
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rafael M. Santos | -- | 1914 | 2022-09-05 16:43:27 | | | |
| 2 | Sirius Huang | Meta information modification | 1914 | 2022-09-06 02:41:01 | | |
Video Upload Options
As a principal part of the atmosphere–lithosphere interface, soil plays a key role in regulating the atmospheric CO2 concentration and global climate. Comprising two major pools (carbonate in soils and bicarbonate in groundwater), soil inorganic carbon (SIC) is deemed as the primary carbon (C) sink and source in areas with low mean annual rainfall. SIC may originate from soil parent material or from the formation of secondary carbonate when divalent cations from an extraneous source are supplied. The latter may result in pedogenic carbonate (PC) formation, increasing soil C content and sequestering atmospheric carbon. Since the sequestration of atmospheric CO2 through formation of pedogenic carbonate is gaining popularity as a method to support climate change mitigation efforts and to claim carbon credits, the mechanisms influencing the formation and migration of pedogenic carbonate need to be well understood.
1. Introduction
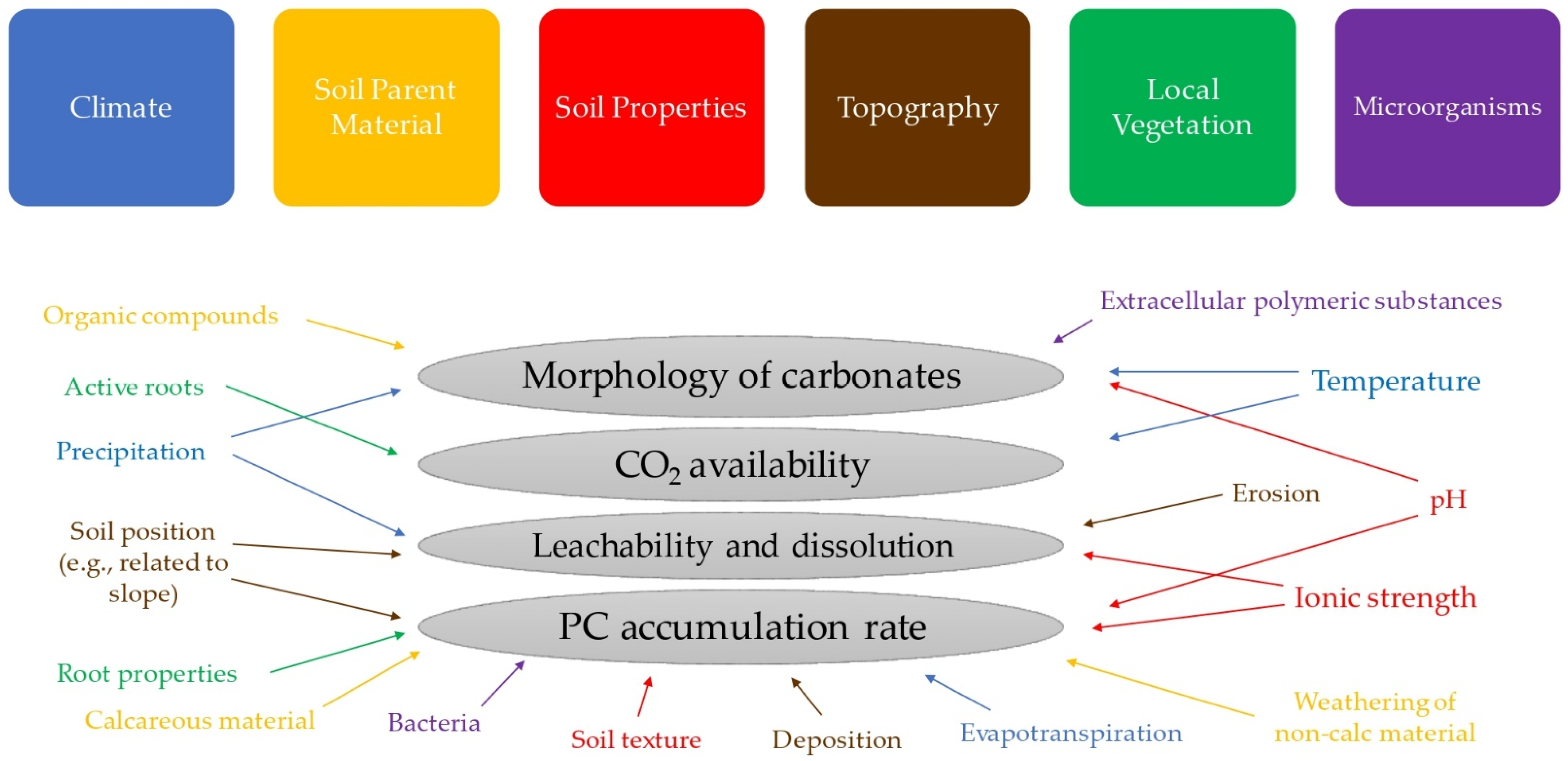
2. Pedogenic Carbonate Formation Mechanisms
3. Factors Affecting Pedogenic Carbonate Formation and Recrystallization
References
- Zhang, W.; Wang, X.; Lu, T.; Shi, H.; Zhao, Y. Influences of soil properties and hydrological processes on soil carbon dynamics in the cropland of North China Plain. Agric. Ecosyst. Environ. 2020, 295, 106886.
- Jin, Z.; Dong, Y.; Wang, Y.; Wei, X.; Wang, Y.; Cui, B.; Zhou, W. Natural vegetation restoration is more beneficial to soil surface organic and inorganic carbon sequestration than tree plantation on the Loess Plateau of China. Sci. Total Environ. 2014, 485–486, 615–623.
- Raheb, A.; Heidari, A.; Mahmoodi, S. Organic and inorganic carbon storage in soils along an arid to dry sub-humid climosequence in northwest of Iran. Catena 2017, 153, 66–74.
- Zamanian, K.; Pustovoytov, K.; Kuzyakov, Y. Pedogenic carbonates: Forms and formation processes. Earth-Sci. Rev. 2016, 157, 1–17.
- Díaz-Hernández, J.L. Is soil carbon storage underestimated? Chemosphere 2010, 80, 346–349.
- Ahmad, W.; Singh, B.; Dalal, R.; Dijkstra, F.A. Carbon dynamics from carbonate dissolution in Australian agricultural soils. Soil Res. 2015, 53, 144–153.
- You, M.; Han, X.; Hu, N.; Du, S.; Doane, T.A.; Li, L.-J. Profile storage and vertical distribution (0–150 cm) of soil inorganic carbon in croplands in northeast China. Catena 2019, 185, 104302.
- Lal, R. Carbon Management in Agricultural Soils. Mitig. Adapt. Strat. Glob. Chang. 2006, 12, 303–322.
- Wang, X.; Wang, J.; Shi, H.; Guo, Y. Carbon Sequestration in Arid Lands: A Mini Review; Springer: Singapore, 2018; ISBN 9789811070228.
- Monger, H.C.; Kraimer, R.A.; Khresat, S.; Cole, D.R.; Wang, X.; Wang, J. Sequestration of inorganic carbon in soil and groundwater. Geology 2015, 43, 375–378.
- Bughio, M.A.; Wang, P.; Meng, F.; Qing, C.; Kuzyakov, Y.; Wang, X.; Junejo, S.A. Neoformation of pedogenic carbonates by irrigation and fertilization and their contribution to carbon sequestration in soil. Geoderma 2016, 262, 12–19.
- Ferdush, J.; Paul, V. A review on the possible factors influencing soil inorganic carbon under elevated CO2. Catena 2021, 204, 105434.
- Li, Y.; Fu, C.; Zeng, L.; Zhou, Q.; Zhang, H.; Tu, C.; Wei, J.; Li, L.; Luo, Y. Carbon accumulation in the red clay layer of the subsoil in a major river delta: Contribution of secondary carbonate. Catena 2019, 186, 104391.
- Sanderman, J. Can management induced changes in the carbonate system drive soil carbon sequestration? A review with particular focus on Australia. Agric. Ecosyst. Environ. 2012, 155, 70–77.
- Diaz-Hernandez, J.; Navas, A.S.; Delgado, A.; Yepes, J.; Garcia-Casco, A. Textural and isotopic evidence for Ca-Mg carbonate pedogenesis. Geochim. Cosmochim. Acta 2017, 222, 485–507.
- Prokof’Eva, T.; Shishkov, V.; Kiriushin, A. Calcium carbonate accumulations in Technosols of Moscow city. J. Soils Sediments 2020, 21, 2049–2058.
- Monger, H.C. Soils as Generators and Sinks of Inorganic Carbon in Geologic Time. In Soil Carbon; Hartemink, A.E., McSweeney, K., Eds.; Springer: Cham, Switzerland, 2014; pp. 27–36.
- Leogrande, R.; Vitti, C.; Castellini, M.; Mastrangelo, M.; Pedrero, F.; Vivaldi, G.; Stellacci, A. Comparison of Two Methods for Total Inorganic Carbon Estimation in Three Soil Types in Mediterranean Area. Land 2021, 10, 409.
- Mikhailova, E.A.; Bryant, R.B.; Galbraith, J.M.; Wang, Y.; Post, C.J.; Khokhlova, O.S.; Schlautman, M.A.; Cope, M.P.; Shen, Z. Pedogenic Carbonates and Radiocarbon Isotopes of Organic Carbon at Depth in the Russian Chernozem. Geosciences 2018, 8, 458.
- Wang, Y.; Li, Y.; Ye, X.; Chu, Y.; Wang, X. Profile storage of organic/inorganic carbon in soil: From forest to desert. Sci. Total Environ. 2010, 408, 1925–1931.
- Golubtsov, V.A.; Cherkashina, A.A.; Khokhlova, O.S. Carbonate Profile of Soils in the Baikal Region: Structure, Age, and Formation Conditions. Eurasian Soil Sci. 2019, 52, 1515–1532.
- Dietrich, F.; Diaz, N.; Deschamps, P.; Ngatcha, B.N.; Sebag, D.; Verrecchia, E.P. Origin of calcium in pedogenic carbonate nodules from silicate watersheds in the Far North Region of Cameroon: Respective contribution of in situ weathering source and dust input. Chem. Geol. 2017, 460, 54–69.
- Nyachoti, S.; Jin, L.; Tweedie, C.E.; Ma, L. Insight into factors controlling formation rates of pedogenic carbonates: A combined geochemical and isotopic approach in dryland soils of the US Southwest. Chem. Geol. 2019, 527, 118503.
- Mikhailova, E.; Goddard, M.; Post, C.; Schlautman, M.; Galbraith, J. Potential Contribution of Combined Atmospheric Ca2+ and Mg2+ Wet Deposition within the Continental U.S. to Soil Inorganic Carbon Sequestration. Pedosphere 2013, 23, 808–814.
- Da, J.; Zhang, Y.G.; Wang, H.; Balsam, W.; Ji, J. An Early Pleistocene atmospheric CO2 record based on pedogenic carbonate from the Chinese loess deposits. Earth Planet. Sci. Lett. 2015, 426, 69–75.
- Carmi, I.; Kronfeld, J.; Moinester, M. Sequestration of atmospheric carbon dioxide as inorganic carbon in the unsaturated zone under semi-arid forests. Catena 2018, 173, 93–98.
- Wang, X.; Wang, J.; Xu, M.; Zhang, W.; Fan, T.; Zhang, J. Carbon accumulation in arid croplands of northwest China: Pedogenic carbonate exceeding organic carbon. Sci. Rep. 2015, 5, 11439.
- Chendev, Y.G.; Novykh, L.L.; Sauer, T.J.; Petin, A.N.; Zazdravnykh, E.A.; Burras, C.L. Evolution of Soil Carbon Storage and Morphometric Properties of Afforested Soils in the US. Great Plains. In Soil Carbon; Springer: Cham, Switzerland, 2014; pp. 475–482.
- Khokhlova, O.; Myakshina, T.; Kuznetsova, A. Origins of hard carbonate nodules in arable Chernozems in the Central Russian Upland. Eur. J. Soil Sci. 2020, 72, 326–342.
- Durand, N.; Monger, H.C.; Canti, M.G.; Verrecchia, E.P. Calcium Carbonate Features; Elsevier B.V.: Amsterdam, The Netherlands, 2018; ISBN 9780444635228.
- Li, Y.; Zhang, W.; Aydin, A.; Deng, X. Formation of calcareous nodules in loess–paleosol sequences: Reviews of existing models with a proposed new “per evapotranspiration model”. J. Southeast Asian Earth Sci. 2018, 154, 8–16.
- Laudicina, V.A.; Scalenghe, R.; Pisciotta, A.; Parello, F.; Dazzi, C. Pedogenic carbonates and carbon pools in gypsiferous soils of a semiarid Mediterranean environment in south Italy. Geoderma 2013, 192, 31–38.
- Millière, L.; Gussone, N.; Moritz, T.; Bindschedler, S.; Verrecchia, E. Origin of strontium and calcium in pedogenic needle fibre calcite (NFC). Chem. Geol. 2019, 524, 329–344.
- Gile, L.H. Eolian and Associated Pedogenic Features of the Jornada Basin Floor, Southern New Mexico. Soil Sci. Soc. Am. J. 1999, 63, 151–163.
- Jiménez-Ballesta, R.; Bravo, S.; Amorós, J.A.; Pérez-De-Los-Reyes, C.; García-Pradas, J.; Sanchez, M.; García-Navarro, F.J. A morphological approach to evaluating the nature of vineyard soils in semiarid Mediterranean environment. Eur. J. Soil Sci. 2021, 73, 13201.
- Hasinger, O.; Spangenberg, J.E.; Millière, L.; Bindschedler, S.; Cailleau, G.; Verrecchia, E.P. Carbon dioxide in scree slope deposits: A pathway from atmosphere to pedogenic carbonate. Geoderma 2015, 247–248, 129–139.
- Gocke, M.; Pustovoytov, K.; Kuzyakov, Y. Effect of CO2 concentration on the initial recrystallization rate of pedogenic carbonate-Revealed by 14C and 13C labeling. Geoderma 2010, 155, 351–358.
- Zhao, X.; Zhao, C.; Stahr, K.; Kuzyakov, Y.; Wei, X. The effect of microorganisms on soil carbonate recrystallization and abiotic CO2 uptake of soil. Catena 2020, 192, 104592.
- Zhao, X.; Zhao, C.; Wang, J.; Stahr, K.; Kuzyakov, Y. CaCO3 recrystallization in saline and alkaline soils. Geoderma 2016, 282, 1–8.
- Gallagher, T.M.; Sheldon, N.D. Combining soil water balance and clumped isotopes to understand the nature and timing of pedogenic carbonate formation. Chem. Geol. 2016, 435, 79–91.
- Golubtsov, V.; Bronnikova, M.; Khokhlova, O.; Cherkashina, A.; Turchinskaia, S. Morphological and isotopic study of pedogenic carbonate coatings from steppe and forest-steppe areas of Baikal region, South-Eastern Siberia. Catena 2020, 196, 104817.
- Díaz-Hernández, J.; Sánchez-Navas, A.; Reyes, E. Isotopic evidence for dolomite formation in soils. Chem. Geol. 2013, 347, 20–33.
- Liu, Z.; Zhang, Y.; Fa, K.; Zhao, H.; Qin, S.; Yan, R.; Wu, B. Desert soil bacteria deposit atmospheric carbon dioxide in carbonate precipitates. Catena 2018, 170, 64–72.
- Liu, Z.; Sun, Y.; Zhang, Y.; Qin, S.; Sun, Y.; Mao, H.; Miao, L. Desert soil sequesters atmospheric CO2 by microbial mineral formation. Geoderma 2019, 361, 114104.
- Liu, X.; Monger, H.C.; Whitford, W.G. Calcium carbonate in termite galleries–biomineralization or upward transport? Biogeochemistry 2006, 82, 241–250.
- Mujinya, B.; Mees, F.; Boeckx, P.; Bodé, S.; Baert, G.; Erens, H.; Delefortrie, S.; Verdoodt, A.; Ngongo, M.; Van Ranst, E. The origin of carbonates in termite mounds of the Lubumbashi area, D.R. Congo. Geoderma 2011, 165, 95–105.
- Fa, K.; Liu, Z.; Zhang, Y.; Qin, S.; Wu, B.; Liu, J. Abiotic carbonate dissolution traps carbon in a semiarid desert. Sci. Rep. 2016, 6, 23570.




