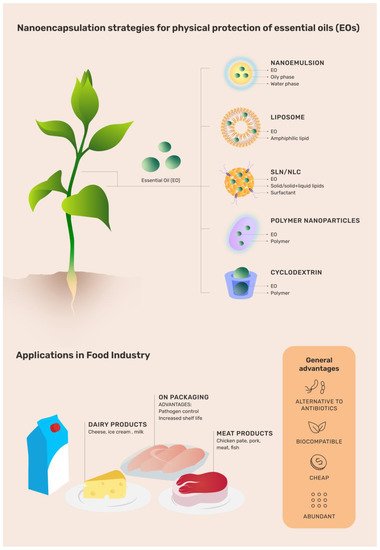
Illustrative chart regarding the use and advantages of different organic nanoparticles loading essential oils (EOs) throughout the food production process. EO: essential oil; SLN: solid lipid nanoparticle; NLC: nanostructured lipid carriers.
Food-grade nanoparticulate products are already available on the market and include nano-additives and nano-ingredients made from ω-3 fatty acids, incorporated into yogurts
[27][42] and fruit juices
[28][43]. They have the function of not only enriching the product, but also promoting the sensory acceptability of fish oil
[29][44]. Other products developed by Aquanova
® include nanoemulsions loading vitamins. Other nanoencapsulated vitamins (Lypo-Spheric Vitamin, Livon Laboratories, Inc. Company Profile, Henderson, NV, USA) manufactured by LivOn Labs offer protection against their degradation in the gastrointestinal system, increasing the bioavailability
[30][45]. There are also some reports related to the NP application for sensory enhancements, to inhibit flavor loss (infused pure cocoa nanoclusters, RBC Life Sciences
® Inc, Irving, TX, USA)
[31][46], incorporate natural colorants (β-carotene, curcumin; NutraLease
®, Ma’ale Adumim, Israel)
[32][47], and offer nanotechnological advantages, such as Nestlé
® patented water-in-oil emulsion (10–500 nm), to facilitate microwave thawing of frozen products
[33][48]. The possibilities of applications are wide, including expressive fat reduction (from 16% to 1%) in ice cream production (Unilever
®, London, UK)
[34][49] and incorporation of antimicrobial compounds
[34][49] in packaging, and in decontamination of industrial equipment
[32][47], demonstrating the diversity of application in food matrices and a dynamic approach to food and feed quality assurance
[18][33].
3. Nanostructured Delivery Systems (NDSs)
The nanostructured delivery systems (NDSs) are molecularly composed of different biomaterials and are processed as several forms in order to interact specifically with the targets.
There are a lot of benefits of the EOs nanoencapsulation approach, such as bioactive protection to the external environment (e.g., products rich in unsaturated lipids can be peroxided over time); sustained release; desirable shelf time; to mask eventual bioactive unpleasant odor and/or flavor; and act as smart packaging with moisture maintenance, protection against pathogens, and monitoring of conditions during distribution
[35][36][37][72,81,106]. Different NPs loading EOs have been proposed for pathogen control or incorporated as adjuvants in food-based products
[26][41]. Furthermore, nanoencapsulation presents itself as a method to protect against formation of degraded compounds with toxic derivatives, such as the conversion of safrole (4-allyl-1,2-methylene dioxybenzene), derived from plants of the Lauraceae family (including nutmeg and black pepper) with hepatocarcinogenic effects
[36][38][81,107].
3.1. Liposomes
Liposomes are spherical vesicles composed of phospholipids spontaneously oriented as lipid bilayers (unilamellar or multilamellar) in aqueous solutions, given by the hydrophobic interactions of nonpolar acyl chains
[39][108]. The amphiphilic nature of lipids is currently exploited to encapsulate food ingredients of different polarities, creating an efficient physical barrier system and protecting bioactives from environmental conditions
[40][109]. The lipid bilayers allow interaction with the hydrophobic encapsulated compounds, being intrinsically related to NDS stability (fluidity, permeability, and polarity, among others), as well as encapsulation efficiency and sustained release profile
[41][110].
In general, the analyzed reports showed that the EO-loaded liposomes provided optimized antimicrobial activity. Sebaaly et al. (2021) developed multilamellar liposomes composed of dipalmitoyl phosphatidylcholine (DPPC) encapsulating EO from
Z. tingoassuiba, with a mean diameter of 9.37 ± 4.69 µm
[42][111]. The in vitro antimicrobial activity was evaluated by the disc diffusion test. Antimicrobial activity against
Staphylococcus aureus,
Micrococcus luteus, and
Streptococcus mutans, important pathogens related to the food infections, was observed
[41][110].
3.2. Nanoemulsions (NEs)
Nanoemulsions (NEs) are stable systems composed of two immiscible phases
[43][112], being processed as water-in-oil or oil-in-water, stabilized by amphiphilic surfactant and co-surfactant. They exhibit excellent structural properties, with particle size between 50–250 nm and a monodisperse distribution
[44][113]. Food-grade NEs containing bioactive components can be used to develop biodegradable packaging films. Promising results were provided of NEs that prevented foodborne diseases. In this sense, carvacrol-based NE was tested in minimal processes as an alternative to calcium hypochlorite use against
Escherichia coli O157:H7 and
Salmonella Typhimurium in contaminated broccoli and radish seeds. A system containing 0.4% carvacrol was able to inactivate contamination in low levels (about 2–3 log CFU/g) in radish seeds. The treatment was not effective for broccoli seeds
[45][52].
3.3. Solid Lipid Nanoparticles (SLNs)
SLNs emerged in 1991, firstly described by Professors Müller and Gasco, as an alternative to traditional colloidal systems such as liposomes
[46][47][115,116]. This system is based on a lipid matrix composed of solid lipid stabilized by a surfactant
[15][47][30,116]. SLNs have high affinity to load hydrophobic active into the solid matrix
[48][117]. SLNs allow to improve the stability of loaded EOs under various adverse conditions, including food processing, heating, high pressure, drying, UV radiation, and bile salt
[49][118]. Therefore, such a system was designed for applications in food distribution because it has interesting advantages to be used in this sector, such as the effective uptake of fat-soluble assets, sustained release profile, and long-term stability
[50][51][119,120].
3.4. Nanostructured Lipid Carriers (NLCs)
NLCs are the second generation of lipid nanoparticles, composed of a lipid matrix formed by a blend between solid and liquid lipids in room temperature, stabilized by a surfactant
[46][115]. They are also considered to be safe and promising nanocarriers for the delivery of hydrophobic compounds, despite their hydrophilicity (colloid liquid systems), which is paramount for the establishment of water-based foods. Therefore, NLCs have industrial relevance as these nanocarriers also combine specific advantages for food uses, such as the ability to be successfully incorporated into transparent and opaque foods and beverages
[46][115]. The encapsulation of EOs by NLCs has been proposed to exhibit higher stability, minimized toxicity, and preserve the therapeutic properties of pristine EOs
[52][124]. EO-loaded NLC formulations have been widely described to increase the antimicrobial activity of EOs and protect them against environmental damage conditions (light, hydrolysis, and evaporation, among others)
[16][53][31,125].
3.5. Polymer Nanocapsules (NCs)
NCs are NDSs formed by a shell (polymer wall) and core (oil core) counterparts that have interesting physicochemical properties for application in food matrices, such as those derived from aliphatic polyester (ε-caprolactone), which are stable in storage and allow a sustained release of loaded actives. Such core–shell structure brings benefits that include the difficulty of contact with external substances and is better suited to lipophilic foods
[54][128].
The encapsulation of EOs by polymer nanocapsules (NC) aims to improve hydrophilicity of insoluble compounds, protection against degradation, prevention of loss of volatile compounds caused by evaporation, and a sustained release. Their antimicrobial activity of NC loading Thymus capitatus EO with high amount of bioactives (43% thymol) was reported
[5][8]. The association of polymers and thymol EO has immediate applicability against important pathogens in the FI (
S. aureus,
L. monocytogenes,
B. cereus,
S. typhi,
S. dysenteriae, and
E. coli).
3.6. Cyclodextrins (CDs)
Cyclodextrins (CDs) are cyclic oligosaccharides that have glucose units linked by α-(1,4)-glycosidic bonds, derived from the enzymatic degradation of starch
[41][110], mainly by
B. macerans [19][34]. They present a cone-shaped morphology with a hydrophobic cavity and 7.9 Å diameter, and are able to form inclusion complexes with low-aqueous-solubility molecules
[55][50].
The inclusion complexes formed by CDs and EOs improve the EO solubility and physicochemical stability, providing a sustained release of these compounds
[56][131], also acting as physical barrier to protect the EO in procedures such as freezing, thawing, and/or microwaving during food processing
[19][34]. In the FI, they are used to improve flavor and sorption of bitter compounds. Recently, CDs have been noticed as constituents of active packaging of edible products
[57][132].
3.7. Chitosan-Based Delivery Systems (CHT)
Chitosan (CHT) is a linear cationic polysaccharide formed by (1,4)-amino-deoxy-b-d-glucan chains derived from a deacetylated chitin of crabs and crustaceans, and is biocompatible, biodegradable, and cheap
[58][64]. The use of CHT as a drug carrier has additional advantages as an NDS, such as the protection of the selected active, changes in temperature, and sustained release at the site of interest
[59][136], given by the electrostatic interactions with anionic biological membranes. As a biopolymer, it can be processed as several forms and is abundant, cheap, and biodegradable
[60][137].
CHT application in association with EOs has demonstrated higher antimicrobial activity against foodborne pathogens than biopolymer films and coatings
[58][64]. Specifically, it has demonstrated its advantageous use as an NDS in food preservatives, as it allows a sustained release of active ingredients in the production of food films and coatings
[58][64]. Its inherent antimicrobial activity has been attributed to the electrostatic forces between the cationic amino groups (NH
3) of CHT and the negatively charged residues of the fatty acids located on the bacterial
[61][138].
4. Nanopackaging and Smart Control of Food Pathogens
EOs are widely explored for use in food packaging due to their potential antimicrobial activity
[62][98]. Antimicrobial packaging is also able to maintain both the nutritional and sensory quality of food, extending its shelf life. Such approach seems to be an effective alternative to the addition of synthetic preservatives in the packages, as it will be in contact with the food products. Therefore, EOs are currently incorporated into the packages, aiming to decrease the maximum population growth rate and/or prolong the lag phase of the target microorganism by contact inactivation. When used in adequate concentrations, in addition to inhibiting foodborne pathogenic bacteria (
Listeria,
Salmonella,
Aeromonas,
Clostridium botulinum,
Enterobacter, and
Staphylococci) and their toxins, EOs can also improve the sensory properties of the food products
[63][144]. However, the EOs incorporation in active and smart packaging needs to meet crucial safety requirements. For instance, the slow denaturation of EOs from the packaging material to the food surface allows maintaining a high concentration of EOs over a long period of time when compared to the direct addition of EOs in food, the result of which differs by the rapid diffusion of the bioactive with a consequent reduction in antimicrobial activity
[64][145].
The application of nanotechnology in packaging development presents interesting opportunities to exploit the antimicrobial properties of EOs
[65][146]. This strategy has been widely explored, mainly because they act as a physical barrier leading to decreased spoilage and improved inhibition of pathogenic microorganism growth
[66][147]. Nanofilms composed of a thin layer of polymers, created using layer-by-layer deposition techniques, have potential applications in the field of active food packaging. They have been applied directly on the product, whereas non-edible coatings have a protective function specifically on the packaging. Their applicability also extends to surfaces that are in contact with food during processing or to prevent the formation of biofilms
[13][28].