miR-944 is localized in intron 4 of TP63. ΔNp63 in intron 3 of TP63 recruits the transcription factor AP-2 to promote miR-944 gene expression, which mediates epidermal differentiation induction by ΔNp63. miR-944 is dysregulated in various cancers. In squamous cell carcinoma. miR-944 can target and inhibit 27 protein-coding genes, thereby regulating cell cycle, proliferation, apoptosis, epithelial mesenchymal transition, cancer cell invasion and migration, and other cell behaviors. The genes targeted by miR-944 are involved in three signaling pathways, including the Wnt/β-catenin pathway, Jak/STAT3 pathway, and PI3K/AKT pathway. miR-944 was regulated by a total of 11 competing endogenous RNAs, including 6 circular RNAs and 5 long non-coding RNAs. Abnormally expressed miR-944 can act as an independent prognostic factor and is closely related to tumor invasion, lymph node metastasis, TNM staging, and drug resistance. miR-944 is expected to become a critical biomarker with great clinical application value in cancer.
- miR-944
- ceRNA
- dysregulation
- diagnosis
1. Introduction
2. miR-944 and Its Host Gene TP63
3. Aberrant Expression of miR-944 in Cancer
| Physiological System | Cancer | miR-944 Expression | Cell Line | Tissue or Serum | Ref. |
|---|---|---|---|---|---|
| Nervous system | GBM/LGG | Downregulated | HA1800 versus SHG44, U87MG, and U251MG | Paracancerous tissues versus glioma tissues from 5 patients | [28] |
| Respiratory system | NPC | Downregulated | NP69 versus C666-1, CNE1, CNE2, and HNE1 | Paracancerous tissues versus tumor tissues from 20 NPC patients | [29] |
| Downregulated | NP69 versus CNU46, SUNE1, HONE1, 6–10 B, CNE1, CNE2, and HNE1 | Nasopharyngeal mucosa tissues from 30 healthy people versus primary tumor tissues from 30 NPC patients | [7] | ||
| LUAD | Downregulated | 16HBE versus A549, H1299, SK-Lu-1, and PC-9 | Paracancerous tissues versus LUAD tissues from 25 patients | [30] | |
| LUSC | Upregulated | — | Paracancerous tissues from patients versus SCC tissues from patients | [21] | |
| NSCLC | Downregulated | BEAS-2B versus H522 and H1975 | — | [15] | |
| Downregulated | BEAS-2B versus H358, H1299, PC-9, and A549 | Paracancerous tissues versus tumor tissues from 65 NSCLC patients | [31] | ||
| Downregulated | BEAS-2B versus A549, H226, H292, ANP973, and H1299 | Paracancerous tissues versus tumor tissues from 60 NSCLC patients | [32] | ||
| Downregulated | — | Paracancerous tissues versus tumor tissues from 9 NSCLC patients | [2] | ||
| Digestive system | TSCC | Downregulated | normal gingival epithelial cells versus SCC-9, CAL-27, and SCC-15 | Paracancerous tissues versus TSCC tissues from 57 patients | [33] |
| ECa | Downregulated | — | Paracancerous tissues versus adenocarcinoma tissues from 59 eca patients; serum exosomes from healthy persons versus serum exosomes from 59 eca patients | [20] | |
| GC | Downregulated | GES-1 versus AGS, MKN-1, HGC-27, MKN-45, SGC-7901, and BGC-823 | — | [34] | |
| Downregulated | GES-1 versus SGC-7901, MGC-803, MKN-28, and BGC-823 | Paracancerous tissues versus tumor tissues from 40 GC patients | [35] | ||
| HCC | Downregulated | L02 versus Hep3B, Bel-7402, SMMC-7721, Huh7, and SK-HEP-1 | Paracancerous tissues versus tumor tissues from 61 HCC patients | [36] | |
| CRC | Downregulated | HIEC and HEK293 versus HCT116, Caco-2, HT29, SW620, and SW480 | — | [9] | |
| Downregulated | COS7 versus HCT116, LoVo, RKO, HCT15, HT29, SW480, and SW620 | — | [37] | ||
| Downregulated | — | Paracancerous tissues versus fresh CRC tissues from 140 CRC patients | [8] | ||
| Downregulated | CCC-HIE-2 versus HT-29, HCT116, SW480, and SW620 | Paracancerous tissues versus fresh CRC tissues from 100 CRC patients | [38] | ||
| Reproductive system | EC | Upregulated | — | Normal endometrial tissues from 20 non-cancer patients versus tumor tissues from 68 EC patients | [22] |
| CxCa | Upregulated | — | Paracancerous tissues versus tumor tissues from 27 cxca patients | [25] | |
| Upregulated | — | Serum specimens from 24 women with localized disease versus serum specimens from 25 women with metastatic disease | [24] | ||
| Upregulated | HcerEpiC versus HeLa, CaSki, SiHa, and C33A | Paracancerous tissues versus fresh cxca tissues from 70 cxca patients | [23] | ||
| Upregulated | — | 50 FFPE normal cervical tissue samples versus 66 FFPE cxca tissue samples | [5] | ||
| BrC | Downregulated | MCF-10A versus MDA-MB-231, MCF-7, MDA-MB-453, ZR-75, and T47-D | Paracancerous tissues versus locally invasive breast tumors tissues from brc patients | [27] | |
| Upregulated | — | Paracancerous tissues versus tumor tissues from 40 brc patients; serum samples from 30 healthy people versus serum samples from 30 brc patients | [26] | ||
| Motor system | SaOS | Downregulated | hFOB1.19 versus MG-63, SAOS-2, HOS, and U2OS | Paracancerous tissues versus tumor tissues from 38 saos patients | [39] |
| COF | Downregulated | — | Bone tissues from 10 healthy people versus bone tissues from 9 COF patients | [3] |
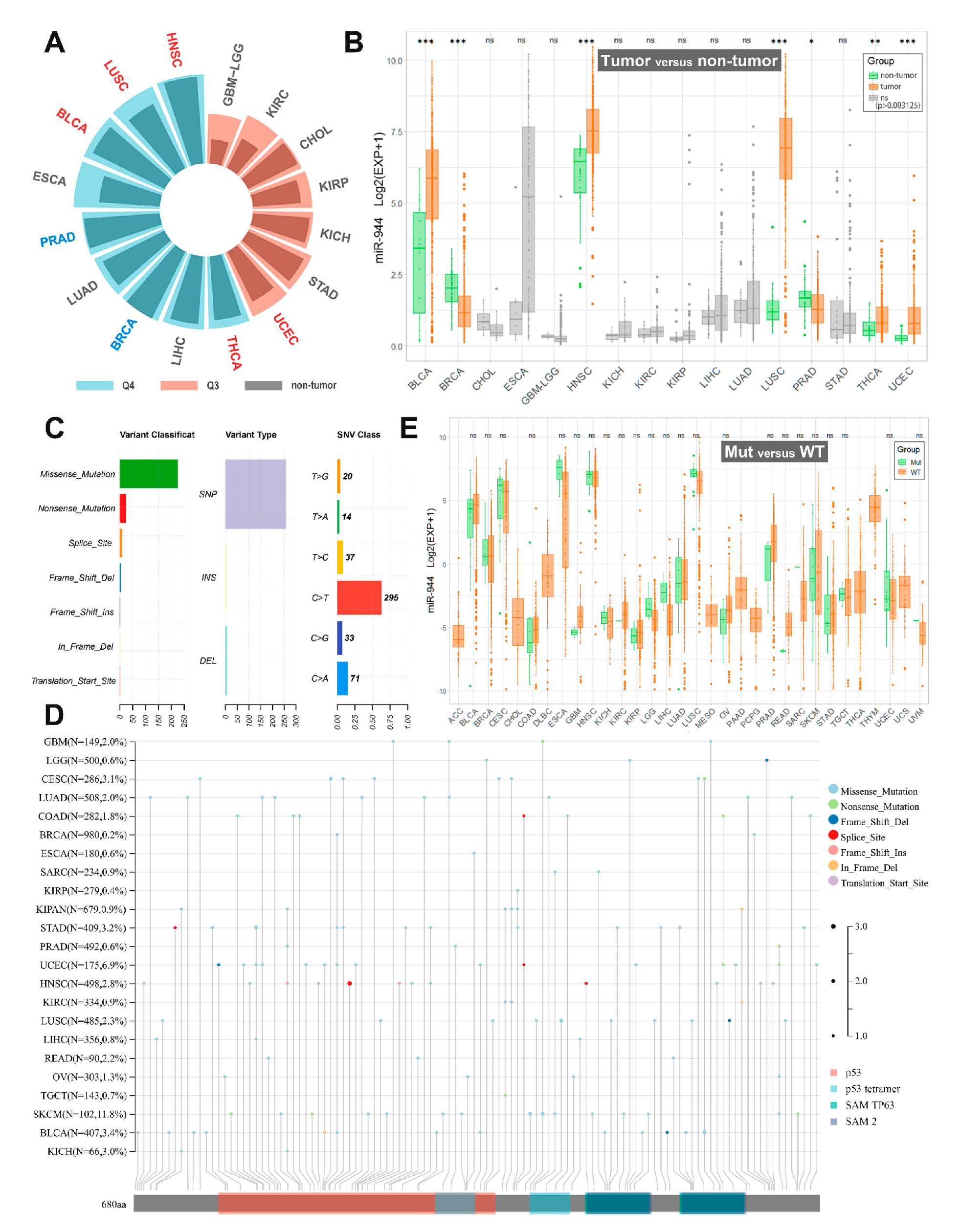
| TCGA Cancers | Sample Size (T/N) | miR-944 Expression in TCGA | miR-944 Expression in the Present Studies |
|---|---|---|---|
| BLCA | 405/18 | Upregulated; Q4 | Not studied |
| BRCA | 624/74 | Downregulated; Q4 | Downregulated in BrC tissues and BrC cells (MDA-MB-231, MCF-7, MDA-MB-453, ZR-75, and T47-D) [27]; and Upregulated in BrC tissues and serum sample of BrC patients [26] |
| CHOL | 20/8 | ns; Q3 | Not studied |
| ESCA | 176/8 | ns; Q4 | Downregulated in ECa tissues and serums of ECa patients [20] |
| GBM/LGG | 209/3 | ns; Q3 | Downregulated in GBM/LGG tissues and GBM/LGG cells (SHG44, U87MG, and U251MG) [28] |
| HNSC | 485/44 | Upregulated; Q4 | Not studied |
| KICH | 49/8 | ns; Q3 | Not studied |
| KIRC | 108/19 | ns; Q3 | Not studied |
| KIRP | 155/23 | ns; Q3 | Not studied |
| LIHC | 324/47 | ns; Q4 | Downregulated in HCC tissues and HCC cells (Hep3B, Bel-7402, SMMC-7721, Huh7, and SK-HEP-1) [36] |
| LUAD | 430/40 | ns; Q4 | Downregulated in LUAD tissues and LUAD cells (A549, H1299, SK-Lu-1, and PC-9) [30] |
| LUSC | 334/44 | Upregulated; Q4 | Upregulated in LUSC tissues [21] |
| PRAD | 437/50 | Downregulated; Q4 | Not studied |
| STAD | 303/26 | ns; Q3 | Downregulated in GC tissues and GC cells (AGS, MKN-1, HGC-27, MKN-45, SGC-7901, MGC-803, BGC-823, and MKN-28) [34][35] |
| THCA | 420/50 | Upregulated; Q4 | Not studied |
| UCEC | 330/26 | Upregulated; Q3 | Upregulated in EC tissues [22] |
4. Co-Expression of TP63 Transcripts and miR-944
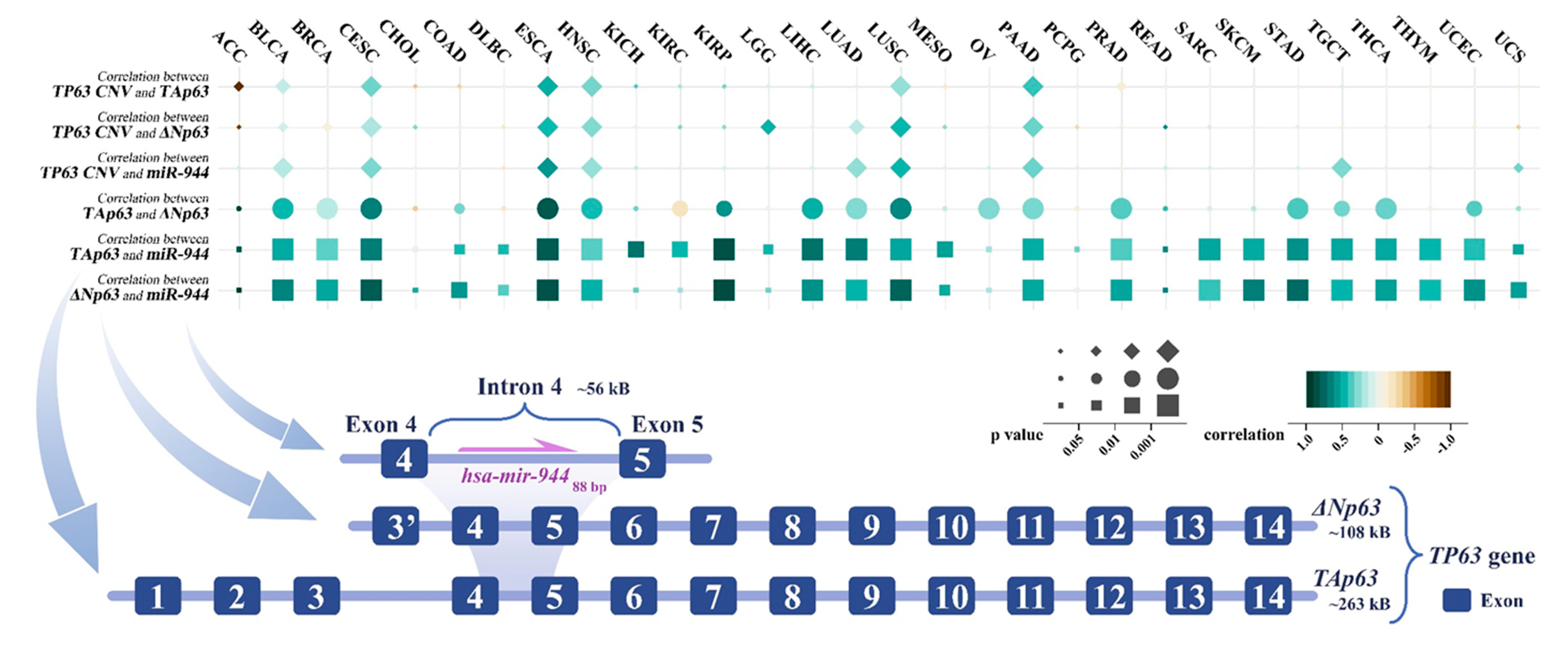
This entry is adapted from the peer-reviewed paper 10.3390/cancers14174232
References
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524.
- Lee, H.Y.; Han, S.S.; Rhee, H.; Park, J.H.; Lee, J.S.; Oh, Y.M.; Choi, S.S.; Shin, S.H.; Kim, W.J. Differential expression of microRNAs and their target genes in non-small-cell lung cancer. Mol. Med. Rep. 2015, 11, 2034–2040.
- Pereira, T.; Brito, J.A.R.; Guimaraes, A.L.S.; Gomes, C.C.; de Lacerda, J.C.T.; de Castro, W.H.; Coimbra, R.S.; Diniz, M.G.; Gomez, R.S. MicroRNA profiling reveals dysregulated microRNAs and their target gene regulatory networks in cemento-ossifying fibroma. J. Oral Pathol. Med. 2018, 47, 78–85.
- Zou, Y.; Zhong, C.; Hu, Z.; Duan, S. MiR-873-5p: A Potential Molecular Marker for Cancer Diagnosis and Prognosis. Front. Oncol. 2021, 11, 743701.
- Park, S.; Kim, J.; Eom, K.; Oh, S.; Kim, S.; Kim, G.; Ahn, S.; Park, K.H.; Chung, D.; Lee, H. microRNA-944 overexpression is a biomarker for poor prognosis of advanced cervical cancer. BMC Cancer 2019, 19, 419.
- Zhong, C.; Xie, Z.; Zeng, L.H.; Yuan, C.; Duan, S. MIR4435-2HG Is a Potential Pan-Cancer Biomarker for Diagnosis and Prognosis. Front. Immunol. 2022, 13, 855078.
- Liu, R.; Zhou, M.; Zhang, P.; Zhao, Y.; Zhang, Y. Cell proliferation and invasion is promoted by circSERPINA3 in nasopharyngeal carcinoma by regulating miR-944/MDM2 axis. J. Cancer 2020, 11, 3910–3918.
- Tang, J.; Gao, W.; Liu, G.; Sheng, W.; Zhou, J.; Dong, Q.; Dong, M. miR-944 Suppresses EGF-Induced EMT in Colorectal Cancer Cells by Directly Targeting GATA6. Oncol. Targets Ther. 2021, 14, 2311–2325.
- Wen, L.; Li, Y.; Jiang, Z.; Zhang, Y.; Yang, B.; Han, F. miR-944 inhibits cell migration and invasion by targeting MACC1 in colorectal cancer. Oncol. Rep. 2017, 37, 3415–3422.
- Lv, W.; Tan, Y.; Xiong, M.; Zhao, C.; Wang, Y.; Wu, M.; Wu, Y.; Zhang, Q. Analysis and validation of m6A regulatory network: A novel circBACH2/has-miR-944/HNRNPC axis in breast cancer progression. J. Transl. Med. 2021, 19, 527.
- Zhang, L.; Wu, Y.; Hou, C.; Li, F. Circ_0072088 knockdown contributes to cisplatin sensitivity and inhibits tumor progression by miR-944/LASP1 axis in non-small cell lung cancer. J. Gene Med. 2022, 24, e3414.
- Chen, X.; Guo, Z.; Fan, S.; Sun, L.; Li, H.; Zhou, J.; Li, Y. Integrating microRNA and mRNA expression in rapamycin-treated T-cell acute lymphoblastic leukemia. Pathol. Res. Pract. 2019, 215, 152494.
- Xi, L.; Liu, Q.; Zhang, W.; Luo, L.; Song, J.; Liu, R.; Wei, S.; Wang, Y. Circular RNA circCSPP1 knockdown attenuates doxorubicin resistance and suppresses tumor progression of colorectal cancer via miR-944/FZD7 axis. Cancer Cell Int. 2021, 21, 153.
- Long, X.; Zheng, M.; Yang, Y.; Chen, Y.; Zhang, X.; Zhang, H. circ_ZFR Is Linked to Paclitaxel Resistance in Cervical Cancer via miR-944 Sponging and IL-10 Upregulation. Anal. Cell Pathol. 2022, 2022, 4807287.
- Zhu, H.; Lu, Q.; Lu, Q.; Shen, X.; Yu, L. Matrine Regulates Proliferation, Apoptosis, Cell Cycle, Migration, and Invasion of Non-Small Cell Lung Cancer Cells Through the circFUT8/miR-944/YES1 Axis. Cancer Manag. Res. 2021, 13, 3429–3442.
- Lin, B.Z.; Wan, S.Y.; Lin, M.Y.; Chang, C.H.; Chen, T.W.; Yang, M.H.; Lee, Y.J. Involvement of Differentially Expressed microRNAs in the PEGylated Liposome Encapsulated (188)Rhenium-Mediated Suppression of Orthotopic Hypopharyngeal Tumor. Molecules 2020, 25, 3609.
- Kim, K.H.; Cho, E.G.; Yu, S.J.; Kang, H.; Kim, Y.J.; Kim, S.H.; Lee, T.R. DeltaNp63 intronic miR-944 is implicated in the DeltaNp63-mediated induction of epidermal differentiation. Nucleic Acids Res. 2015, 43, 7462–7479.
- Prieto-Garcia, C.; Hartmann, O.; Reissland, M.; Braun, F.; Fischer, T.; Walz, S.; Schulein-Volk, C.; Eilers, U.; Ade, C.P.; Calzado, M.A.; et al. Maintaining protein stability of Np63 via USP28 is required by squamous cancer cells. EMBO Mol. Med. 2020, 12, e11101.
- Kim, J.; Park, S.; Chang, Y.; Park, K.H.; Lee, H. Synergetic Effects of Intronic Mature miR-944 and DeltaNp63 Isoforms on Tumorigenesis in a Cervical Cancer Cell Line. Int. J. Mol. Sci. 2020, 21, 5612.
- Warnecke-Eberz, U.; Chon, S.H.; Holscher, A.H.; Drebber, U.; Bollschweiler, E. Exosomal onco-miRs from serum of patients with adenocarcinoma of the esophagus: Comparison of miRNA profiles of exosomes and matching tumor. Tumour Biol. 2015, 36, 4643–4653.
- Ma, J.; Mannoor, K.; Gao, L.; Tan, A.; Guarnera, M.A.; Zhan, M.; Shetty, A.; Stass, S.A.; Xing, L.; Jiang, F. Characterization of microRNA transcriptome in lung cancer by next-generation deep sequencing. Mol. Oncol. 2014, 8, 1208–1219.
- He, Z.; Xu, H.; Meng, Y.; Kuang, Y. miR-944 acts as a prognostic marker and promotes the tumor progression in endometrial cancer. Biomed. Pharmacother. 2017, 88, 902–910.
- Chen, Y.; Gu, Y.; Gu, Y.; Wu, J. Long Noncoding RNA LINC00899/miR-944/ESR1 Axis Regulates Cervical Cancer Cell Proliferation, Migration, and Invasion. J. Interferon Cytokine Res. 2021, 41, 220–233.
- Palatnik, A.; Ye, S.; Kendziorski, C.; Iden, M.; Zigman, J.S.; Hessner, M.J.; Rader, J.S. Identification of a serum-induced transcriptional signature associated with metastatic cervical cancer. PLoS ONE 2017, 12, e0181242.
- Xie, H.; Lee, L.; Scicluna, P.; Kavak, E.; Larsson, C.; Sandberg, R.; Lui, W.O. Novel functions and targets of miR-944 in human cervical cancer cells. Int. J. Cancer 2015, 136, E230–E241.
- He, H.; Tian, W.; Chen, H.; Jiang, K. MiR-944 functions as a novel oncogene and regulates the chemoresistance in breast cancer. Tumour Biol. 2016, 37, 1599–1607.
- Flores-Perez, A.; Marchat, L.A.; Rodriguez-Cuevas, S.; Bautista, V.P.; Fuentes-Mera, L.; Romero-Zamora, D.; Maciel-Dominguez, A.; de la Cruz, O.H.; Fonseca-Sanchez, M.; Ruiz-Garcia, E.; et al. Suppression of cell migration is promoted by miR-944 through targeting of SIAH1 and PTP4A1 in breast cancer cells. BMC Cancer 2016, 16, 379.
- Jiang, J.; Lu, J.; Wang, X.; Sun, B.; Liu, X.; Ding, Y.; Gao, G. Glioma stem cell-derived exosomal miR-944 reduces glioma growth and angiogenesis by inhibiting AKT/ERK signaling. Aging 2021, 13, 19243–19259.
- Ji, J.; Peng, Y.; Niu, T.; Lin, Y.; Lin, Y.; Li, X.; Wu, X.; Huang, Z.; Zhong, L.; Zhang, S. miR-944 inhibits cell migration and invasion by targeting MACC1 in nasopharyngeal carcinoma. Int. J. Clin. Exp. Pathol. 2018, 11, 1167–1174.
- An, J.C.; Shi, H.B.; Hao, W.B.; Zhu, K.; Ma, B. miR-944 inhibits lung adenocarcinoma tumorigenesis by targeting STAT1 interaction. Oncol. Lett. 2019, 17, 3790–3798.
- Lv, J.; Li, Q.; Ma, R.; Wang, Z.; Yu, Y.; Liu, H.; Miao, Y.; Jiang, S. Long Noncoding RNA FGD5-AS1 Knockdown Decrease Viability, Migration, and Invasion of Non-Small Cell Lung Cancer (NSCLC) Cells by Regulating the MicroRNA-944/MACC1 Axis. Technol. Cancer Res. Treat. 2021, 20, 1533033821990090.
- Geng, H.; Li, S.; Xu, M. Long Noncoding RNA SNHG6 Functions as an Oncogene in Non-Small Cell Lung Cancer via Modulating ETS1 Signaling. Oncol. Targets Ther. 2020, 13, 921–930.
- Lin, C.; Zou, Y.; Li, R.; Liu, D. Long noncoding RNA PRNCR1 exerts oncogenic effects in tongue squamous cell carcinoma in vitro and in vivo by sponging microRNA944 and thereby increasing HOXB5 expression. Int. J. Mol. Med. 2020, 46, 119–130.
- Ma, S.; Gu, X.; Shen, L.; Chen, Y.; Qian, C.; Shen, X.; Ju, S. CircHAS2 promotes the proliferation, migration, and invasion of gastric cancer cells by regulating PPM1E mediated by hsa-miR-944. Cell Death Dis. 2021, 12, 863.
- Pan, T.; Chen, W.; Yuan, X.; Shen, J.; Qin, C.; Wang, L. miR-944 inhibits metastasis of gastric cancer by preventing the epithelial-mesenchymal transition via MACC1/Met/AKT signaling. FEBS Open Bio 2017, 7, 905–914.
- Zheng, H.; Zou, A.E.; Saad, M.A.; Wang, X.Q.; Kwok, J.G.; Korrapati, A.; Li, P.; Kisseleva, T.; Wang-Rodriguez, J.; Ongkeko, W.M. Alcohol-dysregulated microRNAs in hepatitis B virus-related hepatocellular carcinoma. PLoS ONE 2017, 12, e0178547.
- Kim, Y.J.; Lee, J.H.; Jin, S.; Kim, J.H.; Kim, S.H. Primate-specific miR-944 activates p53-dependent tumor suppression in human colorectal cancers. Cancer Lett. 2019, 440–441, 168–179.
- Tang, J.T.; Zhao, J.; Sheng, W.; Zhou, J.P.; Dong, Q.; Dong, M. Ectopic expression of miR-944 impairs colorectal cancer cell proliferation and invasion by targeting GATA binding protein 6. J. Cell Mol. Med. 2019, 23, 3483–3494.
- Yan, T.; Zhu, S.; Zhang, J.; Lu, G.; Lv, C.; Wei, Y.; Luo, M. MicroRNA944 targets vascular endothelial growth factor to inhibit cell proliferation and invasion in osteosarcoma. Mol. Med. Rep. 2018, 18, 5221–5228.
