Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bassam Odeh Al-Naami | -- | 4099 | 2022-09-06 14:23:51 | | | |
| 2 | Vivi Li | -3 word(s) | 4096 | 2022-09-07 04:10:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Fazio, R.D.; Mattei, V.; Al-Naami, B.; Vittorio, M.D.; Visconti, P. Methodologies and Wearable Devices to Monitor Sleep Dysfunctions. Encyclopedia. Available online: https://encyclopedia.pub/entry/26920 (accessed on 07 February 2026).
Fazio RD, Mattei V, Al-Naami B, Vittorio MD, Visconti P. Methodologies and Wearable Devices to Monitor Sleep Dysfunctions. Encyclopedia. Available at: https://encyclopedia.pub/entry/26920. Accessed February 07, 2026.
Fazio, Roberto De, Veronica Mattei, Bassam Al-Naami, Massimo De Vittorio, Paolo Visconti. "Methodologies and Wearable Devices to Monitor Sleep Dysfunctions" Encyclopedia, https://encyclopedia.pub/entry/26920 (accessed February 07, 2026).
Fazio, R.D., Mattei, V., Al-Naami, B., Vittorio, M.D., & Visconti, P. (2022, September 06). Methodologies and Wearable Devices to Monitor Sleep Dysfunctions. In Encyclopedia. https://encyclopedia.pub/entry/26920
Fazio, Roberto De, et al. "Methodologies and Wearable Devices to Monitor Sleep Dysfunctions." Encyclopedia. Web. 06 September, 2022.
Copy Citation
Sleep is crucial for human health from metabolic, mental, emotional, and social points of view; obtaining good sleep in terms of quality and duration is fundamental for maintaining a good life quality. Several systems have been proposed in the scientific literature and on the market to derive metrics used to quantify sleep quality as well as detect sleep disturbances and disorders. In this field, wearable systems have an important role in the discreet, accurate, and long-term detection of biophysical markers useful to determine sleep quality.
sleep dysfunction
sensors
wearable devices
EEG
ECG
EOG
polysomnography
sleep staging
1. Introduction
Sleep is a fundamental biological process for human life, as it plays a fundamental role in maintaining physical, psychological, emotional, and social health thanks to its restorative, regenerative, conservative, consolidating, and protective functions [1][2][3]. In fact, sleep’s poor quality or its insufficient duration can have both short- and long-term repercussions, such as a decrease in life’s quality, difficulty in concentrating, learning problems, bad mood, excessive daytime sleepiness, accidents of various types, weakening of the immune system, propensity to contract infections and diseases, etc. [4][5][6]. Numerous negative health impacts, such as an elevated risk of hypertension, diabetes, obesity, depression, heart attack, and stroke, have been associated with the cumulative long-term effects of sleep disturbances and sleep deprivation [7]. The most common sleep disorders, according to their prevalence, are the following: insomnia, sleep apnea, restless legs syndrome (RLS), hypersomnia, parasomnia, circadian rhythm disorders, obstructive sleep apnea, nocturnal, epilepsy, etc. [8][9][10][11]. Therefore, monitoring sleep and related disorders is crucial in preventing diseases [12][13][14].
Moreover, sleep health is a complex sleep-wakefulness pattern adaptable to individual, social, and environmental needs and supports physical and mental health [15]. Subjective satisfaction, optimal timing, adequate duration, high efficiency, and maintained attentiveness throughout waking hours are characteristics of healthy sleep. Thus, quantifying sleep health requires a set of measurable quantities related to physical, mental, and neurobehavioral well-being. These lasts can be classified into five dimensions: sleep duration, continuity, timing, alertness, sleepness, and satisfaction/quality. Some of them are based on subjective evaluation (e.g., satisfaction/quality); thus, sleep health can be quantified through subjective reports.
Wearable devices offer a valuable contribution in this area, as they allow collection, analysis, and transmission during sleep of vital and functional parameters, including body temperature, heart rate, respiratory rate, blood oxygen saturation, etc. [16][17][18][19][20][21]. Furthermore, these solutions are discreet and not bulky (contrary to the polysomnography exam, which remains the gold standard for recording electroencephalographic, respiratory, cardiac, and eye movements activities) and allow sleep monitoring even from home. Moreover, the recent spread of COVID-19 had a negative impact on sleep due to increased anxiety, insecurity, stress, fear, loneliness, depression, etc. These sleep alterations affect a wide portion of the global population, not only subjects who tested positive for this infection [22][23][24].
2. Importance of Sleep Monitoring
This section outlines an overview of sleep mechanisms and related disorders, stressing the importance of sleep monitoring to prevent such diseases [25][26][27]. Sleep is characterized scientifically by the individual behavior while asleep and the accompanying physiological changes occurring in the waking brain’s electrical cycles during sleep. A reduction or lack of mobility, slow eye movements, a distinctive sleeping body posture, lower reactivity to external stimuli, longer reaction time, heightened arousal threshold, diminished cognitive function, and unconscious but reversible state are the behavioral phenomena featuring sleep. Moreover, physiological changes may be measured using specific clinical tests involving the acquisition of several biosignals, such as EEG, EOG, EMG, or ECG.
Sleep is classified into two main states: non-rapid eye movement (NREM) sleep, which is distinguished into three stages (i.e., N1, N2, N3), and rapid eye movement (REM) sleep. The first is defined by a gradual decrease in reactivity to stimulus, slow rolling eye movements, slightly reduced mobility, reversible unconscious state, and synchronized EEG characterized by slow-wave activity associated with spindles and K-complexes, as well as diminished muscular tone. The latter is characterized by fast eye movements, reduced reactivity to stimulus, muscular atonia, fast rhythms, and myoclonic jerks. In addition, other symptoms are desynchronized EEG featured by theta waves, periodic swings in blood pressure and heart rhythm, irregular respiration, phasic tongue movements, and may include a few episodes of apnea or hypopnea [25]. The two states alternate in a cyclic way (from 4 to 6 cycles during sleep, each lasting from 90 to 110 min, Figure 1). From 20% to 25% of total sleep duration in adults is spent in REM sleep, while the remaining 75–80% is spent in NREM sleep. In a healthy adult, there is a gradual progression from wakefulness to sleep onset, NREM sleep, and REM sleep [25]. Nevertheless, this trend is heavily affected by the nervous system’s maturation, changing from childhood to adulthood, as discussed in [25]. The hypothalamus’s suprachiasmatic nuclei master clock regulates the sleep-and-wake circadian rhythm. In addition, the neuroanatomical substrates of NREM sleep are primarily found in the ventrolateral preoptic nucleus of the hypothalamus, while those of REM sleep are found in the pons.
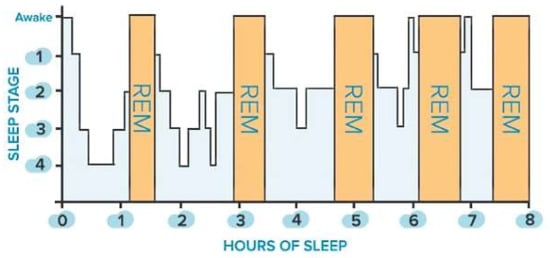
Figure 1. Sleep stages of a healthy adult: non-rapid eye movement (NREM, blue bars) and rapid eye movement (REM, orange bars) stages.
Functional anomalies in the autonomic and somatic nervous systems can induce significant repercussions on sleep, often resulting in sleep disorders. These lasts can be classified into two distinct categories: dyssomnias and parasomnia. The first category comprises the disorders that cause trouble falling or staying asleep, such as insomnia, obstructive sleep apnea, RLS, periodic limb movement, and circadian rhythm disorders. In contrast, the second category includes disorders involving irregular actions executed during sleep, such as arousal disorders, parasomnias associated with REM sleep, and enuresis [28]. It is known that sleep is essential since its functions are assumed to be restorative, conservative, adaptive, thermoregulatory, and memory consolidative. Therefore, sleep deprivation has short and long-term negative repercussions, whether caused by lifestyle or sleep disorders. Short-term consequences include diminished attention and focus, decreased quality of life, higher absenteeism with lower productivity, and workplace, home, and road accidents. Long-term consequences include increased morbidity and mortality from increasing automotive accidents, coronary artery disease, heart failure, high blood pressure, obesity, type 2 diabetes mellitus, stroke, cognitive impairment, and depression. Nevertheless, long-term and short-term repercussions are still a research topic.
Good sleep quality and duration are very important for astronauts, who, instead, often suffer from sleep disorders due to microgravity, isolation, monotonous repetition, workload, low temperatures, noise, interruption of sleep for operational necessities, irregular working schedule, reduced homeostatic regulation, etc. [29]. Among the problems deriving from a poor duration and quality of sleep, it is possible to identify reduced cognitive performance, reduction in concentration and motivation, stress, depression, anxiety, cardiovascular deconditioning, muscle atrophy, and space adaptation syndrome (SAS) as well as a decreased immune response [30][31]. For this reason, NASA (National Aeronautics and Space Administration) has added sleep deprivation among the risk factors that can compromise the cognitive and operational performance and health of astronauts and the safety of missions in orbit. Researchers and scientists are still studying the countermeasures that can be used to avoid the mentioned problems. In [29], some countermeasures for sleep disorders have been proposed, such as creating a good environment in the cabin, adjusting work-rest schedules appropriately, training crew members, intervening pharmacologically, carrying out light treatment, providing adequate psychological support, practicing Tai Chi and using TCM (traditional Chinese medicine).
PSG, which stands for polysomnography, is the gold standard for sleep monitoring, recording several physiologic parameters related to sleep [29]. PSG involves the acquisition of several vital signs, such as EEG, EOG, ECG, EMG, and anterior tibialis EMG, respiratory effort, airflow, and pulse oximetry (i.e., the amount of oxygen in the blood). Electrodermal activity (EDA) is an excellent indicator of the sympathetic nerve’s variations, affected by the sweat glands and blood vessels in the skin. EDA can be a useful tool for describing the various sleep phases compared to other physiological measurements. In detail, the REM phase is featured by high sympathetic nerve activity combined with low muscle tone [32]; moreover, low sympathetic activity is associated with the NREM phase [33][34]. EDA can also be efficiently and reliably acquired using wrist wearable devices during the sleep and wake stages [35]. The collected data can be provided as input for a machine learning algorithm to identify the sleep stage.
3. Overview of Commercial Wearable Devices for Detecting Sleep Disorders
After introducing several wearable devices for sleep monitoring proposed in the scientific literature, this section will illustrate an overview of commercial devices on the market. The most popular devices put comfort ahead of precision. They are not well-adapted to monitor sleep disorders because they solely use heart rate, breathing, and body movement measures to determine sleep quality. In fact, these devices are aimed at healthy users, but they cannot be considered sufficiently reliable for diagnostic sleep disorders.
In [36], Goldstein et al., presented a device called BrainBit, which can be placed on a user’s forehead to track sleep. The device has four EEG channels, with mechanisms for removing EMG and EOG signal components to monitor the electrical brain activity and bioelectricity of the forehead, face, head, and eye muscles [37]. The headband is composed of flexible and non-stretchable ribbon on which the golden dry electrodes, the battery, an integrated electronic board, and an elastic fabric band are placed, as shown in Figure 2.
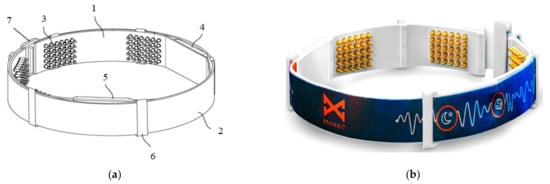
Figure 2. Schematic (a) and real (b) views of BrainBit (1: ribbon, 2: elastic band, 3: electrodes, 4: electronic module, 5: removable battery, 6: eyelet, 7: clasp) [36].
The four electrodes are placed on the temporal (positions T3 and T4) and occipital (positions O1 and O2) lobe regions; in contrast, the reference sensor is positioned on the forehead. Using the BLE technology, BrainBit transmits the collected biological signals to a PC or a mobile app, where software filters the data and obtains the signals’ spectrum. The great disadvantage of dry electrodes is the rapid degradation of their contact impedance, thus worsening the signal quality [38].
Another commercial device to monitor sleep is Philips’ SmartSleep [39]; it is a lightweight and comfortable wearable sleep headband but not suitable for people with sleep disorders. SmartSleep is also used to track astronauts’ sleep and improve its quality. The device is an EEG-based smart mask to detect deep sleep conditions based on the EEG signal in real-time. When the integrated sensors detect the user in the deep sleep phase, the device emits audio tones to increase the slow waves that characterize this sleep stage. During night use, the device has Bluetooth and Wi-Fi modules turned off. Only when the consumer no longer wears it, this last sends the data to the smartphone app, deriving and visualizing the sleep metrics, such as the amount of time in deep sleep, the time spent falling asleep, the number of times where sleep is interrupted, time spent awake, etc. This operation modality is the major limitation of such a device, especially if real-time sleep quality and staging supervision is required. The device consists of a fabric band, a wet sensor placed behind the right ear (up to three times), and two embedded speakers.
Muse S (Gen 2), in [40], is a headband that allows collecting EEG data and heart parameters (HR and HRV) during sleep, using silver electrodes and a PPG (Photoplethysmographic) sensor, as well as keeping track of the time spent in each stage of sleep, detecting the body position and respiratory activity through inertial sensors. The device is also equipped with “digital sleeping pills” (DSP), which emit immersive sound signals designed to help users fall asleep or fall back asleep if sleep has been interrupted. The device can be used for up to 10 consecutive hours. The fabric headband is lightweight (i.e., only 41 g) and fits different head sizes (from 48 to 63 cm in circumference). The device transmits data via Bluetooth 4.2 to the Muse app. A problem with this device lies in the electrodes’ flat conformation, which could lead to incorrect readings when the area of the body has hair. Tests were conducted to verify the device’s performance, comparing the Muse S to laboratory monitoring devices. Tests have shown that the commercial device can provide detailed and accurate information. A recent 2021 study led by Western University, Cambridge Brain Science, Hatch, and Interaxon that used Muse S EEG-sleep support technology showed a 20% improvement in the Pittsburgh Sleep Quality Index relative to controls wanting to improve their sleep [41].
In [42], Dreem 2 is presented; it allows measuring brain activity, heart rate, respiratory rate, and body movement. The device is a headband that allows the screening, diagnosis, and treatment of sleep disorders directly at the user’s home. The headband integrates six custom-designed EEG electrodes (four frontal and two rear) to monitor brain activity, a PPG sensor to monitor the HR discreetly, and an accelerometer to detect head movements and breathing patterns. In detail, the Dreem 2 can detect the breathing frequency by analyzing the real-time audio recorded during the night by an integrated microphone. However, this detection method is sensitive to the background and glitches, which proper filtering techniques can overcome [43].
Some tests have compared this device to PSG, demonstrating that Dreem 2 is reliable and, therefore, a valid alternative to laboratory tests. The Insomnia Severity Index (ISI) was estimated both before and after using the device to evaluate the effectiveness of Dreem 2 in curing insomnia. The authors applied the CBT-I (Cognitive Behavioral Therapy for Insomnia) exercises and guidelines to design an effective in-app digital program that, when used with the Dreem headband, can encourage patients to take the necessary steps toward healthier sleep. The results demonstrated that 71% of users no longer have insomnia, 91% fall asleep easier taking 21 min less to fall asleep, and 78% wake up fewer times during sleep (Figure 3) [44].
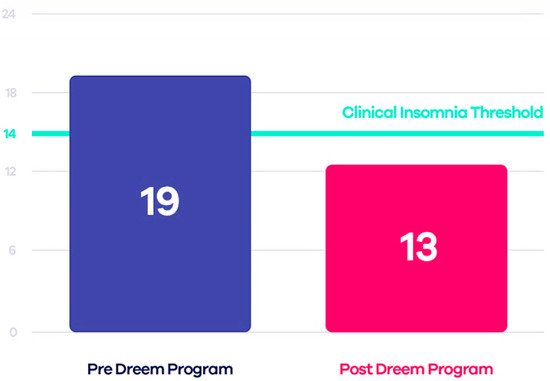
Figure 3. Comparison between ISI scores before and after using Dreem 2 program [42].
iBand+, presented in [45], is an AI-based (artificial intelligence) headband for detecting EEG signals and head movements. It consists of two stereo speakers placed under the pillow and RGB LEDs (light-emitting diodes) on the headband. The device helps develop lucid dreaming discipline, improve sleep quality, and wake sleeping users gradually. In a lucid dream, the dreamer is aware that he is dreaming and can take control of it without waking up. With the brainwave entrainment technique, which is a method to sync brainwaves with a particular steady rhythmic stimulus, iBand+ helps the user’s brain associate some pulsing sounds and flickering light stimulus with dreams. Lucid dreaming discipline can help users decrease nightmares and stress, improving concentration and creativity. The device also tracks sleep stages, and once the user has fallen asleep, it turns off audiovisual signals and emits white noise, improving sleep quality. In the morning, through a smart alarm, the headband wakes the user up by emitting sounds during the light sleep stage.
Neuroon Open, in [46], is a wearable mask developed by a Poland startup that detects EEG and EOG signals, blood oxygenation (through PPG sensor), and body temperature; it helps users wake up full of energy and facilitates lucid dreams. Indeed, these data are processed by a suitable sleep staging algorithm run on a custom mobile application; it also calculates the sleep efficiency and provides useful suggestions to enhance sleep habits to obtain a better night’s sleep. The device consists of an adjustable strap with a pulse oximeter, electrodes, EEG and EOG sensors, thermometers, LEDs, and a triaxial accelerometer (Figure 4).
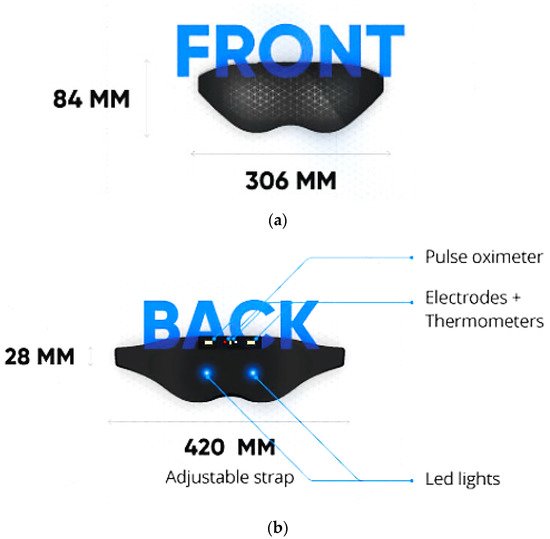
Figure 4. Front (a) and rear (b) views of Neuroon Open mask [46].
Through BLE 4.0 (designed and marketed by Bluetooth Special Interest Group—BSIG, Kirkland, WA, USA), collected data are transmitted to a mobile device, where Neuroon app allows users to check their sleep analysis (also in real-time) and read personalized tips on improving sleep quality. In addition, through Neuroon app, the users can measure their subjective evaluation of sleep quality every morning. They are given an A/B test, and the combination of self-evaluation and physiological data provides information about the best sleep pattern that suits them. Moreover, this device can help users induce lucid dreaming through light, vibration, and acoustic stimuli and wake up naturally by simulating the light of gradually rising dawn. If the user does not wake up, the device adds vibrations and an audio signal from the mobile phone.
Also, the Somni mask, developed by Somni Ltd. (Delft, The Netherlands) startup, monitors sleep by tracking the eye and head movements [47]. Furthermore, the device should create lucid dreams without disturbing sleep by emitting auditory and visual signals. It is composed of a fabric mask similar to conventional ones and, on it, there is an electronic section with a button to turn the device on/off and test the light signals, audio input, and a micro-USB input (Figure 5a). It is not equipped with speakers, so users must connect the headphones to hear the soothing melodies. In addition, the device does not have a wireless module to transfer data, but it can be connected to a PC or a USB OTG (On-The-Go) interface. Inside the mask, an eye-tracking device and accelerometer detect head movements (Figure 5b). However, the headband design may not be comfortable for the user since it has an electronic section positioned in the front part of the mask, causing a feeling of weight on the face.
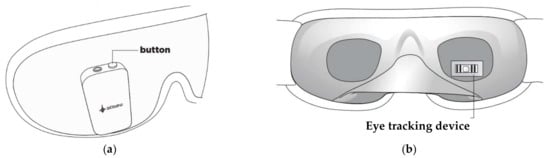
Figure 5. External (a) and internal (b) views of the Somni mask [47].
BrainLink Pro headset, manufactured by Shenzhen Macrotellect Ltd. (Shenzhen, China), monitors brain activity, heart rate, body temperature, and head movements [48]. It consists of three dry metal electrodes, a filter, an amplifier, an A/D converter, a lithium battery lasting 3–4 h, and an adjustable headband that also fits children’s heads. The headband is connected to a smartphone or computer via the BLE 4.0 protocol. The device includes a multi-user EEG acquisition/processing/analysis section called Mindmesh, comprising a Bluetooth gateway, MindMesh EEG sensors, and a front-end acquisition system, allowing the concurrent acquisition of brainwaves of up 50 users. As a result, the device acquires signals with a sampling frequency of 512 Hz, converting them with a 12-bit ADC, ensuring 0.25 µV precision in the signal acquisition and less than 2 ms dynamic response. The device is not oriented to sleep analysis but allows non-invasive detection of EEG signals, which can be used to monitor sleep. Among the device limitations, similarly to other devices previously discussed, the use of dry flat electrodes leads to problems related to contact impedance and applications in hairy body areas. Furthermore, this headband integrates a reference electrode applied to the ear by a clip, which in the long term could create discomfort and irritation to the user.
Sleep Shepherd is a breathable, lightweight, and comfortable headband manufactured by Sleep Shepherd LLC to track head movements, monitor brain activity during sleep, and improve quality [49]. It guides the user toward deep sleep through some binaural beats, played into each ear with two different frequencies. Changing binaural beats’ frequency according to the actual cerebral activity, the device can also wake the user up gradually and during the lightest sleep stage. It comprises EEG sensors and uses NeuroSky’s brain signal filtering technology to remove noises; it also includes a gyroscope and movement sensor for monitoring the user’s movements. The device can be connected wirelessly to a smartphone to display the data collected during the night, such as the duration of sleep, the duration of the deep one, the head movements, the number of times the sleep was interrupted, the time at which the user fell asleep and woke up, etc. However, the device does not stage sleep according to the five standard phases previously described (wake, N1, N2, N3, and REM) but only discerns between awake, light, and deep sleep. Tests were conducted on four healthy awake participants to quantify the accuracy of the NeuroSky sensors and SVM classifier. The participants were divided according to information, mild concentration, and background noise [50]. As a result, classifying the information from noise or mild concentration, the developed system demonstrated high accuracy, with an average of about 86.76.
In [51], Advanced Brain Monitoring Inc. presents the Sleep Profiler headset that monitors the EEG, EOG, and EMG signals and, optionally, ECG, PPG, head position, or quantitative snoring parameters, using a microphone and an accelerometer (Figure 6). In addition, the model SP29 includes a nasal transducer, a cannula, a pulse rate sensor, and an oximeter. The device is very precise, and it can detect sleep spindles (recording their duration and PSD), distinguish the stages of sleep, identify irregular sleep patterns, and monitor insomnia, hypersomnia, and other sleep disorders. Researchers also use it to evaluate dementia, consequences on sleep due to drug use, effects on memory due to poor sleep quality, etc. [51]; however, it cannot replace clinical ECG or EMG. The Sleep Profiler is very lightweight (71 g) and has up to 8 channels, an internal memory of 8 Gb that can record signals over 10–16 h, and a battery lasting about 30 h. The device uses a speaker to send vocal outputs in case of incorrect operation due to the wrong position of the sensors, which could not be well adherent to the skin. It can also transmit signals in real-time to a tablet or a PC using Bluetooth 2.0 or 5.0 protocol to allow experts to monitor signals or just upload data to a cloud server. In detail, the Sleep Profiler implements automatic event detection algorithms to determine an overall supine and non-supine AHI and ODI (Oxygen Desaturation Indices) [52]. The airflow detection rules were created to mimic how humans would identify apneas based on a >90% drop in airflow for 10 or more seconds and hypopneas based on a >30% reduction in airflow volume. The algorithms used sliding scale thresholds based on the strength of the airflow signal to reduce the identification of over-scored events when there is little airflow. With 3% SpO2 desaturation happening between 5 and 120 s from baseline and 1% recovery occurring within 30 s of the nadir, desaturation events required to validate hypopneas were identified using algorithms applied to the least filtered beat-by-beat SpO2 signal (four-beat fast average). Separate counts of desaturation occurrences were made.
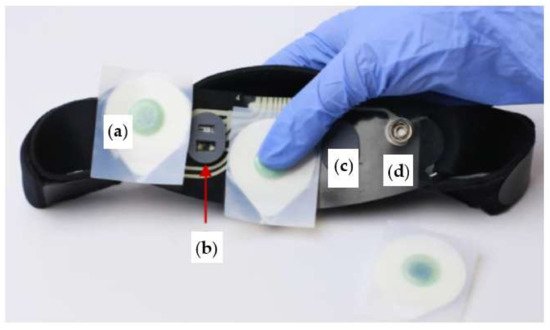
Figure 6. Internal view of Sleep Profiler headband, consisting of EEG sensor (a), optical sensor (b), strip pad (c), and sensor snap (d) [51].
In [53], tests were performed to understand the effectiveness of the Sleep Profiler headband; it was used to acquire 3 EEG signals, sampled at 256 Hz with a ± 1000 µV dynamic range and filtered with 0.1 Hz high-pass and 80 Hz low-pass filters. The detected signals were then evaluated by some experts, who analyzed the waveforms of the EEG signal to confirm detection veracity, especially near transitions from one stage to another. In summarizing, the five experts had a mean interscorer agreement of 75.9%, with 90.1%, 51.3%, 75.5%, 67.2%, and 91.1% for stages awake, N1, N2, N3, and REM stages, respectively; the mean kappa score was 0.70 (range 0.61 to 0.78) across all 10 comparisons. As a result, the performed studies have shown that Sleep Profiler is quite accurate. Even one night of recording is enough to characterize abnormal slow-wave sleep, spindle activity, and irregularities in heart rate.
Finally, Table 1 compares the commercial devices previously discussed from the point of view of the number and typology of detected parameters, integrated sensors, available biofeedbacks or post-interventions to improve sleep, and the cost to establish the best solution for tracking sleep. In conclusion, Sleep Profiler is the device that includes the largest number of sensors that track numerous physiological parameters despite its small size and lightness. The only flaw is that after 50 nights, the sensor strip has to be replaced and, probably, the headband too. Nevertheless, Sleep Profiler uses common Ag/AgCl disposable electrodes, which ensures a larger signal quality but requires their substitution after each use.
Table 1. Comparison between the devices discussed above from the point of view of number and typology of the integrated sensors, gathered parameters, and cost.
| Device | Number of Parameters Detected | Integrated Sensors | Gathered Parameters |
Feedbacks/ Interventions |
Cost |
|---|---|---|---|---|---|
| BrainBit [37] |
4 | EEG, PPG, EMG, EOG | Brain activity, heart rate, body movement, eye movement |
Psychology and cognitive remediation | USD $499 |
| SmartSleep [54] |
1 | EEG | Brain activity | Audio tones to boost the slow wave | USD $399 |
| Muse S [40] |
4 | EEG, PPG, gyroscope, accelerometer | Brain activity, heart rate, breath rate, body movements |
Digital sleeping pills (sleep stories and meditation, ambient soundscape, nature and music biofeedbacks) | USD $399 |
| Dreem 2 [42] |
4 | EEG, PPG, gyroscope, accelerometer | Brain activity, heart rate, breath rate, body movement | CBT-I exercises | N.A. 1 |
| iBand+ [45] |
2 | EEG, accelerometer, gyroscope |
Brain activity, head movement |
Audio tones to induce sleep | USD $449 |
| Neuroon Open [46] |
4 | EEG, EOG, PPG, thermometer, |
Brain activity, eye movement, body temperature, blood oxygenation | Audio tones to induce sleep | N.A. 1 |
| Somni [47] |
2 | EOG, accelerometer | Eye movement, head movement | Audiovisual feedback to induce sleep | N.A. 1 |
| BrainLink Pro [48] |
4 | EEG, PPG, gyroscope thermometer, accelerometer, | Brain activity, heart rate, body temperature, head movement | No | USD $259 |
| Sleep Shepherd [49] |
2 | EEG, gyroscope, movement sensor | Brain activity head movement |
Binaural tones to induce sleep | N.A. 1 |
| Sleep Profiler [51] |
5 | EEG, EOG, EMG, accelerometer, ECG (optional), PPG (optional), nasal transducer (model SP29), pulse rate sensor (model SP29), oximeter (model SP29) | Brain activity, eye movement, head position, heart rate, quantitative snoring | No | N.A. 1 |
1 Not available.
References
- Worley, S.L. The Extraordinary Importance of Sleep. Pharm. Ther. 2018, 43, 758–763.
- Okano, K.; Kaczmarzyk, J.R.; Dave, N.; Gabrieli, J.D.E.; Grossman, J.C. Sleep Quality, Duration, and Consistency Are Associated with Better Academic Performance in College Students. NPJ Sci. Learn. 2019, 4, 16.
- Kapsi, S.; Katsantoni, S.; Drigas, A. The Role of Sleep and Impact on Brain and Learning. Int. J. Recent Contrib. Eng. Sci. IT (IJES) 2020, 8, 59–68.
- Garbarino, S.; Lanteri, P.; Bragazzi, N.L.; Magnavita, N.; Scoditti, E. Role of Sleep Deprivation in Immune-Related Disease Risk and Outcomes. Commun. Biol. 2021, 4, 1304.
- Gottlieb, D.J.; Ellenbogen, J.M.; Bianchi, M.T.; Czeisler, C.A. Sleep Deficiency and Motor Vehicle Crash Risk in the General Population: A Prospective Cohort Study. BMC Med. 2018, 16, 44.
- Peng, Z.; Dai, C.; Ba, Y.; Zhang, L.; Shao, Y.; Tian, J. Effect of Sleep Deprivation on the Working Memory-Related N2-P3 Components of the Event-Related Potential Waveform. Front. Neurosci. 2020, 14, 469.
- Institute of Medicine (US) Committee on Sleep Medicine and Research. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem; The National Academies Collection: Reports Funded by National Institutes of Health; Colten, H.R., Altevogt, B.M., Eds.; National Academies Press: Washington, DC, USA, 2006; ISBN 978-0-309-10111-0.
- Kales, A.; Soldatos, C.R.; Kales, J.D. Sleep Disorders: Insomnia, Sleepwalking, Night Terrors, Nightmares, and Enuresis. Ann. Intern. Med. 1987, 106, 582–592.
- Sack, R.L.; Auckley, D.; Auger, R.R.; Carskadon, M.A.; Wright, K.P., Jr.; Vitiello, M.V.; Zhdanova, I.V. Circadian Rhythm Sleep Disorders: Part I, Basic Principles, Shift Work and Jet Lag Disorders. Sleep 2007, 30, 1460–1483.
- Gigli, G.L.; Adorati, M.; Dolso, P.; Piani, A.; Valente, M.; Brotini, S.; Budai, R. Restless Legs Syndrome in End-Stage Renal Disease. Sleep Med. 2004, 5, 309–315.
- MedlinePlus. Sleep Disorders. Available online: https://medlineplus.gov/sleepdisorders.html (accessed on 15 July 2022).
- Kelly, J.M.; Strecker, R.E.; Bianchi, M.T. Recent Developments in Home Sleep-Monitoring Devices. ISRN Neurol 2012, 2012, 768794.
- Robbins, R.; Seixas, A.; Masters, L.W.; Chanko, N.; Diaby, F.; Vieira, D.; Jean-Louis, G. Sleep Tracking: A Systematic Review of the Research Using Commercially Available Technology. Curr. Sleep Med. Rep. 2019, 5, 156–163.
- Hussain, Z.; Sheng, Q.Z.; Zhang, W.E.; Ortiz, J.; Pouriyeh, S. A Review of the Non-Invasive Techniques for Monitoring Different Aspects of Sleep. ACM Trans. Comput. Healthc. 2022, 3, 24.
- Buysse, D.J. Sleep Health: Can We Define It? Does It Matter? Sleep 2014, 37, 9–17.
- De Fazio, R.; De Vittorio, M.; Visconti, P. A BLE-Connected Piezoresistive and Inertial Chest Band for Remote Monitoring of the Respiratory Activity by an Android Application: Hardware Design and Software Optimization. Future Internet 2022, 14, 183.
- De Fazio, R.; Al-Hinnawi, A.-R.; De Vittorio, M.; Visconti, P. An Energy-Autonomous Smart Shirt Employing Wearable Sensors for Users’ Safety and Protection in Hazardous Workplaces. Appl. Sci. 2022, 12, 2926.
- De Fazio, R.; Stabile, M.; De Vittorio, M.; Velázquez, R.; Visconti, P. An Overview of Wearable Piezoresistive and Inertial Sensors for Respiration Rate Monitoring. Electronics 2021, 10, 2178.
- Imtiaz, S.A. A Systematic Review of Sensing Technologies for Wearable Sleep Staging. Sensors 2021, 21, 1562.
- Schutte-Rodin, S.; Deak, M.C.; Khosla, S.; Goldstein, C.A.; Yurcheshen, M.; Chiang, A.; Gault, D.; Kern, J.; O’Hearn, D.; Ryals, S.; et al. Evaluating Consumer and Clinical Sleep Technologies: An American Academy of Sleep Medicine Update. J. Clin. Sleep Med. 2021, 17, 2275–2282.
- Chinoy, E.D.; Cuellar, J.A.; Jameson, J.T.; Markwald, R.R. Performance of Four Commercial Wearable Sleep-Tracking Devices Tested under Unrestricted Conditions at Home in Healthy Young Adults. Nat. Sci. Sleep 2022, 14, 493–516.
- Alimoradi, Z.; Broström, A.; Tsang, H.W.H.; Griffiths, M.D.; Haghayegh, S.; Ohayon, M.M.; Lin, C.-Y.; Pakpour, A.H. Sleep Problems during COVID-19 Pandemic and Its’ Association to Psychological Distress: A Systematic Review and Meta-Analysis. eClinicalMedicine 2021, 36, 100916.
- Lin, Y.N.; Liu, Z.R.; Li, S.Q.; Li, C.X.; Zhang, L.; Li, N.; Sun, X.W.; Li, H.P.; Zhou, J.P.; Li, Q.Y. Burden of Sleep Disturbance During COVID-19 Pandemic: A Systematic Review. Nat. Sci. Sleep 2021, 13, 933–966.
- Islam, M.K.; Molla, M.M.A.; Hasan, P.; Sharif, M.M.; Hossain, F.S.; Amin, M.R.; Rahman, M.R. Persistence of Sleep Disturbance among Post-COVID Patients: Findings from a 2-Month Follow-up Study in a Bangladeshi Cohort. J. Med. Virol. 2022, 94, 971–978.
- Chokroverty, S. Overview of Sleep & Sleep Disorders. Indian J. Med. Res. 2010, 131, 126–140.
- Thorpy, M.J. Classification of Sleep Disorders. Neurotherapeutics 2012, 9, 687–701.
- Surantha, N.; Kusuma, G.P.; Isa, S.M. Internet of Things for Sleep Quality Monitoring System: A Survey. In Proceedings of the 2016 11th International Conference on Knowledge, Information and Creativity Support Systems (KICSS), Yogyakarta, Indonesia, 10–11 November 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 1–6.
- Wickboldt, A.T.; Bowen, A.F.; Kaye, A.J.; Kaye, A.M.; Bueno, F.R.; Kaye, A.D. Sleep Physiology, Abnormal States, and Therapeutic Interventions. Ochsner J. 2012, 12, 123–134.
- Chesson, A.L., Jr.; Ferber, R.A.; Fry, J.M.; Grigg-Damberger, M.; Hartse, K.M.; Hurwitz, T.D.; Johnson, S.; Kader, G.A.; Littner, M.; Rosen, G.; et al. The Indications for Polysomnography and Related Procedures. Sleep 1997, 20, 423–487.
- Dijk, D.-J.; Neri, D.F.; Wyatt, J.K.; Ronda, J.M.; Riel, E.; Ritz-De Cecco, A.; Hughes, R.J.; Elliott, A.R.; Prisk, G.K.; West, J.B.; et al. Sleep, Performance, Circadian Rhythms, and Light-Dark Cycles during Two Space Shuttle Flights. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2001, 281, 1647–1664.
- Oh, C.-M.; Kim, H.Y.; Na, H.K.; Cho, K.H.; Chu, M.K. The Effect of Anxiety and Depression on Sleep Quality of Individuals with High Risk for Insomnia: A Population-Based Study. Front. Neurol. 2019, 10, 849.
- Silvani, A.; Dampney, R.A.L. Central Control of Cardiovascular Function during Sleep. Am. J. Physiol.-Heart Circ. Physiol. 2013, 305, H1683–H1692.
- Somers, V.K.; Dyken, M.E.; Mark, A.L.; Abboud, F.M. Sympathetic-Nerve Activity during Sleep in Normal Subjects. N. Engl. J. Med. 1993, 328, 303–307.
- Murali, N.S.; Svatikova, A.; Somers, V.K. Cardiovascular Physiology and Sleep. Front. Biosci. 2003, 8, s636–s652.
- Anusha, A.S.; Preejith, S.P.; Akl, T.J.; Sivaprakasam, M. Electrodermal Activity Based Autonomic Sleep Staging Using Wrist Wearable. Biomed. Signal Process. Control 2022, 75, 103562.
- Goldstein, B.; Sakharov, V.; Bulanov, S. Personal Apparatus for Conducting Electroencephalography. U.S. Patent WO2021183940A1, 13 March 2020.
- Brainbit. Brainbit Manual. Available online: http://brainbit.com/ (accessed on 3 March 2022).
- Lopez-Gordo, M.A.; Sanchez-Morillo, D.; Valle, F.P. Dry EEG Electrodes. Sensors 2014, 14, 12847–12870.
- Diep, C.; Garcia-Molina, G.; Jasko, J.; Manousakis, J.; Ostrowski, L.; White, D.; Anderson, C. Acoustic Enhancement of Slow Wave Sleep on Consecutive Nights Improves Alertness and Attention in Chronically Short Sleepers. Sleep Med. 2021, 81, 69–79.
- Muse™. Headband Manual. Available online: https://choosemuse.com/ (accessed on 3 March 2022).
- Western University, Canada. Assessing the Effects of the Muse Sleep Intervention on Sleep; Western University: London, ON, Canada, 2021. Available online: https://clinicaltrials.gov (accessed on 10 February 2022).
- Dreem|Sleep Pioneers. Available online: https://dreem.com/en/ (accessed on 3 March 2022).
- da Costa, T.D.; Vara, M.D.F.F.; Cristino, C.S.; Zanella, T.Z.; Neto, G.N.N.; Nohama, P. Breathing Monitoring and Pattern Recognition with Wearable Sensors; IntechOpen: London, UK, 2019; ISBN 978-1-78984-497-9.
- Arnal, P.J.; Thorey, V.; Debellemaniere, E.; Ballard, M.E.; Bou Hernandez, A.; Guillot, A.; Jourde, H.; Harris, M.; Guillard, M.; Van Beers, P.; et al. The Dreem Headband Compared to Polysomnography for Electroencephalographic Signal Acquisition and Sleep Staging. Sleep 2020, 43, zsaa097.
- IBand+ EEG Headband|Sleep Improvement & Lucid Dreaming Wearable Device. Available online: https://www.ibandplus.com/ (accessed on 4 March 2022).
- Neuroon Open: World’s Smartest Sleep Tracker. Available online: https://www.indiegogo.com/projects/2172509 (accessed on 4 March 2022).
- Somni Mask—The Easiest Way to Lucid Dreaming. Available online: https://somni.org/ (accessed on 14 March 2022).
- BrainLink by Macrotellect|Healthy Brainwaves for Everyone. Available online: http://www.macrotellect.com/ (accessed on 15 March 2022).
- Sleep Shepherd: Sleep Optimizer and Tracker. Available online: https://sleepshepherd.com/ (accessed on 14 March 2022).
- Kim, Y.; Moon, J.; Lee, H.-J.; Bae, C.-S.; Sohn, S. Integration of Electroencephalography Based Services into Consumer Electronics. In Proceedings of the 2012 IEEE 16th International Symposium on Consumer Electronics, Harrisburg, PA, USA, 4–6 June 2012; pp. 1–2.
- Sleep Profiler™ Specifications. Available online: https://www.advancedbrainmonitoring.com/products/sleep-profiler#section-specification (accessed on 14 March 2022).
- Levendowski, D.J.; Hamilton, G.S.; St. Louis, E.K.; Penzel, T.; Dawson, D.; Westbrook, P.R. A Comparison between Auto-Scored Apnea-Hypopnea Index and Oxygen Desaturation Index in the Characterization of Positional Obstructive Sleep Apnea. Nat. Sci. Sleep 2019, 11, 69–78.
- Levendowski, D.J.; Ferini-Strambi, L.; Gamaldo, C.; Cetel, M.; Rosenberg, R.; Westbrook, P.R. The Accuracy, Night-to-Night Variability, and Stability of Frontopolar Sleep Electroencephalography Biomarkers. J. Clin. Sleep Med. 2017, 13, 791–803.
- SmartSleep Deep Sleep Headband. Available online: https://www.usa.philips.com/c-e/smartsleep/deep-sleep-headband.html (accessed on 28 February 2022).
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.2K
Entry Collection:
Remote Sensing Data Fusion
Revisions:
2 times
(View History)
Update Date:
07 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




