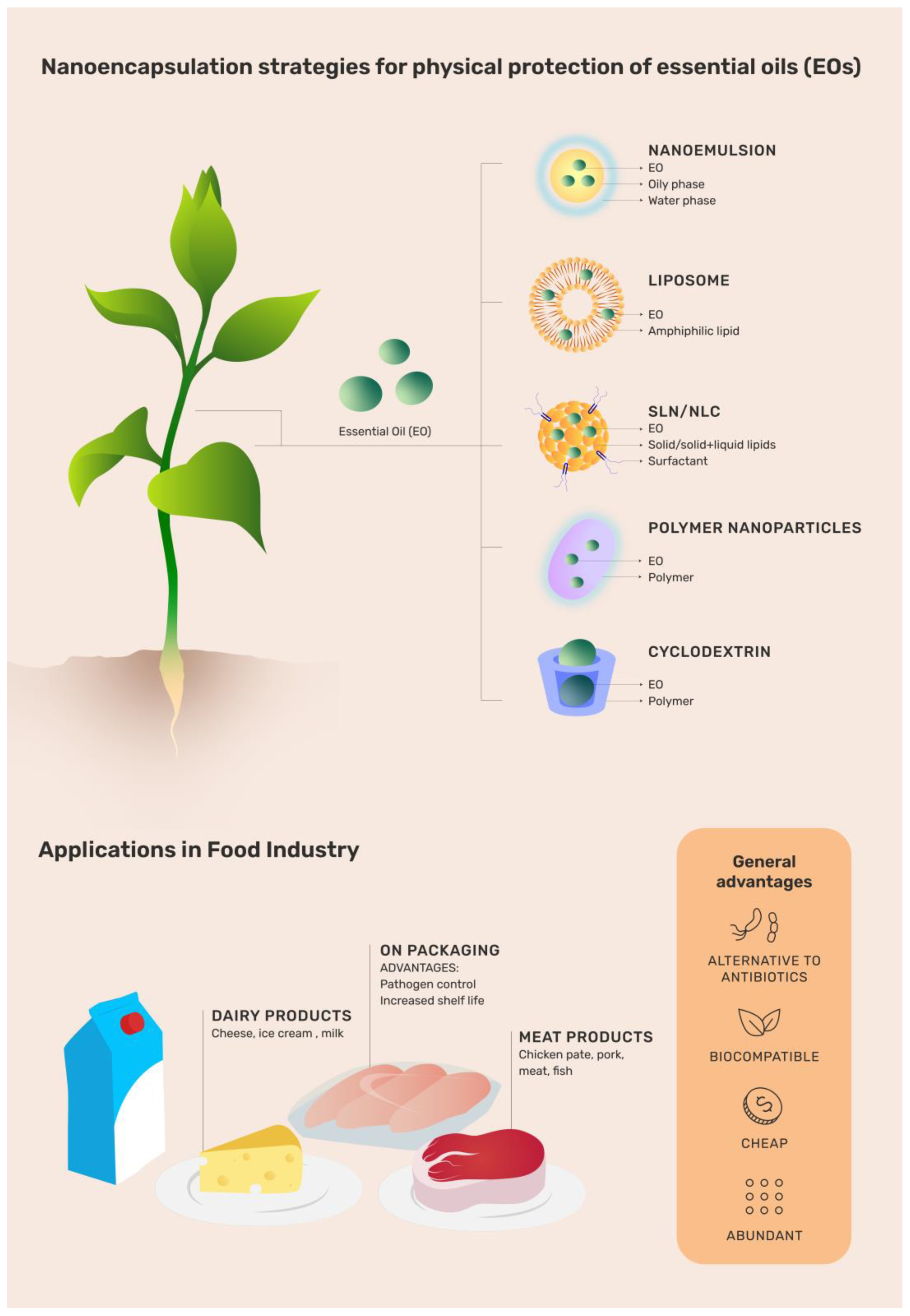
2. Production Chain in the Food Industry (FI)
3. Nanostructured Delivery Systems (NDSs)
3.1. Liposomes
3.2. Nanoemulsions (NEs)
3.3. Solid Lipid Nanoparticles (SLNs)
3.4. Nanostructured Lipid Carriers (NLCs)
3.5. Polymer Nanocapsules (NCs)
3.6. Cyclodextrins (CDs)
3.7. Chitosan-Based Delivery Systems (CHT)
4. Nanopackaging and Smart Control of Food Pathogens
References
- Filipiak, W.; Mochalski, P.; Filipiak, A.; Ager, C.; Cumeras, R.; Davis, C.E.; Agapiou, A.; Unterkofler, K.; Troppmair, J. A Compendium of Volatile Organic Compounds (VOCs) Released by Human Cell Lines. Curr. Med. Chem. 2016, 23, 2112–2131.
- Capelezzo, A.P.; Mohr, L.C.; Dalcanton, F.; de Mello, J.M.M.; Fiori, M.A. β-Cyclodextrins as Encapsulating Agents of Essential Oils. In Cyclodextrin. A Versatile Ingredient; Poonam, A., Neelima, D., Eds.; IntechOpen: London, UK, 2018.
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32.
- Thormar, H. Lipids and Essential Oils as Antimicrobial Agents; Thormar, H., Ed.; John and Wiley and Sons: Hoboken, NJ, USA, 2011; ISBN 9780470741788.
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Consoli, G.M.L.; Cafiso, V.; Stefani, S.; Geraci, C. Essential oils encapsulated in polymer-based nanocapsules as potential candidates for application in food preservation. Food Chem. 2018, 269, 286–292.
- Leitão, S.G.; De Oliveira, D.R.; Sülsen, V.; Martino, V.; Barbosa, Y.G.; Bizzo, H.R.; Lopes, D.; Viccini, L.F.; Salimena, F.R.G.; Peixoto, P.H.P.; et al. Analysis of the chemical composition of the essential oils extracted from Lippia lacunosa Mart. & Schauer and Lippia rotundifolia Cham. (Verbenaceae) by gas chromatography and gas chromatography-mass spectrometry. J. Braz. Chem. Soc. 2008, 19, 1388–1393.
- Yan, D.; Wong, Y.F.; Tedone, L.; Shellie, R.; Marriott, P.J.; Whittock, S.; Koutoulis, A. Chemotyping of new hop (Humulus lupulus L.) genotypes using comprehensive two-dimensional gas chromatography with quadrupole accurate mass time-of-flight mass spectrometry. J. Chromatogr. A 2018, 1536, 110–121.
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential Oils Loaded in Nanosystems: A Developing Strategy for a Successful Therapeutic Approach. Evid. Based Complement. Altern. Med. 2014, 2014, 651593.
- Garcia, A.; Barbas, C. Gas Chromatography-Mass Spectrometry (GC-MS)-Based Metabolomics. In Metabolic Profiling; Humana Press: Totowa, NJ, USA, 2010; pp. 191–204.
- Satyal, P.; Jones, T.H.; Lopez, E.M.; McFeeters, R.L.; Ali, N.A.A.; Mansi, I.; Al-Kaf, A.G.; Setzer, W.N. Chemotypic Characterization and Biological Activity of Rosmarinus officinalis. Foods 2017, 6, 20.
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.; Arabi, S.A.; Liu, D.; Guo, M. Emerging chitosan-essential oil films and coatings for food preservation—A review of advances and applications. Carbohydr. Polym. 2021, 273, 118616.
- Chivandi, E.; Dangarembizi, R.; Nyakudya, T.T.; Erlwanger, K.H. Use of Essential Oils as a Preservative of Meat. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2016; pp. 85–91.
- Joye, I.J.; Davidov-Pardo, G.; McClements, D.J. Nanotechnology in Food Processing. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 49–55.
- Kumar, A.; Singh, P.; Gupta, V.; Prakash, B. Application of nanotechnology to boost the functional and preservative properties of essential oils. In Functional and Preservative Properties of Phytochemicals; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 241–267. ISBN 9780128185933.
- Prakash, B.; Kujur, A.; Yadav, A.; Kumar, A.; Singh, P.P.; Dubey, N.K. Nanoencapsulation: An efficient technology to boost the antimicrobial potential of plant essential oils in food system. Food Control 2018, 89, 1–11.
- Ribeiro, L.N.D.M.; de Paula, E.; Rossi, D.A.; Martins, F.A.; de Melo, R.T.; Monteiro, G.P.; Breitkreitz, M.C.; Goulart, L.R.; Fonseca, B.B. Nanocarriers from Natural Lipids With In Vitro Activity Against Campylobacter jejuni. Front. Cell. Infect. Microbiol. 2021, 10, 571040.
- FDA. Guidance for Industry Use of Nanomaterials in Food for Animals; FDA: Silver Spring, MD, USA, 2015; p. 10.
- Melo, R.T.; Galvão, N.N.; Guidotti-Takeuchi, M.; Peres, P.A.B.M.; Fonseca, B.B.; Profeta, R.; Azevedo, V.A.C.; Monteiro, G.P.; Brenig, B.; Rossi, D.A. Molecular Characterization and Survive Abilities of Salmonella Heidelberg Strains of Poultry Origin in Brazil. Front. Microbiol. 2021, 12, 674147.
- Pereira, A.G.; Carpena, M.; Oliveira, P.G.; Mejuto, J.; Prieto, M.; Gandara, J.S. Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host-Guest Complexes. Int. J. Mol. Sci. 2021, 22, 1339.
- Wu, Z. Antimicrobial use in food animal production: Situation analysis and contributing factors. Front. Agric. Sci. Eng. 2018, 5, 301.
- Quinto, E.J.; Caro, I.; Villalobos-Delgado, L.H.; Mateo, J.; De-Mateo-Silleras, B.; Redondo-Del-Río, M.P. Food Safety through Natural Antimicrobials. Antibiotics 2019, 8, 208.
- Iriti, M.; Vitalini, S.; Varoni, E.M. Humans, Animals, Food and Environment: One Health Approach against Global Antimicrobial Resistance. Antibiotics 2020, 9, 346.
- Kon, K.V.; Rai, M.K. Plant essential oils and their constituents in coping with multidrug-resistant bacteria. Expert Rev. Anti-Infect. Ther. 2012, 10, 775–790.
- Ruddaraju, L.K.; Pammi, S.V.N.; Guntuku, G.S.; Padavala, V.S.; Kolapalli, V.R.M. A review on anti-bacterials to combat resistance: From ancient era of plants and metals to present and future perspectives of green nano technological combinations. Asian J. Pharm. Sci. 2019, 15, 42–59.
- Pérez-Rodríguez, F.; Taban, B.M. A State-of-Art Review on Multi-Drug Resistant Pathogens in Foods of Animal Origin: Risk Factors and Mitigation Strategies. Front. Microbiol. 2019, 10, 2091.
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58.
- Ghorbanzade, T.; Jafari, S.M.; Akhavan, S.; Hadavi, R. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chem. 2017, 216, 146–152.
- Ilyasoglu, H.; El, S.N. Nanoencapsulation of EPA/DHA with sodium caseinate–gum arabic complex and its usage in the enrichment of fruit juice. LWT 2013, 56, 461–468.
- McClements, D.J.; Öztürk, B. Utilization of Nanotechnology to Improve the Handling, Storage and Biocompatibility of Bioactive Lipids in Food Applications. Foods 2021, 10, 365.
- Dasgupta, N.; Ranjan, S. An Introduction to Food Grade Nanoemulsions; Springer: Singapore, 2018; Volume 1, ISBN 978-981-10-6985-7.
- Ranjan, S.; Dasgupta, N.; Chakraborty, A.R.; Samuel, S.M.; Ramalingam, C.; Shanker, R.; Kumar, A. Nanoscience and nanotechnologies in food industries: Opportunities and research trends. J. Nanoparticle Res. 2014, 16, 2464.
- Silva, H.D.; Cerqueira, M.; Vicente, A.A. Nanoemulsions for Food Applications: Development and Characterization. Food Bioprocess Technol. 2012, 5, 854–867.
- Kirby, C.J. Nanotechnology in the Food Sector. In Food Processing Handbook, 2nd ed.; Brennan, G.J., Grandison, A.S., Eds.; John and Wiley and Sons: Hoboken, NJ, USA, 2009; Volume 1, pp. 693–726.
- Gupta, S.V.S. Nanotechnology and Food Science: Tomorrow Design the Food. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3553–3561.
- Beyki, M.; Zhaveh, S.; Khalili, S.T.; Rahmani-Cherati, T.; Abollahi, A.; Bayat, M.; Tabatabaei, M.; Mohsenifar, A. Encapsulation of Mentha piperita essential oils in chitosan-cinnamic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Ind. Crop. Prod. 2014, 54, 310–319.
- Ferreira, C.D.; Nunes, I.L. Oil nanoencapsulation: Development, application, and incorporation into the food market. Nanoscale Res. Lett. 2019, 14, 9.
- Ribeiro, L.N.M.; Alcântara, A.C.S.; Da Silva, G.H.R.; Franz-Montan, M.; Nista, S.V.G.; Castro, S.R.; Couto, V.M.; Guilherme, V.A.; De Paula, E. Advances in Hybrid Polymer-Based Materials for Sustained Drug Release. Int. J. Polym. Sci. 2017, 2017, 1231464.
- Forensic Science. In Handbook of Analytical Separations; Elsevier: Amsterdam, The Netherlands, 2008.
- de Paula, E.; Oliveira, J.D.; de Lima, F.F.; de Morais Ribeiro, L.N. Liposome-Based Delivery of Therapeutic Agents. In Controlled Drug Delivery Systems; CRC Press: Boca Raton, FL, USA, 2020; pp. 299–324.
- Taylor, T.M.; Weiss, J.; Davidson, P.M.; Bruce, B.D. Liposomal Nanocapsules in Food Science and Agriculture. Crit. Rev. Food Sci. Nutr. 2005, 45, 587–605.
- Hammoud, Z.; Gharib, R.; Fourmentin, S.; Elaissari, A.; Greige-Gerges, H. New findings on the incorporation of essential oil components into liposomes composed of lipoid S100 and cholesterol. Int. J. Pharm. 2019, 561, 161–170.
- Sebaaly, C.; Trifan, A.; Sieniawska, E.; Greige-Gerges, H. Chitosan-Coating Effect on the Characteristics of Liposomes: A Focus on Bioactive Compounds and Essential Oils: A Review. Processes 2021, 9, 445.
- Ingle, A.P.; Shende, S.; Gupta, I.; Rai, M. Recent trends in the development of nano-bioactive compounds and delivery systems. In Biotechnological Production of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 409–431.
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49.
- Landry, K.S.; Micheli, S.; McClements, D.J.; McLandsborough, L. Effectiveness of a spontaneous carvacrol nanoemulsion against Salmonella enterica Enteritidis and Escherichia coli O157:H7 on contaminated broccoli and radish seeds. Food Microbiol. 2015, 51, 10–17.
- Müller, R.H.; Alexiev, U.; Sinambela, P.; Keck, C.M. Nanostructured Lipid Carriers (NLC): The Second Generation of Solid Lipid Nanoparticles. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 161–185. ISBN 978-3-662-45012-3.
- Trevaskis, N.L.; Charman, W.N.; Porter, C.J.H. Lipid-based delivery systems and intestinal lymphatic drug transport: A mechanistic update. Adv. Drug Deliv. Rev. 2008, 60, 702–716.
- Pink, D.L.; Loruthai, O.; Ziolek, R.M.; Wasutrasawat, P.; Terry, A.E.; Lawrence, M.J.; Lorenz, C.D. On the Structure of Solid Lipid Nanoparticles. Small 2019, 15, e1903156.
- Azar, F.A.N.; Pezeshki, A.; Ghanbarzadeh, B.; Hamishehkar, H.; Mohammadi, M. Nanostructured lipid carriers: Promising delivery systems for encapsulation of food ingredients. J. Agric. Food Res. 2020, 2, 100084.
- Katouzian, I.; Esfanjani, A.F.; Jafari, S.M.; Akhavan, S. Formulation and application of a new generation of lipid nano-carriers for the food bioactive ingredients. Trends Food Sci. Technol. 2017, 68, 14–25.
- Shegokar, R.; Athawale, R.; Kurup, N.; Yang, R.; Chougule, M.B. Lipid-Based Nanoparticles for Targeted Drug Delivery of Anticancer Drug. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Academic Press: Cambridge, MA, USA, 2017; pp. 287–321.
- Ribeiro, L.N.; Alcantara, A.C.; Franz-Montan, M.; Couto, V.M.; Nista, S.V.; de Paula, E. Nanostructured organic-organic bio-hybrid delivery systems. In Biomedical Application of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 341–374.
- Khezri, K.; Farahpour, M.R.; Rad, S.M. Efficacy of Mentha pulegium essential oil encapsulated into nanostructured lipid carriers as an in vitro antibacterial and infected wound healing agent. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124414.
- Esmaeili, A.; Gholami, M. Optimization and preparation of nanocapsules for food applications using two methodologies. Food Chem. 2015, 179, 26–34.
- Detoni, C.B.; Cabral-Albuquerque, E.C.M.; Hohlemweger, S.V.A.; Sampaio, C.; Barros, T.F.; Velozo, E.S. Essential oil from Zanthoxylum tingoassuiba loaded into multilamellar liposomes useful as antimicrobial agents. J. Microencapsul. 2009, 26, 1–8.
- Chiriac, A.; Rusu, A.; Nita, L.; Chiriac, V.; Neamtu, I.; Sandu, A. Polymeric Carriers Designed for Encapsulation of Essential Oils with Biological Activity. Pharmaceutics 2021, 13, 631.
- Petitjean, M.; García-Zubiri, I.X.; Isasi, J.R. History of cyclodextrin-based polymers in food and pharmacy: A review. Environ. Chem. Lett. 2021, 19, 3465–3476.
- Yuan, G.; Chen, X.; Li, D. Chitosan films and coatings containing essential oils: The antioxidant and antimicrobial activity, and application in food systems. Food Res. Int. 2016, 89, 117–128.
- Mujtaba, M.; Khawar, K.M.; Camara, M.C.; Carvalho, L.B.; Fraceto, L.F.; Morsi, R.E.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Ullah, H.; et al. Chitosan-based delivery systems for plants: A brief overview of recent advances and future directions. Int. J. Biol. Macromol. 2020, 154, 683–697.
- Bonetti, F.M.R.; de Paula, E.; Fonseca, B.B.; da Silva, G.R.; da Silva, L.S.S.; de Moura, L.D.; Breitkreitz, M.C.; da Silva, G.H.R.; Ribeiro, L.N.D.M. Hybrid Nanobeads for Oral Indomethacin Delivery. Pharmaceutics 2022, 14, 583.
- Möller, H.; Grelier, S.; Pardon, A.P.; Coma, V. Antimicrobial and Physicochemical Properties of Chitosan−HPMC-Based Films. J. Agric. Food Chem. 2004, 52, 6585–6591.
- Zarzycki, P.K.; ena Fenert, B.; Głód, B.K. 17—Cyclodextrins-based nanocomplexes for encapsulation of bioactive compounds in food, cosmetics, and pharmaceutical products: Principles of supramolecular complexes formation, their influence on the antioxidative properties of target chemicals, and rec. In Encapsulations; Grumezescu, A.M., Ed.; Nanotechnology in the Agri-Food Industry; Academic Press: Cambridge, MA, USA, 2016; pp. 717–767. ISBN 978-0-12-804307-3.
- Vergis, J.; Gokulakrishnan, P.; Agarwal, R.K.; Kumar, A. Essential Oils as Natural Food Antimicrobial Agents: A Review. Crit. Rev. Food Sci. Nutr. 2013, 55, 1320–1323.
- Quintavalla, S.; Vicini, L. Antimicrobial food packaging in meat industry. Meat Sci. 2002, 62, 373–380.
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K.; Rather, I.A. Application of Nanotechnology in Food Science: Perception and Overview. Front. Microbiol. 2017, 8, 1501.
- Anvar, A.A.; Ahari, H.; Ataee, M. Antimicrobial Properties of Food Nanopackaging: A New Focus on Foodborne Pathogens. Front. Microbiol. 2021, 12, 690706.




