Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Georgia Xiromerisiou | -- | 4175 | 2022-09-10 10:18:42 | | | |
| 2 | Peter Tang | -20 word(s) | 4155 | 2022-09-13 03:57:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mysiris, D.S.; Vavougios, G.D.; Karamichali, E.; Papoutsopoulou, S.; Stavrou, V.T.; Papayianni, E.; Boutlas, S.; Mavridis, T.; Foka, P.; Zarogiannis, S.G.; et al. SARS-CoV-2 Infection and Parkinson’s Disease Overlaps. Encyclopedia. Available online: https://encyclopedia.pub/entry/27080 (accessed on 08 February 2026).
Mysiris DS, Vavougios GD, Karamichali E, Papoutsopoulou S, Stavrou VT, Papayianni E, et al. SARS-CoV-2 Infection and Parkinson’s Disease Overlaps. Encyclopedia. Available at: https://encyclopedia.pub/entry/27080. Accessed February 08, 2026.
Mysiris, Dimitrios S., George D. Vavougios, Eirini Karamichali, Stamatia Papoutsopoulou, Vasileios T. Stavrou, Eirini Papayianni, Stylianos Boutlas, Theodoros Mavridis, Pelagia Foka, Sotirios G. Zarogiannis, et al. "SARS-CoV-2 Infection and Parkinson’s Disease Overlaps" Encyclopedia, https://encyclopedia.pub/entry/27080 (accessed February 08, 2026).
Mysiris, D.S., Vavougios, G.D., Karamichali, E., Papoutsopoulou, S., Stavrou, V.T., Papayianni, E., Boutlas, S., Mavridis, T., Foka, P., Zarogiannis, S.G., Gourgoulianis, K., & Xiromerisiou, G. (2022, September 10). SARS-CoV-2 Infection and Parkinson’s Disease Overlaps. In Encyclopedia. https://encyclopedia.pub/entry/27080
Mysiris, Dimitrios S., et al. "SARS-CoV-2 Infection and Parkinson’s Disease Overlaps." Encyclopedia. Web. 10 September, 2022.
Copy Citation
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disease after Alzheimer’s disease, globally. Dopaminergic neuron degeneration in substantia nigra pars compacta and aggregation of misfolded alpha-synuclein are the PD hallmarks, accompanied by motor and non-motor symptoms. Several viruses have been linked to the appearance of a post-infection parkinsonian phenotype. Coronavirus disease 2019 (COVID-19), caused by emerging severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection, has evolved from a novel pneumonia to a multifaceted syndrome with multiple clinical manifestations, among which neurological sequalae appear insidious and potentially long-lasting.
Parkinson’s disease
SARS-CoV-2
exosomes
neuroinflammation
inflammation
parkinsonism
alpha-synuclein
post-COVID-19
neurodegeneration
virus
1. Introduction
Parkinsonism is a clinical syndrome defined by the presence of resting tremor, bradykinesia, rigidity and postural instability [1]. These motor symptoms are characteristically observed in Parkinson’s disease (PD) [2], which remains the primary cause of parkinsonism, but there are other disorders with the same symptoms that mirror it [3][4]. PD is the second most prevalent neurodegenerative disease worldwide after Alzheimer’s disease (AD) and constitutes a debilitating, progressive motor disorder characterized by degeneration of the nigrostriatal dopaminergic pathway [5]. The prevalence of PD is estimated to be approximately 0.5–1% among those 65–69 years of age, rising to 3% among persons of 80 years and older [6], with an annual incidence rate of approximately 11–19/100,000 cases per year [7][8]. Although PD is generally an idiopathic disorder, there is 5–10% of PD cases that report a family history or display a clear Mendelian inheritance [9][10]. The incremental loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and striatum is the mechanistic cause of motor manifestations, with 60–70% dopaminergic neuron loss required for the appearance of motor symptoms [11]. However, prior to motor manifestation onset, patients may display non-motor symptoms such as hyposmia, gastrointestinal dysfunction, and sleep disorders [12]. The neuropathological hallmark of PD is the misfolding and aggregation of alpha-synuclein (α-syn), which is the major protein component of Lewy bodies (LB). Indeed, formation of α-syn protein clumps within neural cells triggers the initiation of neurodegeneration processes [5].
PD is a disease of multicomplex etiology, involving the interaction of aging, genetics, and environmental variables, as well as infectious agents, such as viral infections [13][14]. Additionally, there is now a wide range of data to support the existence of viral parkinsonism, which often manifests following recovery from viral infections [4]. Although the precise mechanisms remain unclear, viruses have been implicated as potential etiological or trigger factors for both PD pathogenesis [15][16] and viral parkinsonism [4]. Recent data suggest that the emerging human severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for the ongoing pandemic that has already killed more than 6.4 M people worldwide [17], may be one of these viruses [18][19][20].
On cellular and molecular level, mitochondrial dysfunction, defective autophagy, oxidative stress, and neuroinflammation are all thought to play a role in PD pathogenesis and they are linked to the accumulation and spread of misfolded α-syn [21][22][23]. The “prion-like” cell-to-cell dissemination of amyloidogenic proteins, such as α-syn, principally refers to the formation and subsequent spread of self-propagating pathological α-syn aggregates throughout brain regions and has lately garnered considerable attention in the quest to understand PD pathophysiology [24][25][26][27]. Several in vitro studies, both in animals and continuous human cell lines, have supported this reminiscent of, yet distinct from prion diseases, mechanism of misfolded α-syn spread [28][29][30]. Exosomes, the nanosized vesicles and masters of intercellular communication [31], have been proposed to serve as an efficient “vehicle” of transportation for such proteins [32], mainly because they are a priori involved in several homeostatic procedures in the central nervous system (CNS) including myelination maintenance, synaptic plasticity, antigen presentation, signal transduction, neurogenesis, and trophic support for neurons [33][34]. Interestingly, many viruses, including SARS-CoV-2, have been shown to regulate exosomal biogenesis and cargo content upon release from infected host cells [35][36].
2. Viral Ιnfections as Τriggers for Parkinsonism and PD Development
Several studies have demonstrated that viruses may contribute to the etiology of PD and parkinsonism, despite the fact that the underlying molecular and cellular mechanisms remain obscure. The first recorded association between viral infections and parkinsonism was observed during the Spanish flu and the appearance of encephalitis lethargica, an unknown disease with parkinsonian phenotype in survivors [37]. Major human viruses, such as hepatitis C virus (HCV) [38], herpes simplex virus-1 (HSV-1) [39], human immunodeficiency virus (HIV) [40], varicella-zoster virus (VZV) [41], West Nile virus (WNV) [42], Japanese encephalitis virus (JEV) [43][44], and Epstein–Barr virus (EBV) [45], have all been cited as risk factors for PD development or parkinsonism [3]. Notably, the role of influenza A virus (IAV) in the etiology of the transient parkinsonian phenotype [46] and in PD development [3] has been documented in several in vivo and especially in vitro studies. A case-control study found that an influenza diagnosis was linked to PD development 10 years following infection onset [47], while IAV was found postmortem in the substantia nigra of PD patients [48]. Furthermore, H5N1 infection in a mouse model resulted in Parkinson’s phenomenology, sustained microglial activation, and α-syn aggregation, leading to dopaminergic neuron loss in SNpc [49]. Similarly, H1N1 infection in mice resulted in persistent microglial activation as a sign of chronic virus-induced neuroinflammation that could potentially lead to neurodegeneration [50]. More recently, another in vitro study has demonstrated that H1N1 replication can directly disrupt protein homeostasis, inducing α-syn aggregates in Lund human mesencephalic dopaminergic cells, but failing to regulate TAR DNA-binding protein 43 (TDP-43) or tau protein. Those results clearly hint at a selective effect of H1N1 virus on α-syn misfolding [51].
The key pathophysiological processes by which viruses contribute to parkinsonism development remain unclear; however, direct neuronal damage, sustained neuroinflammation, cerebral edema due to virus-mediated damage of brain endothelium, and induction of α-syn aggregation have all been proposed as crucial neurobiological pathways of dopaminergic neuron loss and α-syn pathology [3]. Notably, due to its tendency to entrap viral particles and reduce viral replication, α-syn has been postulated to be a natural antiviral defense mechanism for neurons [52]. This notion was supported by in vivo experiments, where WNV-infected α-syn-knockout mice showed decreased survival compared to the control group [53]. Additionally, it has been suggested that viruses can cause α-syn aggregation and oligomerization through molecular mimicry mechanisms [54][55]. Taken together, these observations strongly support the notion that virus-mediated neuronal deposition of pathological α-syn may induce neurotoxicity and PD pathology.
The relationship between other members of the human Coronaviridae family, such as OC43 and 229E, and PD has been previously described, since antibodies against these coronaviruses were found in the cerebrospinal fluid (CSF) of PD patients [56]. The novel coronavirus SARS-CoV-2 emerged in China at the end of 2019 and triggered an outbreak of atypical viral pneumonia [57]. Due to its enhanced transmissibility, this unusual coronavirus disease, also known as coronavirus disease 2019 (COVID-19), marched fast over the world, constituting a huge public health burden [58][59]. SARS-CoV-2 spreads via infected secretions, such as saliva and respiratory droplets, through direct, indirect, or close contact with infected patients, even if COVID-19 symptomatology is absent [60][61]. While symptoms of COVID-19 are primarily systemic or respiratory, several studies demonstrate the presence of a broad spectrum of neuropsychiatric consequences including anosmia, ageusia, altered consciousness, headache, seizures, and paresthesias [62][63][64]. Several studies have shown that COVID-19-related neurological sequelae might persist long after the acute phase of infection [65]. The term “long” or “post”-COVID-19 syndrome refers to a syndrome observed after the acute infection period and it is characterized by the presence of a combination of COVID-19-related symptoms lasting for more than 12 weeks [66]. These symptoms cannot be explained by an alternative diagnosis and are considered a disability under the Americans with Disabilities Act (ADA) [67]. The post-COVID-19 syndrome includes a plethora of neurological manifestations such as fatigue, brain fog, cognitive impairment, and olfactory dysfunctions [68][69][70], many of which are also present in PD [2]. Thus, since SARS-CoV-2 shares immunopathological similarities with other viruses linked to parkinsonism, such as influenza [71], and because of COVID-19-related neurological consequences, it is reasonable to suspect that these persistent symptoms might be a prologue to a post-COVID-19 new-onset neurological disease.
3. SARS-CoV-2 Infection and PD Overlaps
3.1. Clinical Co-Manifestations
To date, only few cases of parkinsonism have been reported in literature following COVID-19 infection [19][20][72][73][74]. In these studies, the authors speculate a possible causative link between COVID-19 infection and a post-COVID new-onset parkinsonian phenotype, but they do not address the possibility of prodromal, pre-symptomatic PD, which became symptomatic as a result of biological or psychological stress processes associated with COVID-19. In the latter case, SARS-CoV-2 infection could act as a trigger that unmasks an underlying PD phenotype, possibly by stimulating neuroinflammatory and neurodegenerative cascades. In addition, SARS-CoV-2 infection has been demonstrated to significantly worsen motor and non-motor symptoms in people with pre-existing PD [75][76]. Considering the prevalence of post-COVID-19 syndrome [77][78], a multicenter study found that 23 out of 27 PD patients developed post-COVID-19 symptoms, with the most common long term effects of COVID-19 being the deterioration of motor function and the requirement for increased levodopa daily dose, followed by fatigue, cognitive disturbances including brain fog, and sleep disorders [79].
Probably the clinical symptoms most commonly shared between PD and COVID-19 are gustatory and especially olfactory dysfunctions. Indeed, both olfactory and gustatory impairments are among the earliest non-motor PD features [80][81]. Surprisingly, these are common early onset symptoms of COVID-19 and it has been observed that hyposmia–anosmia and dysgeusia could persist long after viral load decline, constituting a key clinical manifestation of the long COVID-19 syndrome [82][83]. Due to lack of evidence regarding the definite CNS infiltration, the olfactory route is discussed as a way for SARS-CoV-2 to gain access to the CNS. Indeed, a postmortem study demonstrated that the highest levels of SARS-CoV-2 RNA and spike protein (S protein) among various brain areas were found in the olfactory mucosal–nervous milieu, as well as in neuroanatomical areas related to the olfactory tract. In this regard, the olfactory mucosa could serve as an “anatomical bridge” for SARS-CoV-2 CNS invasion through axonal transport [84]. Furthermore, angiotensin-converting enzyme 2 (ACE2), an essential cell surface receptor responsible for S protein-mediated entry of SARS-CoV-2, was found to be expressed by epithelial cells of the human olfactory mucosa [85]. The extent of α-syn pathology in other brain regions has been substantially linked with the pathological burden in the olfactory bulb, suggesting that PD pathology extends along olfactory pathways [86]. The Braak hypothesis proposes that LB are initially found in olfactory structures, such as the olfactory bulb, and then they gradually spread towards the brain stem and ultimately to the cerebral cortex, strengthening the scenario that the earliest lesions could develop at non nigral areas [87][88]. Accordingly, Beach and colleagues have demonstrated that the olfactory bulb constitutes a primary affected area in α-synucleinopathies, including PD. In fact, it was suggested that the extent of α-synucleinopathy in the olfactory bulb strongly predicts the neuropathological confirmation of PD and reflects the severity of α-synucleinopathy in other brain regions [89]. Based on these studies, one could hypothesize that the olfactory route might pose a way for SARS-CoV-2 to gain access to the CNS, where it can modify neuropathological pathways pertinent to PD development.
Another common pathology shared between PD and COVID-19 is the deregulation and dysfunction of the gastrointestinal (GI) tract. GI symptoms and intestinal inflammation may emerge years before clinical indications of PD become apparent [90][91]. Specifically, gastrointestinal dysbiosis has been proposed to be involved in PD pathogenesis [92] and the enteric nervous system has been previously identified as a primary region for abnormal α-syn aggregation, which may then spread from the periphery to the CNS [93][94][95]. Specifically, the dorsal motor nucleus of the vagus nerve (DMV) receives signals from vagal parasympathetic neurons that project to the entire GI system. The DMV is involved in the PD–neuroanatomical pathway, since a monosynaptic nigro–vagal pathway that connects the SNpc to the DMV has been identified in the rat [96]. In postmortem PD studies, the DMV and the vagus nerve itself are among the most frequently afflicted structures [97][98] and they constitute principal areas of LB accumulation, even at the earliest stages of disease development [87]. In vitro research has shown that pathological α-syn may spread from the gut to the brain through the vagus nerve, with DMV being the first area of the brain to be impacted. From there, α-syn can spread to other PD brain regions including the SNpc, resulting in dopaminergic neuron loss and the appearance of the parkinsonian phenotype [99]. Interestingly, the vagus nerve has been proposed as a pathway through which SARS-CoV-2 can retrogradely invade the CNS, thus enhancing its neuroinvasiveness [100][101].
Importantly, other GI manifestations, such as diarrhea, emerged as common clinical symptoms of COVID-19, while SARS-CoV-2 RNA detection in fecal samples may persist post-infection [102]. On top of that, gut microbiota imbalance due to extrapulmonary SARS-CoV-2 infection has also been observed in COVID-19 [103][104]. This warrants further investigation because GI microbiota equilibrium plays an important role in several physiological processes ensuring brain integrity and neurogenesis [105][106]. Taken together, the above observations suggest that SARS-CoV-2 infection could promote PD development and progression through a virus-exerted dysfunction of the GI system.
3.2. Inflammatory and Molecular Overlapping Pathways
Common inflammatory events unraveling during PD development and observed in the acute phase of SARS-CoV-2 infection, as well as after COVID-19 remission, may indicate a link between these two disorders. Virus-mediated sustained or aberrant neuroinflammation could be a decisive pathobiological process for the initiation of a neurodegenerative disease, such as PD, long after recovery from the viral infection [107][108][109]. Indeed, growing evidence indicates that SARS-CoV-2 induces neuroinflammation [110] through its neurotropic, neuroinvasive, and neurovirulence effects [111][112] or even via immune-mediated pathways [113]. SARS-CoV-2 infection also triggers systemic inflammatory responses and induces cytokine release [114]. Severe COVID-19 is characterized by a cytokine storm syndrome, which is a major cause of mortality [114][115]. Several studies have demonstrated the presence of inflammatory mediators, such as increased levels of pro- and anti-inflammatory interleukins (IL-1, IL-2, IL-6, IL-10) and tumor necrosis factor-alpha (TNF-α) in the serum of COVID-19 patients [116][117][118][119]. Interestingly, a small prospective observational study had previously found that high levels of IL-6 were linked to a higher chance of developing PD [120]. Evidently, an exacerbated systemic infection that causes a huge release of inflammatory mediators, including cytokines, chemokines, and antibodies, could lead to increased blood–brain barrier (BBB) permeability [121]. Functional and structural integrity of the BBB is pivotal in maintaining brain homeostasis [122]. A neurovascular unit (NVU) consists of multiple cell types, including brain microvascular endothelial cells (BMVECs), astrocytes, pericytes, microglia, and neurons, connected together with extracellular matrix components, and is a rigorous regulator of BBB permeability [123]. NVU disruption has been previously associated with neurodegenerative diseases [124]. In particular, BMVECs constitute an important component of NVU and are intricately interconnected through tight junction (TJ) proteins. However, inflammation affects BBB integrity and stability mainly through cytokine-induced degradation of TJ proteins [125]. SARS-CoV-2-mediated brain endothelial inflammation, upregulation of inflammatory mediators, and most significantly, disruption of BBB stability, have also been observed in human BMVECs [126]. According to in vitro studies, SARS-CoV-2 was shown to infect human BMVECs and cause a decrease in TJ protein expression [126][127]. Furthermore, incubation of human BMVECs with S protein resulted in enhanced ACE2 expression, thereby facilitating viral entry and inducing neuroinflammation [128].
When BBB becomes impaired, pro-inflammatory cytokines and factors, innate immune cells from the periphery, and SARS-CoV-2 could possibly pass through and infiltrate the CNS. In that case, the CNS professional immune cells, microglia and astrocytes, may also become activated [129][130]. Neuroinflammation is then likely to set in fast, leading to elevated production of cytokines, chemokines, reactive oxygen species (ROS), and secondary messengers [131]. Microglia, which are highly susceptible to pro-inflammatory stimuli, are concentrated in areas harboring dopaminergic neurons, making them particularly vulnerable to inflammatory mediators [132][133]. Interestingly, the S1 subunit of S protein was found to efficiently trigger neuroinflammation, including microglia activation, release of multiple pro-inflammatory cytokines, and cause behavioral deficits in rats [134]. Consequently, these neuroinflammatory cascades lead to enhanced apoptotic activity, increased ROS levels, mitochondrial dysfunction, and eventually neurodegeneration [135][136].
Finally, cellular senescence is a core homeostatic event that provides yet another, age- and state-dependent substrate for neurodegeneration and the development of diseases like AD and PD [137][138]. Cellular senescence in the aging brain affects both neuronal and non-neuronal cells, and it is characterized by a broad array of interconnected disruptions, such as disruptions in autophagy, bioenergetics, and mitochondrial dynamics, as well as the onset of low-grade inflammation [138]. This cumulative array of dysfunction culminates in the accumulation of proteopathic seeds, including tau, amyloids, and α-syn, and tissue-wide remodeling [137]. It has been shown that SARS-CoV-2 infection induces “immunosenescence” and enhances the senescence-associated secretory phenotype (SASP) in infected tissues, via disruption of host antiviral mechanisms, such as interferon signaling pathways [139][140][141]. Taken together, all the aforementioned studies strongly indicate that the COVID-19 cytokine storm and innate immunity dysregulation may cause neuroinflammation and, in consequence, neurodegeneration.
Neuropathological findings in postmortem brain tissues from COVID-19 patients further support the involvement of COVID-19-related neuroinflammatory processes in PD development. A postmortem brain study of 43 COVID-19 patients has shown activation of microglia and CNS infiltration by cytotoxic T-lymphocytes, more apparent in the brainstem [142]. Regardless of COVID-19 disease severity, significant inflammatory responses such as astrogliosis, microglia activation, and perivascular T-lymphocyte infiltration were observed postmortem in both white and gray matter of patient brains [143]. Performing single-nucleus RNA sequencing and immunohistochemistry on tissue from a group of individuals who died with COVID-19 and a group of individuals who died from other causes, Yang and colleagues revealed glia transcriptomic changes that indicated a COVID-19-associated activation of inflammatory pathways. The ensuing dysregulation of homeostatic pathways could potentially lead to neurodegeneration [144]. Specifically, microglia and astrocytic subpopulations were enriched by inflammatory genes and deregulated neuroprotective ones that had been previously linked to PD and other human neurodegenerative diseases, such as the glial fibrillary acidic protein (GFAP), the interferon-induced transmembrane protein-3 (IFITM3), and others [145][146].
Another mechanism that may contribute to PD pathogenesis involves the renin–angiotensin system and ACE2, which are implicated in the pathophysiology of COVID-19 and may play a role in neuroinflammation-mediated neurodegeneration in PD [147][148]. ACE2 is highly expressed in several brain areas [149], including striatum [150], the substantia nigra, the olfactory bulb [151], and the brain endothelium [126][152][153]. Induced pluripotent stem cells (IPCS) derived from midbrain dopaminergic neurons were shown to be vulnerable to SARS-CoV-2 infection in vitro [154], unravelling the potentially direct neurotrophic effect of SARS-CoV-2 in strategic PD areas. Furthermore, SARS-CoV-2-induced Toll-like receptor (TLR) overactivation led to ACE2 upregulation and promoted the neurotrophic and neuroinflammatory outcomes of SARS-CoV-2 infection [155]. TLRs belong to the family of innate immune receptors and play an important role in the activation of innate immunity, including activation of glial cells. TLR-mediated stimulation of intracellular signaling pathways culminates in the release of proinflammatory mediators such as IL-6, IL-1, TNF-a, and nuclear factor-κB (NF-κB) [156]. Protein-to-protein interaction between SARS-CoV-2 S protein and TLR-4 has been previously recorded [157]. SARS-CoV-2-mediated overactivation of the TLRs may lead to hyperinflammation, ACE2 upregulation and microglia switching from the neuroprotective to the neurotoxic phenotype [155][158]. In sequel, sustained gliosis and prolonged neuroinflammation could lead to α-syn aggregation and finally loss of dopaminergic neurons in the SNpc [108].
Aside from neuroinflammation, dysregulation of several homeostatic molecular pathways has been identified in PD onset and development. These alterations also occur during host–virus interactions as the virus attempts to direct critical cellular infrastructure towards completion of its own lifecycle. SARS-CoV-2 viral proteins were shown to post-translationally reconfigure the biological function of 24 host proteins expressed in lung. The latter act as perturbators and interact with 44 CNS proteins that are known to be implicated in PD pathogenesis [159]. Specifically, SARS-CoV-2-mediated deregulation of Rab7a and nucleoporin-62 (NUP62) could be strongly involved in PD pathogenesis, because Rab7 lysosomal protein decreases α-syn aggregation and associated neurotoxicity [160], while NUP62 is crucial for autophagosome development [161]. Furthermore, SARS-CoV-2 proteins can interact and bind to a variety of human protein trafficking molecules. Protein trafficking, translation, transcription, and ubiquitination regulation are all coordinated by these biomolecules, leading to neuroprotection, protection of BBB integrity, and neurogenesis [162]. A recent study demonstrated a direct interaction between SARS-CoV-2 nucleocapsid protein (N-protein) and α-syn, which led to the aggregation of the latter into amyloid fibrils, a highly pathogenic form of the protein, linked to PD. Co-administration of SARS-CoV-2 N protein and α-syn to a PD cell model resulted in twice the neuron loss due to neurotoxicity compared to control cells treated with α-syn alone [163].
Other important cellular processes implicated in the loss of dopaminergic neurons in SNpc are thought to be oxidative stress and mitochondrial dysfunction, endoplasmic reticulum stress, and the impairment of protein degradation systems [164][165][166].
A key molecular factor in PD development and progression is mitochondrial dysfunction and oxidative stress [167][168]. An imbalance between ROS generation and cellular antioxidant activity leads to oxidative stress and ROS can further affect mitochondria, attenuating adenosine triphosphate (ATP) production as well as causing damage to mitochondrial DNA [169]. In addition to causing direct cellular damage, oxidative stress can speed up neuron degeneration by inducing inflammatory or apoptotic pathways, such as NF-κB or caspase activation [170]. In PD studies, mitochondrial dysfunction may occur months before the onset of striatal dopaminergic neuron loss [171] and PD patients have been well documented to possess reduced or deficient mitochondrial complex I activity in the SNpc [172][173]. In mice, accumulation of wild-type α-syn in dopaminergic neurons reduced mitochondrial complex I activity and elevated ROS production, leading to cell death [174]. SARS-CoV-2 seems to interact with and manipulate mitochondria in order to hijack and evade mitochondria-mediated immune response for its own replication and survival [175][176]. In this effort, SARS-CoV-2 may induce mitochondrial impairment [177][178], mitochondria-mediated oxidative stress, and mitochondrial damage through mitochondrial membrane depolarization, mitochondrial permeability transition pore opening, and enhanced ROS release [179][180][181]. Furthermore, the virus prevents mitophagy by blocking the binding of p62 and microtubule-associated protein 1A/1B-light chain 3 (LC3), thereby hindering viral RNA breakdown [181].
Finally, mitochondria aid the antiviral immune response by allowing release of pro-inflammatory cytokines [182]. ACE2 has been suggested to regulate mitochondrial function [183]. Its expression is decreased when SARS-CoV-2 S protein binds to ACE2 on microglia cells, causing ATP reduction and activation of the ROS-generating enzyme NADPH oxidase [184]. The ensuing increase in ROS production and oxygen consumption may lead to neuroinflammation and loss of neighbor dopaminergic neurons [185].
Endoplasmic reticulum (ER) stress has been linked to neurodegenerative diseases, including PD [186][187]. ER homeostasis disruption and extended ER stress lead to misfolded protein accumulation and may stimulate particular proapoptotic pathways through the activation of the transcription factor C/EBP homologous protein (CHOP) and cysteine proteases caspase-4/12 [188][189]. Growing evidence suggests that SARS-CoV-2 proteins interact with the ER compartment and may induce ER stress [190][191]. SARS-CoV-2 open reading frame 8 (ORF8) is capable of inducing ER stress by triggering the activating transcription factor 6 (ATF6) and inositol-requiring enzymes 1 (IRE1) branches of the ER stress pathway [192], potentially leading to α-syn accumulation [193]. Aside from initiating apoptotic pathways, ER stress is a powerful stimulator of NF-κB activation and inflammatory gene transcription [194][195]. SARS-CoV-2 also appears to activate NF-κB, causing inflammation, possibly through ER stress or via interaction with the non-structural protein Nsp5 [196]. Notably, NF-κB is a crucial transcription factor that regulates inflammation and dopaminergic neurons loss in PD patients [197]. Hence, deregulation of this signaling pathway has been linked to PD onset and pathology [198] by favoring α-syn accumulation, aggregation, and spreading, oxidative stress-induced neuron apoptosis, neuroinflammation, and dopaminergic neuron loss [135][199][200].
When aggregation and deposition of misfolded α-syn elicit dopaminergic neuron loss, protein degradation systems come to the rescue. The ubiquitin–proteasome system (UPS) and the autophagy–lysosomal pathway (ALP) are important proteolytic systems in neurons and critical for refolding or elimination of misfolded proteins; therefore, they play a significant role in cellular homeostasis [201]. Impairment or even failure of these systems may contribute to PD pathogenesis and progression [21][202]. SARS-CoV-2 virulent components, such as ORF proteins, seem to modify autophagy formation and function, leading to SARS-CoV-2-induced autophagy disruption and potentially neuron damage [203][204]. Specifically, ORF3a was shown to impede autophagosome–lysosome (A-L) fusion and ALP formation by interacting directly with the VPS39 subunit of the homotypic fusion and protein sorting (HOPS) complex. ORF3a further damages lysosomes and impairs their function. Remarkably, this feature of HOPS-VPS39-mediated A-L fusion inhibition appears to be unique to SARS-CoV-2, since the quite similar ORF3a of SARS-CoV was ineffective in inhibiting A-L fusion [205]. Furthermore, another study found that although ORF7a protein stimulates autophagy, it also limits A-L fusion progression by downregulating the SNAP29 protein via caspase 3 (CASP3) activation, providing a mechanism through which SARS-CoV-2 uses the autophagic system to facilitate its own propagation [206]. Interestingly, a SARS-CoV-2 papain-like protease has been identified to directly cleave serine/threonine unc-51-like kinase (ULK1) and prevent ULK1-ATG13 complex formation [207]. ULK1 is an upstream autophagy orchestrator, which phosphorylates key regulatory proteins in autophagosome formation [208]. In this regard, ULK1 cleavage is expected to completely inhibit the ALP function, due to lack of autophagosome formation. Evidently, autophagy is crucially involved in the regulation of the antiviral immune response. The striking correlation between SARS-CoV-2-induced aberrant inflammation and the observed autophagy defects [209] suggests that the virus-induced cytokine storm could be mediated by the failure of autophagy mechanisms to maintain cellular homeostasis.
Overall, SARS-CoV-2 seems to interfere and disrupt several host cellular and molecular pathways involved in proper neuronal functions, potentially promoting PD pathogenesis. A summary of these overlaps is depicted in (Figure 1).
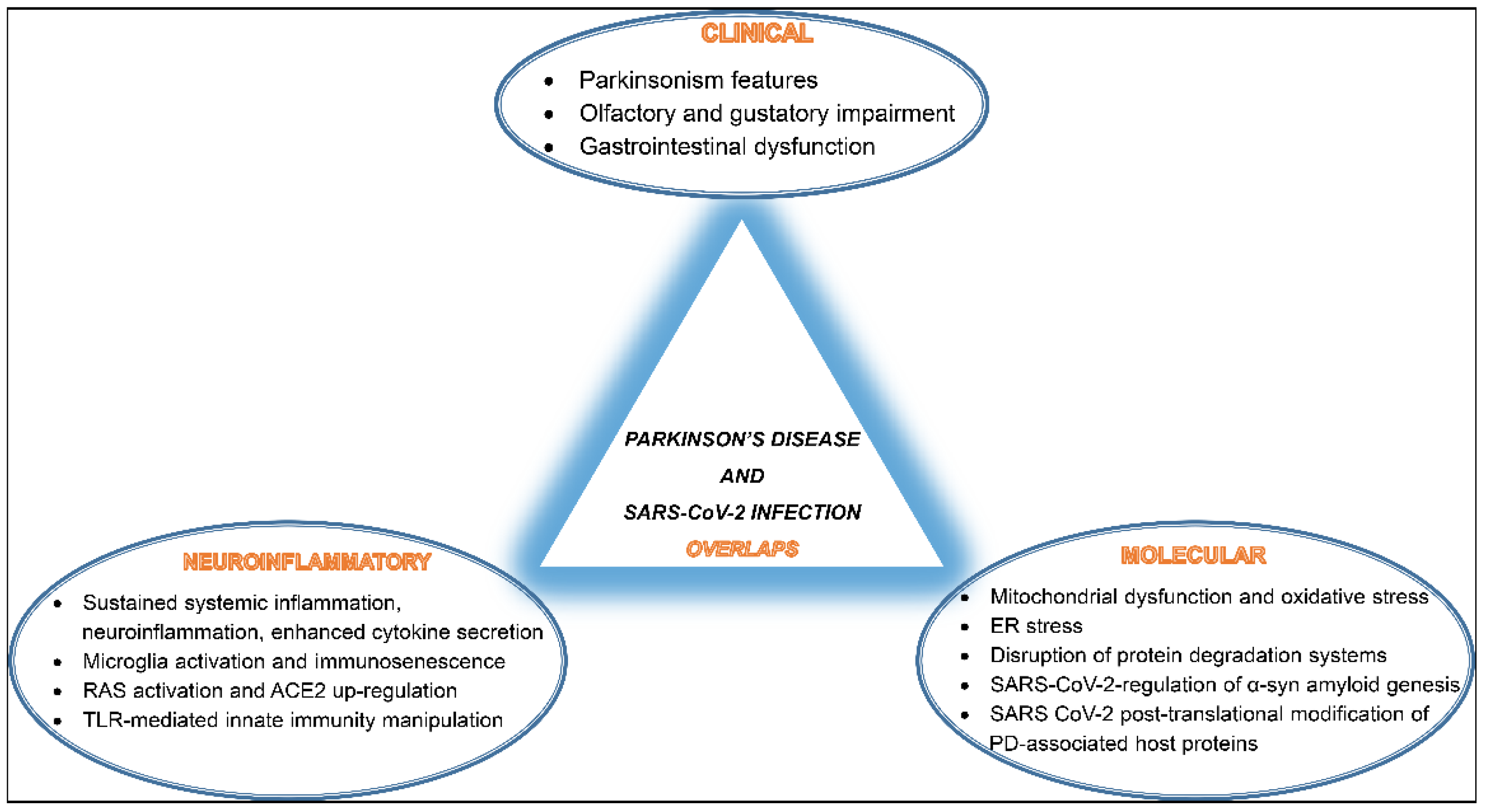
Figure 1. A schematic diagram of SARS-CoV-2 infection and Parkinson’s disease (PD) development overlaps listing shared clinical manifestations, common neuroinflammatory events, and mutually activated molecular pathways.
References
- Keener, A.M.; Bordelon, Y.M. Parkinsonism. Proc. Semin. Neurol. 2016, 36, 330–334.
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376.
- Limphaibool, N.; Iwanowski, P.; Holstad, M.J.V.; Kobylarek, D.; Kozubski, W. Infectious Etiologies of Parkinsonism: Pathomechanisms and Clinical Implications. Front. Neurol. 2019, 10, 652.
- Jang, H.; Boltz, D.A.; Webster, R.G.; Smeyne, R.J. Viral parkinsonism. Biochim. Biophys. Acta 2009, 1792, 714–721.
- Kouli, A.; Torsney, K.M.; Kuan, W.L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 2018.
- Lee, A.; Gilbert, R.M. Epidemiology of Parkinson Disease. Neurol. Clin. 2016, 34, 955–965.
- Hirsch, L.; Jette, N.; Frolkis, A.; Steeves, T.; Pringsheim, T. The Incidence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 2016, 46, 292–300.
- Twelves, D.; Perkins, K.S.; Counsell, C. Systematic review of incidence studies of Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2003, 18, 19–31.
- Karimi-Moghadam, A.; Charsouei, S.; Bell, B.; Jabalameli, M.R. Parkinson Disease from Mendelian Forms to Genetic Susceptibility: New Molecular Insights into the Neurodegeneration Process. Cell. Mol. Neurobiol. 2018, 38, 1153–1178.
- Warner, T.T.; Schapira, A.H. Genetic and environmental factors in the cause of Parkinson’s disease. Ann. Neurol. 2003, 53 (Suppl. 3), S16–S23; discussion S23–S25.
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909.
- Khoo, T.K.; Yarnall, A.J.; Duncan, G.W.; Coleman, S.; O’Brien, J.T.; Brooks, D.J.; Barker, R.A.; Burn, D.J. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology 2013, 80, 276–281.
- Pang, S.Y.; Ho, P.W.; Liu, H.F.; Leung, C.T.; Li, L.; Chang, E.E.S.; Ramsden, D.B.; Ho, S.L. The interplay of aging, genetics and environmental factors in the pathogenesis of Parkinson’s disease. Transl. Neurodegener. 2019, 8, 23.
- Smeyne, R.J.; Noyce, A.J.; Byrne, M.; Savica, R.; Marras, C. Infection and Risk of Parkinson’s Disease. J. Parkinson’s Dis. 2021, 11, 31–43.
- Baizabal-Carvallo, J.F.; Alonso-Juarez, M. The role of viruses in the pathogenesis of Parkinson’s disease. Neural Regen. Res. 2021, 16, 1200–1201.
- Yamada, T. Viral etiology of Parkinson’s disease: Focus on influenza A virus. Parkinsonism Relat. Disord. 1996, 2, 113–121.
- WHO. Coronavirus Disease (COVID-19). Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 9 August 2022).
- Brundin, P.; Nath, A.; Beckham, J.D. Is COVID-19 a Perfect Storm for Parkinson’s Disease? Trends Neurosci. 2020, 43, 931–933.
- Méndez-Guerrero, A.; Laespada-García, M.I.; Gómez-Grande, A.; Ruiz-Ortiz, M.; Blanco-Palmero, V.A.; Azcarate-Diaz, F.J.; Rábano-Suárez, P.; Álvarez-Torres, E.; de Fuenmayor-Fernández de la Hoz, C.P.; Vega Pérez, D.; et al. Acute hypokinetic-rigid syndrome following SARS-CoV-2 infection. Neurology 2020, 95, e2109–e2118.
- Faber, I.; Brandão, P.R.P.; Menegatti, F.; de Carvalho Bispo, D.D.; Maluf, F.B.; Cardoso, F. Coronavirus Disease 2019 and Parkinsonism: A Non-post-encephalitic Case. Mov. Disord. Off. J. Mov. Disord. Soc. 2020, 35, 1721–1722.
- Karabiyik, C.; Lee, M.J.; Rubinsztein, D.C. Autophagy impairment in Parkinson’s disease. Essays Biochem. 2017, 61, 711–720.
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinson’s Dis. 2013, 3, 461–491.
- Hirsch, E.C.; Vyas, S.; Hunot, S. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat. Disord. 2012, 18 (Suppl. 1), S210–S212.
- Jucker, M.; Walker, L.C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 2013, 501, 45–51.
- Guo, J.L.; Lee, V.M. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat. Med. 2014, 20, 130–138.
- Jan, A.; Gonçalves, N.P.; Vaegter, C.B.; Jensen, P.H.; Ferreira, N. The Prion-Like Spreading of Alpha-Synuclein in Parkinson’s Disease: Update on Models and Hypotheses. Int. J. Mol. Sci. 2021, 22, 8338.
- Visanji, N.P.; Brooks, P.L.; Hazrati, L.N.; Lang, A.E. The prion hypothesis in Parkinson’s disease: Braak to the future. Acta Neuropathol. Commun. 2013, 1, 2.
- Recasens, A.; Dehay, B.; Bové, J.; Carballo-Carbajal, I.; Dovero, S.; Pérez-Villalba, A.; Fernagut, P.O.; Blesa, J.; Parent, A.; Perier, C.; et al. Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neurodegeneration in mice and monkeys. Ann. Neurol. 2014, 75, 351–362.
- Hansen, C.; Angot, E.; Bergström, A.L.; Steiner, J.A.; Pieri, L.; Paul, G.; Outeiro, T.F.; Melki, R.; Kallunki, P.; Fog, K.; et al. α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J. Clin. Investig. 2011, 121, 715–725.
- Volpicelli-Daley, L.A.; Luk, K.C.; Patel, T.P.; Tanik, S.A.; Riddle, D.M.; Stieber, A.; Meaney, D.F.; Trojanowski, J.Q.; Lee, V.M. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011, 72, 57–71.
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579.
- Rastogi, S.; Sharma, V.; Bharti, P.S.; Rani, K.; Modi, G.P.; Nikolajeff, F.; Kumar, S. The Evolving Landscape of Exosomes in Neurodegenerative Diseases: Exosomes Characteristics and a Promising Role in Early Diagnosis. Int. J. Mol. Sci. 2021, 22, 440.
- Gangoda, L.; Boukouris, S.; Liem, M.; Kalra, H.; Mathivanan, S. Extracellular vesicles including exosomes are mediators of signal transduction: Are they protective or pathogenic? Proteomics 2015, 15, 260–271.
- Yuyama, K.; Igarashi, Y. Physiological and pathological roles of exosomes in the nervous system. Biomol. Concepts 2016, 7, 53–68.
- Caobi, A.; Nair, M.; Raymond, A.D. Extracellular Vesicles in the Pathogenesis of Viral Infections in Humans. Viruses 2020, 12, 1200.
- Gurunathan, S.; Kang, M.H.; Kim, J.H. Diverse Effects of Exosomes on COVID-19: A Perspective of Progress From Transmission to Therapeutic Developments. Front. Immunol. 2021, 12, 716407.
- McCall, S.; Vilensky, J.A.; Gilman, S.; Taubenberger, J.K. The relationship between encephalitis lethargica and influenza: A critical analysis. J. Neurovirology 2008, 14, 177–185.
- Wijarnpreecha, K.; Chesdachai, S.; Jaruvongvanich, V.; Ungprasert, P. Hepatitis C virus infection and risk of Parkinson’s disease: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 9–13.
- Marttila, R.J.; Rinne, U.K. Herpes simplex virus antibodies in patients with Parkinson’s disease. J. Neurol. Sci. 1978, 35, 375–379.
- Mirsattari, S.M.; Power, C.; Nath, A. Parkinsonism with HIV infection. Mov. Disord. Off. J. Mov. Disord. Soc. 1998, 13, 684–689.
- Lai, S.W.; Lin, C.H.; Lin, H.F.; Lin, C.L.; Lin, C.C.; Liao, K.F. Herpes zoster correlates with increased risk of Parkinson’s disease in older people: A population-based cohort study in Taiwan. Medicine 2017, 96, e6075.
- Robinson, R.L.; Shahida, S.; Madan, N.; Rao, S.; Khardori, N. Transient parkinsonism in West Nile virus encephalitis. Am. J. Med. 2003, 115, 252–253.
- Murgod, U.A.; Muthane, U.B.; Ravi, V.; Radhesh, S.; Desai, A. Persistent movement disorders following Japanese encephalitis. Neurology 2001, 57, 2313–2315.
- Das, K.; Ghosh, M.; Nag, C.; Nandy, S.P.; Banerjee, M.; Datta, M.; Devi, G.; Chaterjee, G. Role of familial, environmental and occupational factors in the development of Parkinson’s disease. Neuro-Degener. Dis. 2011, 8, 345–351.
- Espay, A.J.; Henderson, K.K. Postencephalitic parkinsonism and basal ganglia necrosis due to Epstein-Barr virus infection. Neurology 2011, 76, 1529–1530.
- Toovey, S.; Jick, S.S.; Meier, C.R. Parkinson’s disease or Parkinson symptoms following seasonal influenza. Influenza Other Respir. Viruses 2011, 5, 328–333.
- Cocoros, N.M.; Svensson, E.; Szépligeti, S.K.; Vestergaard, S.V.; Szentkúti, P.; Thomsen, R.W.; Borghammer, P.; Sørensen, H.T.; Henderson, V.W. Long-term Risk of Parkinson Disease Following Influenza and Other Infections. JAMA Neurol. 2021, 78, 1461–1470.
- Rohn, T.T.; Catlin, L.W. Immunolocalization of influenza A virus and markers of inflammation in the human Parkinson’s disease brain. PLoS ONE 2011, 6, e20495.
- Jang, H.; Boltz, D.; Sturm-Ramirez, K.; Shepherd, K.R.; Jiao, Y.; Webster, R.; Smeyne, R.J. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc. Natl. Acad. Sci. USA 2009, 106, 14063–14068.
- Sadasivan, S.; Zanin, M.; O’Brien, K.; Schultz-Cherry, S.; Smeyne, R.J. Induction of microglia activation after infection with the non-neurotropic A/CA/04/2009 H1N1 influenza virus. PLoS ONE 2015, 10, e0124047.
- Marreiros, R.; Müller-Schiffmann, A.; Trossbach, S.V.; Prikulis, I.; Hänsch, S.; Weidtkamp-Peters, S.; Moreira, A.R.; Sahu, S.; Soloviev, I.; Selvarajah, S.; et al. Disruption of cellular proteostasis by H1N1 influenza A virus causes α-synuclein aggregation. Proc. Natl. Acad. Sci. USA 2020, 117, 6741–6751.
- Kasen, A.; Houck, C.; Burmeister, A.R.; Sha, Q.; Brundin, L.; Brundin, P. Upregulation of α-synuclein following immune activation: Possible trigger of Parkinson’s disease. Neurobiol. Dis. 2022, 166, 105654.
- Beatman, E.L.; Massey, A.; Shives, K.D.; Burrack, K.S.; Chamanian, M.; Morrison, T.E.; Beckham, J.D. Alpha-Synuclein Expression Restricts RNA Viral Infections in the Brain. J. Virol. 2015, 90, 2767–2782.
- Caggiu, E.; Paulus, K.; Arru, G.; Piredda, R.; Sechi, G.P.; Sechi, L.A. Humoral cross reactivity between α-synuclein and herpes simplex-1 epitope in Parkinson’s disease, a triggering role in the disease? J. Neuroimmunol. 2016, 291, 110–114.
- Caggiu, E.; Paulus, K.; Galleri, G.; Arru, G.; Manetti, R.; Sechi, G.P.; Sechi, L.A. Homologous HSV1 and alpha-synuclein peptides stimulate a T cell response in Parkinson’s disease. J. Neuroimmunol. 2017, 310, 26–31.
- Fazzini, E.; Fleming, J.; Fahn, S. Cerebrospinal fluid antibodies to coronavirus in patients with Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 1992, 7, 153–158.
- Almaghaslah, D.; Kandasamy, G.; Almanasef, M.; Vasudevan, R.; Chandramohan, S. Review on the coronavirus disease (COVID-19) pandemic: Its outbreak and current status. Int. J. Clin. Pract. 2020, 74, e13637.
- Wu, J.T.; Leung, K.; Leung, G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: A modelling study. Lancet 2020, 395, 689–697.
- Hui, D.S.; Azhar, E.I.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; McHugh, T.D.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health-The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020, 91, 264–266.
- Johansson, M.A.; Quandelacy, T.M.; Kada, S.; Prasad, P.V.; Steele, M.; Brooks, J.T.; Slayton, R.B.; Biggerstaff, M.; Butler, J.C. SARS-CoV-2 Transmission From People Without COVID-19 Symptoms. JAMA Netw. Open 2021, 4, e2035057.
- Zhou, L.; Ayeh, S.K.; Chidambaram, V.; Karakousis, P.C. Modes of transmission of SARS-CoV-2 and evidence for preventive behavioral interventions. BMC Infect. Dis. 2021, 21, 496.
- Harapan, B.N.; Yoo, H.J. Neurological symptoms, manifestations, and complications associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19). J. Neurol. 2021, 268, 3059–3071.
- Paterson, R.W.; Brown, R.L.; Benjamin, L.; Nortley, R.; Wiethoff, S.; Bharucha, T.; Jayaseelan, D.L.; Kumar, G.; Raftopoulos, R.E.; Zambreanu, L.; et al. The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings. Brain J. Neurol. 2020, 143, 3104–3120.
- Beghi, E.; Giussani, G.; Westenberg, E.; Allegri, R.; Garcia-Azorin, D.; Guekht, A.; Frontera, J.; Kivipelto, M.; Mangialasche, F.; Mukaetova-Ladinska, E.B.; et al. Acute and post-acute neurological manifestations of COVID-19: Present findings, critical appraisal, and future directions. J. Neurol. 2022, 269, 2265–2274.
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021, 18, e1003773.
- National Institute for Health and Care Excellence (NICE). National Institute for Health and Care Excellence: Clinical Guidelines. In COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19; National Institute for Health and Care Excellence (NICE): London, UK, 2020.
- Stephenson, J. New Federal Guidance Says COVID-19’s Long-term Effects Can Qualify as a Disability. JAMA Health Forum 2021, 2, e212820.
- Mehandru, S.; Merad, M. Pathological sequelae of long-haul COVID. Nat. Immunol. 2022, 23, 194–202.
- Camargo-Martínez, W.; Lozada-Martínez, I.; Escobar-Collazos, A.; Navarro-Coronado, A.; Moscote-Salazar, L.; Pacheco-Hernández, A.; Janjua, T.; Bosque-Varela, P. Post-COVID 19 neurological syndrome: Implications for sequelae’s treatment. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2021, 88, 219–225.
- Shah, W.; Hillman, T.; Playford, E.D.; Hishmeh, L. Managing the long term effects of covid-19: Summary of NICE, SIGN, and RCGP rapid guideline. BMJ 2021, 372, n136.
- Khorramdelazad, H.; Kazemi, M.H.; Najafi, A.; Keykhaee, M.; Zolfaghari Emameh, R.; Falak, R. Immunopathological similarities between COVID-19 and influenza: Investigating the consequences of Co-infection. Microb. Pathog. 2021, 152, 104554.
- Cohen, M.E.; Eichel, R.; Steiner-Birmanns, B.; Janah, A.; Ioshpa, M.; Bar-Shalom, R.; Paul, J.J.; Gaber, H.; Skrahina, V.; Bornstein, N.M.; et al. A case of probable Parkinson’s disease after SARS-CoV-2 infection. Lancet Neurol. 2020, 19, 804–805.
- Rao, A.R.; Hidayathullah, S.M.; Hegde, K.; Adhikari, P. Parkinsonism: An emerging post COVID sequelae. IDCases 2022, 27, e01388.
- Boura, I.; Chaudhuri, K.R. Coronavirus Disease 2019 and Related Parkinsonism: The Clinical Evidence Thus Far. Mov. Disord. Clin. Pract. 2022, 9, 584–593.
- Cilia, R.; Bonvegna, S.; Straccia, G.; Andreasi, N.G.; Elia, A.E.; Romito, L.M.; Devigili, G.; Cereda, E.; Eleopra, R. Effects of COVID-19 on Parkinson’s Disease Clinical Features: A Community-Based Case-Control Study. Mov. Disord. Off. J. Mov. Disord. Soc. 2020, 35, 1287–1292.
- Brown, E.G.; Chahine, L.M.; Goldman, S.M.; Korell, M.; Mann, E.; Kinel, D.R.; Arnedo, V.; Marek, K.L.; Tanner, C.M. The Effect of the COVID-19 Pandemic on People with Parkinson’s Disease. J. Parkinson’s Dis. 2020, 10, 1365–1377.
- Montenegro, P.; Moral, I.; Puy, A.; Cordero, E.; Chantada, N.; Cuixart, L.; Brotons, C. Prevalence of Post COVID-19 Condition in Primary Care: A Cross Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 1836.
- Nasserie, T.; Hittle, M.; Goodman, S.N. Assessment of the Frequency and Variety of Persistent Symptoms Among Patients With COVID-19: A Systematic Review. JAMA Netw. Open 2021, 4, e2111417.
- Leta, V.; Rodríguez-Violante, M.; Abundes, A.; Rukavina, K.; Teo, J.T.; Falup-Pecurariu, C.; Irincu, L.; Rota, S.; Bhidayasiri, R.; Storch, A.; et al. Parkinson’s Disease and Post-COVID-19 Syndrome: The Parkinson’s Long-COVID Spectrum. Mov. Disord. Off. J. Mov. Disord. Soc. 2021, 36, 1287–1289.
- Doty, R.L. Olfactory dysfunction in Parkinson disease. Nat. Rev. Neurol. 2012, 8, 329–339.
- Tarakad, A.; Jankovic, J. Anosmia and Ageusia in Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 133, 541–556.
- Augustin, M.; Schommers, P.; Stecher, M.; Dewald, F.; Gieselmann, L.; Gruell, H.; Horn, C.; Vanshylla, K.; Cristanziano, V.D.; Osebold, L.; et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: A longitudinal prospective cohort study. Lancet Reg. Health. Eur. 2021, 6, 100122.
- Vavougios, G.D. Potentially irreversible olfactory and gustatory impairments in COVID-19: Indolent vs. fulminant SARS-CoV-2 neuroinfection. Brain Behav. Immun. 2020, 87, 107–108.
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175.
- Brann, D.H.; Tsukahara, T.; Weinreb, C.; Lipovsek, M.; Van den Berge, K.; Gong, B.; Chance, R.; Macaulay, I.C.; Chou, H.J.; Fletcher, R.B.; et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020, 6, eabc5801.
- Hubbard, P.S.; Esiri, M.M.; Reading, M.; McShane, R.; Nagy, Z. Alpha-synuclein pathology in the olfactory pathways of dementia patients. J. Anat. 2007, 211, 117–124.
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211.
- Braak, H.; Del Tredici, K. Neuropathological Staging of Brain Pathology in Sporadic Parkinson’s disease: Separating the Wheat from the Chaff. J. Parkinson’s Dis. 2017, 7, S71–S85.
- Beach, T.G.; White, C.L., 3rd; Hladik, C.L.; Sabbagh, M.N.; Connor, D.J.; Shill, H.A.; Sue, L.I.; Sasse, J.; Bachalakuri, J.; Henry-Watson, J.; et al. Olfactory bulb alpha-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol. 2009, 117, 169–174.
- Cersosimo, M.G.; Raina, G.B.; Pecci, C.; Pellene, A.; Calandra, C.R.; Gutiérrez, C.; Micheli, F.E.; Benarroch, E.E. Gastrointestinal manifestations in Parkinson’s disease: Prevalence and occurrence before motor symptoms. J. Neurol. 2013, 260, 1332–1338.
- Devos, D.; Lebouvier, T.; Lardeux, B.; Biraud, M.; Rouaud, T.; Pouclet, H.; Coron, E.; Bruley des Varannes, S.; Naveilhan, P.; Nguyen, J.M.; et al. Colonic inflammation in Parkinson’s disease. Neurobiol. Dis. 2013, 50, 42–48.
- Huang, Y.; Liao, J.; Liu, X.; Zhong, Y.; Cai, X.; Long, L. Review: The Role of Intestinal Dysbiosis in Parkinson’s Disease. Front. Cell. Infect. Microbiol. 2021, 11, 615075.
- Klingelhoefer, L.; Reichmann, H. Pathogenesis of Parkinson disease--the gut-brain axis and environmental factors. Nat. Rev. Neurol. 2015, 11, 625–636.
- Shannon, K.M.; Keshavarzian, A.; Dodiya, H.B.; Jakate, S.; Kordower, J.H. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov. Disord. Off. J. Mov. Disord. Soc. 2012, 27, 716–719.
- Braak, H.; Rüb, U.; Gai, W.P.; Del Tredici, K. Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. 2003, 110, 517–536.
- Anselmi, L.; Toti, L.; Bove, C.; Hampton, J.; Travagli, R.A. A Nigro-Vagal Pathway Controls Gastric Motility and Is Affected in a Rat Model of Parkinsonism. Gastroenterology 2017, 153, 1581–1593.
- Beach, T.G.; Adler, C.H.; Sue, L.I.; Vedders, L.; Lue, L.; White Iii, C.L.; Akiyama, H.; Caviness, J.N.; Shill, H.A.; Sabbagh, M.N.; et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010, 119, 689–702.
- Gelpi, E.; Navarro-Otano, J.; Tolosa, E.; Gaig, C.; Compta, Y.; Rey, M.J.; Martí, M.J.; Hernández, I.; Valldeoriola, F.; Reñé, R.; et al. Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov. Disord. Off. J. Mov. Disord. Soc. 2014, 29, 1010–1018.
- Kim, S.; Kwon, S.H.; Kam, T.I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; et al. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627–641.e7.
- Chaves Andrade, M.; Souza de Faria, R.; Avelino Mota Nobre, S. COVID-19: Can the symptomatic SARS-CoV-2 infection affect the homeostasis of the gut-brain-microbiota axis? Med. Hypotheses 2020, 144, 110206.
- Xu, J.; Wu, Z.; Zhang, M.; Liu, S.; Zhou, L.; Yang, C.; Liu, C. The Role of the Gastrointestinal System in Neuroinvasion by SARS-CoV-2. Front. Neurosci. 2021, 15, 694446.
- Tao, W.; Wang, X.; Zhang, G.; Guo, M.; Ma, H.; Zhao, D.; Sun, Y.; He, J.; Liu, L.; Zhang, K.; et al. Re-detectable positive SARS-CoV-2 RNA tests in patients who recovered from COVID-19 with intestinal infection. Protein Cell 2021, 12, 230–235.
- Kaźmierczak-Siedlecka, K.; Vitale, E.; Makarewicz, W. COVID-19-gastrointestinal and gut microbiota-related aspects. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10853–10859.
- Viana, S.D.; Nunes, S.; Reis, F. ACE2 imbalance as a key player for the poor outcomes in COVID-19 patients with age-related comorbidities-Role of gut microbiota dysbiosis. Ageing Res. Rev. 2020, 62, 101123.
- Mohajeri, M.H.; La Fata, G.; Steinert, R.E.; Weber, P. Relationship between the gut microbiome and brain function. Nutr. Rev. 2018, 76, 481–496.
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932.
- Troncoso-Escudero, P.; Parra, A.; Nassif, M.; Vidal, R.L. Outside in: Unraveling the Role of Neuroinflammation in the Progression of Parkinson’s Disease. Front. Neurol. 2018, 9, 860.
- Marogianni, C.; Sokratous, M.; Dardiotis, E.; Hadjigeorgiou, G.M.; Bogdanos, D.; Xiromerisiou, G. Neurodegeneration and Inflammation-An Interesting Interplay in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8421.
- Majde, J.A. Neuroinflammation resulting from covert brain invasion by common viruses-a potential role in local and global neurodegeneration. Med. Hypotheses 2010, 75, 204–213.
- Almutairi, M.M.; Sivandzade, F.; Albekairi, T.H.; Alqahtani, F.; Cucullo, L. Neuroinflammation and Its Impact on the Pathogenesis of COVID-19. Front. Med. 2021, 8, 745789.
- Bauer, L.; Laksono, B.M.; de Vrij, F.M.S.; Kushner, S.A.; Harschnitz, O.; van Riel, D. The neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2. Trends Neurosci. 2022, 45, 358–368.
- Yachou, Y.; El Idrissi, A.; Belapasov, V.; Ait Benali, S. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: Understanding the neurological manifestations in COVID-19 patients. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2020, 41, 2657–2669.
- Cárdenas, G.; Fragoso, G.; Sciutto, E. Neuroinflammation in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) infection: Pathogenesis and clinical manifestations. Curr. Opin. Pharmacol. 2022, 63, 102181.
- Rowaiye, A.B.; Okpalefe, O.A.; Onuh Adejoke, O.; Ogidigo, J.O.; Hannah Oladipo, O.; Ogu, A.C.; Oli, A.N.; Olofinase, S.; Onyekwere, O.; Rabiu Abubakar, A.; et al. Attenuating the Effects of Novel COVID-19 (SARS-CoV-2) Infection-Induced Cytokine Storm and the Implications. J. Inflamm. Res. 2021, 14, 1487–1510.
- Olbei, M.; Hautefort, I.; Modos, D.; Treveil, A.; Poletti, M.; Gul, L.; Shannon-Lowe, C.D.; Korcsmaros, T. SARS-CoV-2 Causes a Different Cytokine Response Compared to Other Cytokine Storm-Causing Respiratory Viruses in Severely Ill Patients. Front. Immunol. 2021, 12, 629193.
- Islam, H.; Chamberlain, T.C.; Mui, A.L.; Little, J.P. Elevated Interleukin-10 Levels in COVID-19: Potentiation of Pro-Inflammatory Responses or Impaired Anti-Inflammatory Action? Front. Immunol. 2021, 12, 677008.
- Santa Cruz, A.; Mendes-Frias, A.; Oliveira, A.I.; Dias, L.; Matos, A.R.; Carvalho, A.; Capela, C.; Pedrosa, J.; Castro, A.G.; Silvestre, R. Interleukin-6 Is a Biomarker for the Development of Fatal Severe Acute Respiratory Syndrome Coronavirus 2 Pneumonia. Front. Immunol. 2021, 12, 613422.
- Lu, Q.; Zhu, Z.; Tan, C.; Zhou, H.; Hu, Y.; Shen, G.; Zhu, P.; Yang, G.; Xie, X. Changes of serum IL-10, IL-1β, IL-6, MCP-1, TNF-α, IP-10 and IL-4 in COVID-19 patients. Int. J. Clin. Pract. 2021, 75, e14462.
- Mehta, P.; Fajgenbaum, D.C. Is severe COVID-19 a cytokine storm syndrome: A hyperinflammatory debate. Curr. Opin. Rheumatol. 2021, 33, 419–430.
- Chen, H.; O’Reilly, E.J.; Schwarzschild, M.A.; Ascherio, A. Peripheral inflammatory biomarkers and risk of Parkinson’s disease. Am. J. Epidemiol. 2008, 167, 90–95.
- Hsu, R.J.; Yu, W.C.; Peng, G.R.; Ye, C.H.; Hu, S.; Chong, P.C.T.; Yap, K.Y.; Lee, J.Y.C.; Lin, W.C.; Yu, S.H. The Role of Cytokines and Chemokines in Severe Acute Respiratory Syndrome Coronavirus 2 Infections. Front. Immunol. 2022, 13, 832394.
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69.
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185.
- Yu, X.; Ji, C.; Shao, A. Neurovascular Unit Dysfunction and Neurodegenerative Disorders. Front. Neurosci. 2020, 14, 334.
- Klein, R.S.; Garber, C.; Funk, K.E.; Salimi, H.; Soung, A.; Kanmogne, M.; Manivasagam, S.; Agner, S.; Cain, M. Neuroinflammation During RNA Viral Infections. Annu. Rev. Immunol. 2019, 37, 73–95.
- Yang, R.C.; Huang, K.; Zhang, H.P.; Li, L.; Zhang, Y.F.; Tan, C.; Chen, H.C.; Jin, M.L.; Wang, X.R. SARS-CoV-2 productively infects human brain microvascular endothelial cells. J. Neuroinflamm. 2022, 19, 149.
- Raghavan, S.; Kenchappa, D.B.; Leo, M.D. SARS-CoV-2 Spike Protein Induces Degradation of Junctional Proteins That Maintain Endothelial Barrier Integrity. Front. Cardiovasc. Med. 2021, 8, 687783.
- Reynolds, J.L.; Mahajan, S.D. SARS-COV2 Alters Blood Brain Barrier Integrity Contributing to Neuro-Inflammation. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2021, 16, 4–6.
- John, G.R.; Lee, S.C.; Brosnan, C.F. Cytokines: Powerful regulators of glial cell activation. Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2003, 9, 10–22.
- da Fonseca, A.C.; Matias, D.; Garcia, C.; Amaral, R.; Geraldo, L.H.; Freitas, C.; Lima, F.R. The impact of microglial activation on blood-brain barrier in brain diseases. Front. Cell. Neurosci. 2014, 8, 362.
- Rama Rao, K.V.; Kielian, T. Neuron-astrocyte interactions in neurodegenerative diseases: Role of neuroinflammation. Clin. Exp. Neuroimmunol. 2015, 6, 245–263.
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487.
- Koprich, J.B.; Reske-Nielsen, C.; Mithal, P.; Isacson, O. Neuroinflammation mediated by IL-1beta increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson’s disease. J. Neuroinflamm. 2008, 5, 8.
- Frank, M.G.; Nguyen, K.H.; Ball, J.B.; Hopkins, S.; Kelley, T.; Baratta, M.V.; Fleshner, M.; Maier, S.F. SARS-CoV-2 spike S1 subunit induces neuroinflammatory, microglial and behavioral sickness responses: Evidence of PAMP-like properties. Brain Behav. Immun. 2022, 100, 267–277.
- Chaudhry, Z.L.; Klenja, D.; Janjua, N.; Cami-Kobeci, G.; Ahmed, B.Y. COVID-19 and Parkinson’s Disease: Shared Inflammatory Pathways Under Oxidative Stress. Brain Sci. 2020, 10, 807.
- Kumar, D.; Jahan, S.; Khan, A.; Siddiqui, A.J.; Redhu, N.S.; Wahajuddin; Khan, J.; Banwas, S.; Alshehri, B.; Alaidarous, M. Neurological Manifestation of SARS-CoV-2 Induced Inflammation and Possible Therapeutic Strategies Against COVID-19. Mol. Neurobiol. 2021, 58, 3417–3434.
- Sahu, M.R.; Rani, L.; Subba, R.; Mondal, A.C. Cellular senescence in the aging brain: A promising target for neurodegenerative diseases. Mech. Ageing Dev. 2022, 204, 111675.
- Martínez-Cué, C.; Rueda, N. Cellular Senescence in Neurodegenerative Diseases. Front. Cell. Neurosci. 2020, 14, 16.
- Tripathi, U.; Nchioua, R.; Prata, L.; Zhu, Y.; Gerdes, E.O.W.; Giorgadze, N.; Pirtskhalava, T.; Parker, E.; Xue, A.; Espindola-Netto, J.M.; et al. SARS-CoV-2 causes senescence in human cells and exacerbates the senescence-associated secretory phenotype through TLR-3. Aging 2021, 13, 21838–21854.
- Kandhaya-Pillai, R.; Yang, X.; Tchkonia, T.; Martin, G.M.; Kirkland, J.L.; Oshima, J. TNF-α/IFN-γ synergy amplifies senescence-associated inflammation and SARS-CoV-2 receptor expression via hyper-activated JAK/STAT1. Aging Cell 2022, 21, e13646.
- Müller, L.; Di Benedetto, S. How Immunosenescence and Inflammaging May Contribute to Hyperinflammatory Syndrome in COVID-19. Int. J. Mol. Sci. 2021, 22, 12539.
- Matschke, J.; Lütgehetmann, M.; Hagel, C.; Sperhake, J.P.; Schröder, A.S.; Edler, C.; Mushumba, H.; Fitzek, A.; Allweiss, L.; Dandri, M.; et al. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 2020, 19, 919–929.
- Schurink, B.; Roos, E.; Radonic, T.; Barbe, E.; Bouman, C.S.C.; de Boer, H.H.; de Bree, G.J.; Bulle, E.B.; Aronica, E.M.; Florquin, S.; et al. Viral presence and immunopathology in patients with lethal COVID-19: A prospective autopsy cohort study. Lancet Microbe 2020, 1, e290–e299.
- Yang, A.C.; Kern, F.; Losada, P.M.; Agam, M.R.; Maat, C.A.; Schmartz, G.P.; Fehlmann, T.; Stein, J.A.; Schaum, N.; Lee, D.P.; et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature 2021, 595, 565–571.
- Vavougios, G.D.; Breza, M.; Mavridis, T.; Krogfelt, K.A. FYN, SARS-CoV-2, and IFITM3 in the neurobiology of Alzheimer’s disease. Brain Disord. 2021, 3, 100022.
- Clairembault, T.; Kamphuis, W.; Leclair-Visonneau, L.; Rolli-Derkinderen, M.; Coron, E.; Neunlist, M.; Hol, E.M.; Derkinderen, P. Enteric GFAP expression and phosphorylation in Parkinson’s disease. J. Neurochem. 2014, 130, 805–815.
- Rodriguez-Perez, A.I.; Garrido-Gil, P.; Pedrosa, M.A.; Garcia-Garrote, M.; Valenzuela, R.; Navarro, G.; Franco, R.; Labandeira-Garcia, J.L. Angiotensin type 2 receptors: Role in aging and neuroinflammation in the substantia nigra. Brain Behav. Immun. 2020, 87, 256–271.
- Paul, D.; Mohankumar, S.K.; Thomas, R.S.; Kheng, C.B.; Basavan, D. Potential Implications of Angiotensin-converting Enzyme 2 Blockades on Neuroinflammation in SARS-CoV-2 Infection. Curr. Drug Targets 2022, 23, 364–372.
- Williams, A.; Branscome, H.; Khatkar, P.; Mensah, G.A.; Al Sharif, S.; Pinto, D.O.; DeMarino, C.; Kashanchi, F. A comprehensive review of COVID-19 biology, diagnostics, therapeutics, and disease impacting the central nervous system. J. Neurovirol. 2021, 27, 667–690.
- Pavel, A.; Murray, D.K.; Stoessl, A.J. COVID-19 and selective vulnerability to Parkinson’s disease. Lancet Neurol. 2020, 19, 719.
- Klingenstein, M.; Klingenstein, S.; Neckel, P.H.; Mack, A.F.; Wagner, A.P.; Kleger, A.; Liebau, S.; Milazzo, A. Evidence of SARS-CoV2 Entry Protein ACE2 in the Human Nose and Olfactory Bulb. Cells Tissues Organs 2020, 209, 155–164.
- Chen, R.; Wang, K.; Yu, J.; Howard, D.; French, L.; Chen, Z.; Wen, C.; Xu, Z. The Spatial and Cell-Type Distribution of SARS-CoV-2 Receptor ACE2 in the Human and Mouse Brains. Front. Neurol. 2020, 11, 573095.
- Wan, D.; Du, T.; Hong, W.; Chen, L.; Que, H.; Lu, S.; Peng, X. Neurological complications and infection mechanism of SARS-COV-2. Signal Transduct. Target. Ther. 2021, 6, 406.
- Yang, L.; Han, Y.; Nilsson-Payant, B.E.; Gupta, V.; Wang, P.; Duan, X.; Tang, X.; Zhu, J.; Zhao, Z.; Jaffré, F.; et al. A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids. Cell Stem Cell 2020, 27, 125–136.e7.
- Aboudounya, M.M.; Heads, R.J. COVID-19 and Toll-Like Receptor 4 (TLR4): SARS-CoV-2 May Bind and Activate TLR4 to Increase ACE2 Expression, Facilitating Entry and Causing Hyperinflammation. Mediat. Inflamm. 2021, 2021, 8874339.
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461.
- Conte, C. Possible Link between SARS-CoV-2 Infection and Parkinson’s Disease: The Role of Toll-Like Receptor 4. Int. J. Mol. Sci. 2021, 22, 7135.
- Lecours, C.; Bordeleau, M.; Cantin, L.; Parent, M.; Paolo, T.D.; Tremblay, M. Microglial Implication in Parkinson’s Disease: Loss of Beneficial Physiological Roles or Gain of Inflammatory Functions? Front. Cell. Neurosci. 2018, 12, 282.
- Estrada, E. Cascading from SARS-CoV-2 to Parkinson’s Disease through Protein-Protein Interactions. Viruses 2021, 13, 897.
- Wen, H.; Zhan, L.; Chen, S.; Long, L.; Xu, E. Rab7 may be a novel therapeutic target for neurologic diseases as a key regulator in autophagy. J. Neurosci. Res. 2017, 95, 1993–2004.
- Shin, W.H.; Park, J.H.; Chung, K.C. The central regulator p62 between ubiquitin proteasome system and autophagy and its role in the mitophagy and Parkinson’s disease. BMB Rep. 2020, 53, 56–63.
- Khan, M.T.; Irfan, M.; Ahsan, H.; Ahmed, A.; Kaushik, A.C.; Khan, A.S.; Chinnasamy, S.; Ali, A.; Wei, D.Q. Structures of SARS-CoV-2 RNA-Binding Proteins and Therapeutic Targets. Intervirology 2021, 64, 55–68.
- Semerdzhiev, S.A.; Fakhree, M.A.A.; Segers-Nolten, I.; Blum, C.; Claessens, M. Interactions between SARS-CoV-2 N-Protein and α-Synuclein Accelerate Amyloid Formation. ACS Chem. Neurosci. 2022, 13, 143–150.
- Malkus, K.A.; Tsika, E.; Ischiropoulos, H. Oxidative modifications, mitochondrial dysfunction, and impaired protein degradation in Parkinson’s disease: How neurons are lost in the Bermuda triangle. Mol. Neurodegener. 2009, 4, 24.
- Zeng, X.S.; Geng, W.S.; Jia, J.J.; Chen, L.; Zhang, P.P. Cellular and Molecular Basis of Neurodegeneration in Parkinson Disease. Front. Aging Neurosci. 2018, 10, 109.
- Fujita, K.A.; Ostaszewski, M.; Matsuoka, Y.; Ghosh, S.; Glaab, E.; Trefois, C.; Crespo, I.; Perumal, T.M.; Jurkowski, W.; Antony, P.M.; et al. Integrating pathways of Parkinson’s disease in a molecular interaction map. Mol. Neurobiol. 2014, 49, 88–102.
- Henchcliffe, C.; Beal, M.F. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat. Clin. Pract. Neurol. 2008, 4, 600–609.
- Abou-Sleiman, P.M.; Muqit, M.M.; Wood, N.W. Expanding insights of mitochondrial dysfunction in Parkinson’ disease. Nat. Rev. Neurosci. 2006, 7, 207–219.
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659.
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2000, 7, 153–163.
- Subramaniam, S.R.; Vergnes, L.; Franich, N.R.; Reue, K.; Chesselet, M.F. Region specific mitochondrial impairment in mice with widespread overexpression of alpha-synuclein. Neurobiol. Dis. 2014, 70, 204–213.
- Hattori, N.; Tanaka, M.; Ozawa, T.; Mizuno, Y. Immunohistochemical studies on complexes I, II, III, and IV of mitochondria in Parkinson’s disease. Ann. Neurol. 1991, 30, 563–571.
- Parker, W.D., Jr.; Parks, J.K.; Swerdlow, R.H. Complex I deficiency in Parkinson’s disease frontal cortex. Brain Res. 2008, 1189, 215–218.
- Martin, L.J.; Pan, Y.; Price, A.C.; Sterling, W.; Copeland, N.G.; Jenkins, N.A.; Price, D.L.; Lee, M.K. Parkinson’s disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 41–50.
- Gatti, P.; Ilamathi, H.S.; Todkar, K.; Germain, M. Mitochondria Targeted Viral Replication and Survival Strategies-Prospective on SARS-CoV-2. Front. Pharmacol. 2020, 11, 578599.
- Singh, K.K.; Chaubey, G.; Chen, J.Y.; Suravajhala, P. Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am. J. Physiol. Cell Physiol. 2020, 319, C258–C267.
- Burtscher, J.; Cappellano, G.; Omori, A.; Koshiba, T.; Millet, G.P. Mitochondria: In the Cross Fire of SARS-CoV-2 and Immunity. iScience 2020, 23, 101631.
- Morowitz, J.M.; Pogson, K.B.; Roque, D.A.; Church, F.C. Role of SARS-CoV-2 in Modifying Neurodegenerative Processes in Parkinson’s Disease: A Narrative Review. Brain Sci. 2022, 12, 536.
- Suhail, S.; Zajac, J.; Fossum, C.; Lowater, H.; McCracken, C.; Severson, N.; Laatsch, B.; Narkiewicz-Jodko, A.; Johnson, B.; Liebau, J.; et al. Role of Oxidative Stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) Infection: A Review. Protein J. 2020, 39, 644–656.
- Chernyak, B.V.; Popova, E.N.; Prikhodko, A.S.; Grebenchikov, O.A.; Zinovkina, L.A.; Zinovkin, R.A. COVID-19 and Oxidative Stress. Biochem. Biokhimiia 2020, 85, 1543–1553.
- Shang, C.; Liu, Z.; Zhu, Y.; Lu, J.; Ge, C.; Zhang, C.; Li, N.; Jin, N.; Li, Y.; Tian, M.; et al. SARS-CoV-2 Causes Mitochondrial Dysfunction and Mitophagy Impairment. Front. Microbiol. 2021, 12, 780768.
- Tiku, V.; Tan, M.W.; Dikic, I. Mitochondrial Functions in Infection and Immunity. Trends Cell Biol. 2020, 30, 263–275.
- Shi, T.T.; Yang, F.Y.; Liu, C.; Cao, X.; Lu, J.; Zhang, X.L.; Yuan, M.X.; Chen, C.; Yang, J.K. Angiotensin-converting enzyme 2 regulates mitochondrial function in pancreatic β-cells. Biochem. Biophys. Res. Commun. 2018, 495, 860–866.
- Bordt, E.A.; Polster, B.M. NADPH oxidase- and mitochondria-derived reactive oxygen species in proinflammatory microglial activation: A bipartisan affair? Free Radic. Biol. Med. 2014, 76, 34–46.
- Clough, E.; Inigo, J.; Chandra, D.; Chaves, L.; Reynolds, J.L.; Aalinkeel, R.; Schwartz, S.A.; Khmaladze, A.; Mahajan, S.D. Mitochondrial Dynamics in SARS-COV2 Spike Protein Treated Human Microglia: Implications for Neuro-COVID. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2021, 16, 770–784.
- Omura, T.; Kaneko, M.; Okuma, Y.; Matsubara, K.; Nomura, Y. Endoplasmic reticulum stress and Parkinson’s disease: The role of HRD1 in averting apoptosis in neurodegenerative disease. Oxidative Med. Cell. Longev. 2013, 2013, 239854.
- Colla, E. Linking the Endoplasmic Reticulum to Parkinson’s Disease and Alpha-Synucleinopathy. Front. Neurosci. 2019, 13, 560.
- Hitomi, J.; Katayama, T.; Eguchi, Y.; Kudo, T.; Taniguchi, M.; Koyama, Y.; Manabe, T.; Yamagishi, S.; Bando, Y.; Imaizumi, K.; et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J. Cell Biol. 2004, 165, 347–356.
- Marciniak, S.J.; Yun, C.Y.; Oyadomari, S.; Novoa, I.; Zhang, Y.; Jungreis, R.; Nagata, K.; Harding, H.P.; Ron, D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004, 18, 3066–3077.
- Bartolini, D.; Stabile, A.M.; Vacca, C.; Pistilli, A.; Rende, M.; Gioiello, A.; Cruciani, G.; Galli, F. Endoplasmic reticulum stress and NF-kB activation in SARS-CoV-2 infected cells and their response to antiviral therapy. IUBMB Life 2022, 74, 93–100.
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468.
- Rashid, F.; Dzakah, E.E.; Wang, H.; Tang, S. The ORF8 protein of SARS-CoV-2 induced endoplasmic reticulum stress and mediated immune evasion by antagonizing production of interferon beta. Virus Res. 2021, 296, 198350.
- Jiang, P.; Gan, M.; Ebrahim, A.S.; Lin, W.L.; Melrose, H.L.; Yen, S.H. ER stress response plays an important role in aggregation of α-synuclein. Mol. Neurodegener. 2010, 5, 56.
- Chaudhari, N.; Talwar, P.; Parimisetty, A.; Lefebvre d’Hellencourt, C.; Ravanan, P. A molecular web: Endoplasmic reticulum stress, inflammation, and oxidative stress. Front. Cell. Neurosci. 2014, 8, 213.
- Jones, J.T.; Qian, X.; van der Velden, J.L.; Chia, S.B.; McMillan, D.H.; Flemer, S.; Hoffman, S.M.; Lahue, K.G.; Schneider, R.W.; Nolin, J.D.; et al. Glutathione S-transferase pi modulates NF-κB activation and pro-inflammatory responses in lung epithelial cells. Redox Biol. 2016, 8, 375–382.
- Li, W.; Qiao, J.; You, Q.; Zong, S.; Peng, Q.; Liu, Y.; Hu, S.; Liu, W.; Li, S.; Shu, X.; et al. SARS-CoV-2 Nsp5 Activates NF-κB Pathway by Upregulating SUMOylation of MAVS. Front. Immunol. 2021, 12, 750969.
- Hunot, S.; Brugg, B.; Ricard, D.; Michel, P.P.; Muriel, M.P.; Ruberg, M.; Faucheux, B.A.; Agid, Y.; Hirsch, E.C. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease. Proc. Natl. Acad. Sci. USA 1997, 94, 7531–7536.
- Dolatshahi, M.; Ranjbar Hameghavandi, M.H.; Sabahi, M.; Rostamkhani, S. Nuclear factor-kappa B (NF-κB) in pathophysiology of Parkinson disease: Diverse patterns and mechanisms contributing to neurodegeneration. Eur. J. Neurosci. 2021, 54, 4101–4123.
- Bellucci, A.; Bubacco, L.; Longhena, F.; Parrella, E.; Faustini, G.; Porrini, V.; Bono, F.; Missale, C.; Pizzi, M. Nuclear Factor-κB Dysregulation and α-Synuclein Pathology: Critical Interplay in the Pathogenesis of Parkinson’s Disease. Front. Aging Neurosci. 2020, 12, 68.
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Rathore, A.S.; Singh, S.P. NF-κB-Mediated Neuroinflammation in Parkinson’s Disease and Potential Therapeutic Effect of Polyphenols. Neurotox. Res. 2020, 37, 491–507.
- Ebrahimi-Fakhari, D.; Cantuti-Castelvetri, I.; Fan, Z.; Rockenstein, E.; Masliah, E.; Hyman, B.T.; McLean, P.J.; Unni, V.K. Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of α-synuclein. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 14508–14520.
- Dehay, B.; Martinez-Vicente, M.; Caldwell, G.A.; Caldwell, K.A.; Yue, Z.; Cookson, M.R.; Klein, C.; Vila, M.; Bezard, E. Lysosomal impairment in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2013, 28, 725–732.
- Lakshmana, M.K. SARS-CoV-2-induced autophagy dysregulation may cause neuronal dysfunction in COVID-19. Neural Regen. Res. 2022, 17, 1255–1256.
- Singh, K.; Chen, Y.C.; Hassanzadeh, S.; Han, K.; Judy, J.T.; Seifuddin, F.; Tunc, I.; Sack, M.N.; Pirooznia, M. Network Analysis and Transcriptome Profiling Identify Autophagic and Mitochondrial Dysfunctions in SARS-CoV-2 Infection. Front. Genet. 2021, 12, 599261.
- Miao, G.; Zhao, H.; Li, Y.; Ji, M.; Chen, Y.; Shi, Y.; Bi, Y.; Wang, P.; Zhang, H. ORF3a of the COVID-19 virus SARS-CoV-2 blocks HOPS complex-mediated assembly of the SNARE complex required for autolysosome formation. Dev. Cell 2021, 56, 427–442.e5.
- Hou, P.; Wang, X.; Wang, H.; Wang, T.; Yu, Z.; Xu, C.; Zhao, Y.; Wang, W.; Zhao, Y.; Chu, F.; et al. The ORF7a protein of SARS-CoV-2 initiates autophagy and limits autophagosome-lysosome fusion via degradation of SNAP29 to promote virus replication. Autophagy 2022, 144, 110206.
- Mohamud, Y.; Xue, Y.C.; Liu, H.; Ng, C.S.; Bahreyni, A.; Jan, E.; Luo, H. The papain-like protease of coronaviruses cleaves ULK1 to disrupt host autophagy. Biochem. Biophys. Res. Commun. 2021, 540, 75–82.
- Russell, R.C.; Tian, Y.; Yuan, H.; Park, H.W.; Chang, Y.Y.; Kim, J.; Kim, H.; Neufeld, T.P.; Dillin, A.; Guan, K.L. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 2013, 15, 741–750.
- García-Pérez, B.E.; González-Rojas, J.A.; Salazar, M.I.; Torres-Torres, C.; Castrejón-Jiménez, N.S. Taming the Autophagy as a Strategy for Treating COVID-19. Cells 2020, 9, 2679.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
966
Revisions:
2 times
(View History)
Update Date:
13 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




