miR-944 is located in the fourth intron of tumor protein p63 (TP63) in the chromosome 3q28 region
[5] and produced at the 3’ end of the stem-loop structure of pre-mir-944. miR-944 is aberrantly expressed in more than 10 cancers. Targeted inhibition of mRNA by miRNA can be hindered by competing endogenous RNAs (ceRNAs), such as circular RNAs (circRNAs) and long non-coding RNAs (lncRNAs)
[6]. miR-944 is regulated by eleven ceRNAs, including six circRNAs and five lncRNAs. miR-944 can target and suppress 27 protein-coding genes, thereby regulating cancer cell behaviors such as cancer cell cycle, growth, proliferation, epithelial-mesenchymal transition (EMT), cancer cell invasion, and metastasis. The genes targeted by miR-944 are involved in three signaling pathways, including the Wnt/β-catenin pathway, the Jak/STAT3 pathway, and the PI3K/AKT pathway.
In patients with nasopharyngeal carcinoma (NPC)
[7], colorectal cancer (CRC)
[8][9], or breast cancer (BrC)
[10], low expression of miR-944 was not only associated with poorer overall survival (OS) but also with more advanced tumor infiltration, more lymph node metastasis, and more advanced tumor node metastasis (TNM) stage
[9]. miR-944 is associated with resistance to four anticancer drugs, including cisplatin (DDP)
[11], rapamycin (RAPA)
[12], doxorubicin (DOX)
[13], and paclitaxel (PTX)
[14]. miR-944 is involved in the molecular mechanism of action of two anticancer drugs, including the quinazolidine alkaloid matrine
[15] and a drug under clinical trials, 188Re-liposome
[16].
2. miR-944 and Its Host Gene TP63
The p63 transcription factor encoded by TP63, the host gene of miR-944, is a tumor suppressor gene belonging to the p53 family
[17]. N-terminal truncated isoform of p63 (ΔNp63), which is transcribed from an alternative promoter in intron 3 of TP63, can regulate the epithelial properties of cells and play an important role in the terminal differentiation and stemness maintenance of basal epidermal cells
[18]. miR-944 was significantly positively correlated with ΔNp63 expression in cervical cancer (CxCa) and jointly exerted a cancer-promoting effect
[19]. During the differentiation of human epidermal keratinocytes, ΔNp63 can recruit the transcription factor AP-2 to the promoter region of the miR-944 gene, thereby promoting the expression of miR-944 and inducing epidermal differentiation
[17].
3. Aberrant Expression of miR-944 in Cancer
As shown in
Table 1, miR-944 is downregulated in cells and tissues of 11 cancers. Meanwhile, miR-944 is lowly expressed in the plasma of esophageal cancer (ECa)
[20]. It is worth noting that miR-944 is highly expressed in cancer tissues of LUSC
[21] and endometrial carcinoma (EC)
[22], as well as CxCa cell lines, serum, and tissues
[5][23][24][25]. Furthermore, the expression of miR-944 in BrC is controversial. Specifically, miR-944 was highly expressed in serum and tissues of BrC
[26], whereas miR-944 was found to be downregulated in five BrC cell lines and tissues
[27].
Table 1. Aberrant expression of miR-944 in different cancers.
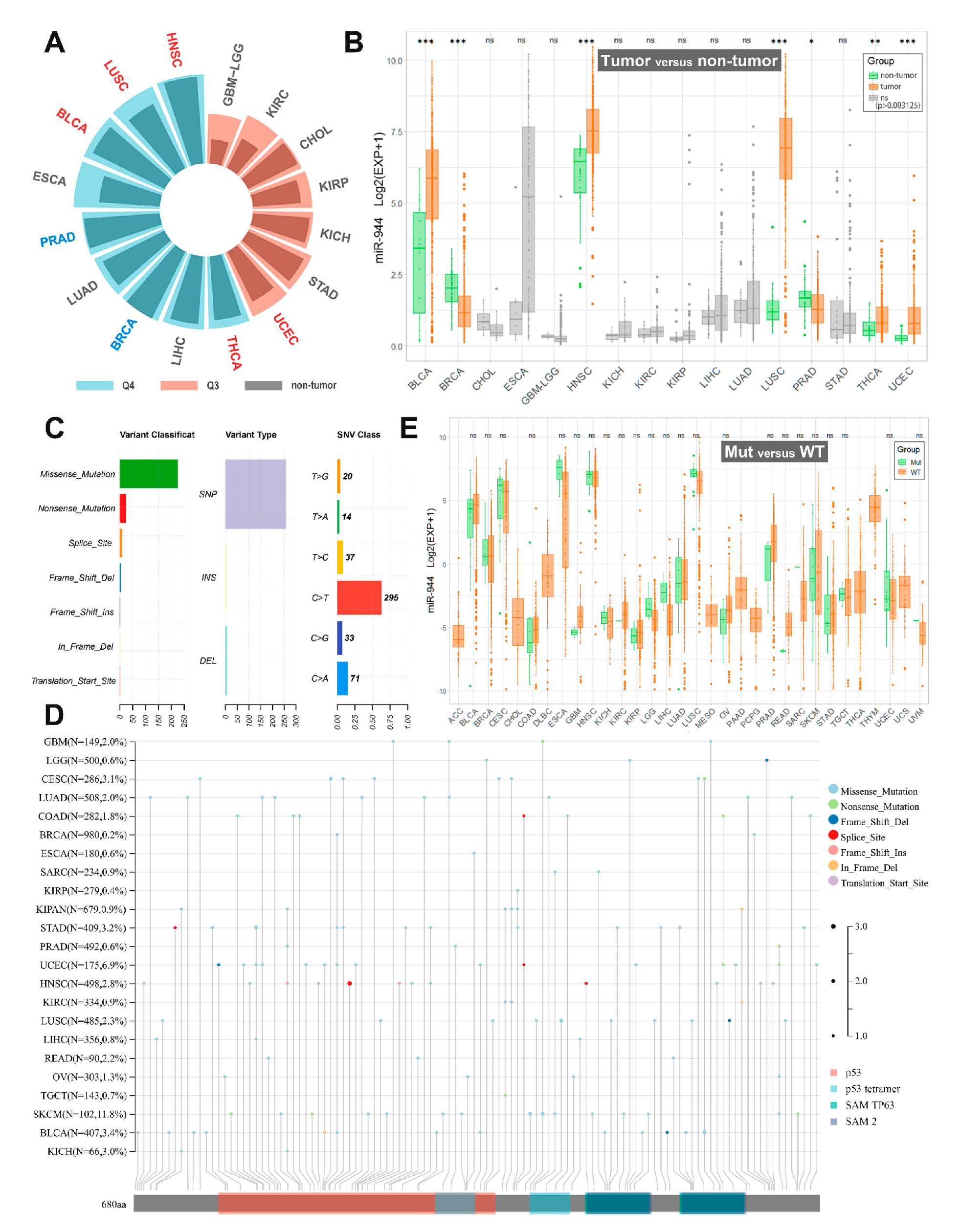
Researchers downloaded the TCGA (pan-cancer) dataset from the UCSC Xena database (
https://xenabrowser.net (accessed on 20 June 2022)). Researchers extracted miR-944 expression data (RPM) in 33 cancer samples and performed log2(RPM+1) transformation. Researchers excluded cancer types with <3 control samples and finally retrieved miR-944 expression data in 16 TCGA cancer types. Researchers calculated the quantile percentage of miR-944 expression among all non-zero-expressed miRNAs in each of these 16 cancer types. As shown in
Figure 1A, miR-944 was highly expressed in nine tumors (0.75–1.0 quantile, Q4), while miR-944 was moderately expressed in other seven tumors (0.5–0.75 quantile, Q3).
Figure 1. Pan-cancer analysis of miR-944 based on TCGA database. (A) Histogram of median quantile expression of miR-944 in non-tumor and tumor groups in the TCGA database. The blue font indicates that miR-944 is significantly low expressed in this cancer type; the red font indicates that miR-944 is significantly highly expressed in this cancer type; (B) comparison of miR-944 expression levels between non-tumor and tumor groups in the TCGA database. *** means p < 0.0000625; ** means p < 0.000625; * means p < 0.003125; ns means no significant difference; (C) overview of SNVs of TP63; (D) mutation types in TP63 protein domains in various cancers; (E) differences in the expression level of miR-944 between the TP63 mutant group (Mut) and the wild group (WT). Ns means no significant difference. ACC, adrenocortical carcinoma; BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL, cholangiocarcinoma; COAD, colon adenocarcinoma; DLBC, lymphoid neoplasm diffuse large B-cell lymphoma; ESCA, esophageal carcinoma; GBM, glioblastoma; HNSC, head and neck squamous cell carcinoma; KICH, kidney chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LGG, brain lower grade glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; MESO, mesothelioma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC, sarcoma; STAD, stomach adenocarcinoma; SKCM, skin cutaneous melanoma; TGCT, testicular germ cell tumor; THCA, thyroid carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial carcinoma; UCS, uterine carcinosarcoma; UVM, uveal melanoma.
Researchers compared differences in miR-944 expression between non-tumor and tumor samples of 16 cancer types (unpaired Wilcoxon test, R version 4.1.3). As shown in Table 2 and Figure 1B, researchers found that miR-944 was significantly upregulated in five tumors (BLCA, HNSC, LUSC, THCA, and UCEC); significantly downregulated in two tumors (BRCA and PRAD) (Figure 1B). Notably, the TCGA analysis demonstrated the association of miR-944 expression with cancer risk in bladder cancer, head and neck squamous cell carcinoma (HNSCC), thyroid cancer, and prostate cancer, which has not been reported yet.
Table 2. Comparison of miR-944 in TCGA dataset with existing data.
Researchers also calculated differences in the expression of miR-944 between patients of different genders or races based on the TCGA database. The results can be found in supplementary materials of original text. There was no significant difference in the expression level of miR-944 between males and females in cancer. In BLCA and ESCA, the level of miR-944 in whites was significantly lower than that in other races.
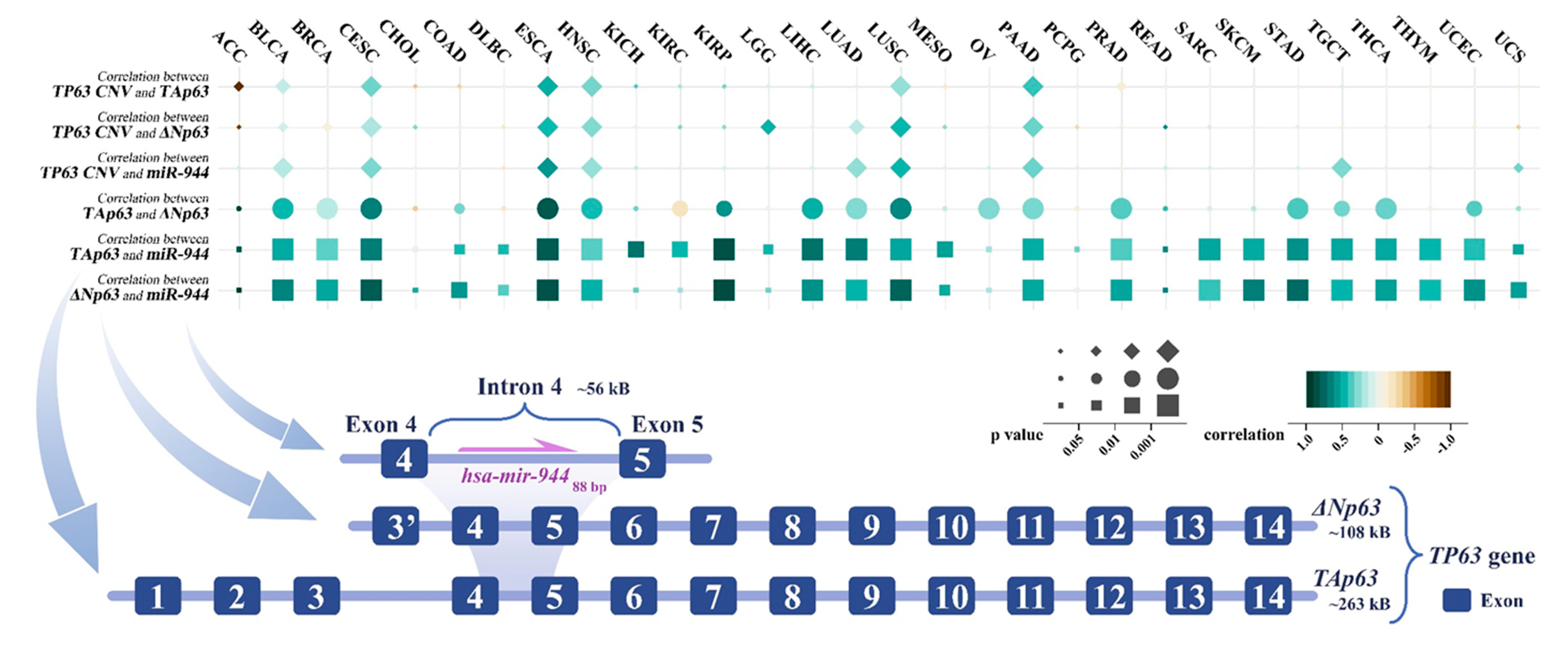
4. Co-Expression of TP63 Transcripts and miR-944
miR-944 is located in the TP63. Previous studies have shown that ΔNp63, but not TAp63, can directly regulate the expression level of miR-944 by recruiting the transcription factor AP-2
[19]. The high correlation between the expression of TAp63 and miR-944 may be due to the common upstream regulators of TAp63 and ΔNp63, resulting in a significant positive correlation between TAp63 and ΔNp63.
In order to explore the relationship between TP63 and miR-944, researchers obtained the expression data of miR-944, TAp63, and ΔNp63 in TCGA (pan-cancer) from the UCSC Xena database (
https://xenabrowser.net (accessed on 20 June 2022)).
Among 30 cancer types, researchers calculated pairwise correlations between miR-944, TP63, and ΔNp63 (Pearson’s correlation test). As shown in Figure 2, miR-944 expression was significantly positively correlated with TAp63 and ΔNp63 in 15 and 16 cancers, respectively (p < 0.01 and r > 0.5). In ACC, CHOL, OV, PCPG, and READ, the expression level of miR-944 was not significantly correlated with TAp63 and ΔNp63 (p > 0.05 or r < 0.3). Among them, the number of ACC (n = 19) and CHOL (n = 20) samples is small, which may lead to the above insignificant correlation. In KIRC and KICH, the expression of miR-944 was not significantly correlated with ΔNp63 (p > 0.05 or r < 0.3) but had a positive correlation with TAp63 (p < 0.01 and r > 0.45), suggesting that there may be a different regulatory mechanism of miR-944 expression in KIRC and KICH.
Figure 2. The correlation of miR-944 with TP63 CNV, TAp63, and ΔNp63. The figure indicates the position of hsa-mir-944 in the TP63 gene and shows the correlation of miR-944 with TP63 CNV and the expression of TAp63 and ΔNp63. ACC, adrenocortical carcinoma; BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL, cholangiocarcinoma; COAD, colon adenocarcinoma; DLBC, lymphoid neoplasm diffuse large B-cell lymphoma; ESCA, esophageal carcinoma; HNSC, head and neck squamous cell carcinoma; KICH, kidney chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LGG, brain lower grade glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; MESO, mesothelioma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC, sarcoma; STAD, stomach adenocarcinoma; SKCM, skin cutaneous melanoma; TGCT, testicular germ cell tumor; THCA, thyroid carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial carcinoma; UCS, uterine carcinosarcoma.