Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bonginkosi A. Thango | -- | 1799 | 2022-08-30 16:54:31 | | | |
| 2 | Amina Yu | -1 word(s) | 1798 | 2022-08-31 04:13:13 | | | | |
| 3 | Amina Yu | Meta information modification | 1798 | 2022-09-01 08:27:23 | | | | |
| 4 | Amina Yu | Meta information modification | 1798 | 2022-09-01 08:33:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Thango, B.A. Solar Photovoltaic in Battery Energy Storage System. Encyclopedia. Available online: https://encyclopedia.pub/entry/26685 (accessed on 28 February 2026).
Thango BA. Solar Photovoltaic in Battery Energy Storage System. Encyclopedia. Available at: https://encyclopedia.pub/entry/26685. Accessed February 28, 2026.
Thango, Bonginkosi Allen. "Solar Photovoltaic in Battery Energy Storage System" Encyclopedia, https://encyclopedia.pub/entry/26685 (accessed February 28, 2026).
Thango, B.A. (2022, August 30). Solar Photovoltaic in Battery Energy Storage System. In Encyclopedia. https://encyclopedia.pub/entry/26685
Thango, Bonginkosi Allen. "Solar Photovoltaic in Battery Energy Storage System." Encyclopedia. Web. 30 August, 2022.
Copy Citation
Solar photovoltaic (PV) is proliferating, it also poses operational challenges attributable to its unpredictability and investment cost. Subsequently, solar PV exhibits variations inherent to renewable energy. These variations can emerge at any interval from seconds to minutes, necessitating the deployment of supplemental energy management devices. PV systems are additionally confronted by the cost differential during peak hours and the power quality given to the power grid. Energy storage technologies are integral parts that can support PV systems to be able to provide energy for longer hours in the absence of sunlight.
load shedding
battery energy storage systems (BESS)
photovoltaic (PV)
1. Solar Photovoltaic (PV) and Battery Energy Storage System
The rooftop solar PV systems convert solar radiation into electrical energy that may be consumed by South African residents, as shown in Figure 1 [1]. Any power that is not utilized is fed into the main grid. To conserve energy generated throughout the day, large-scale batteries can be coupled to solar PV systems. When the system is not producing enough power, particularly at night or in adverse weather conditions, this energy may be consumed. Using the power generated by a solar PV system throughout the day alleviates the amount of power purchased from the grid, lowering the energy costs.
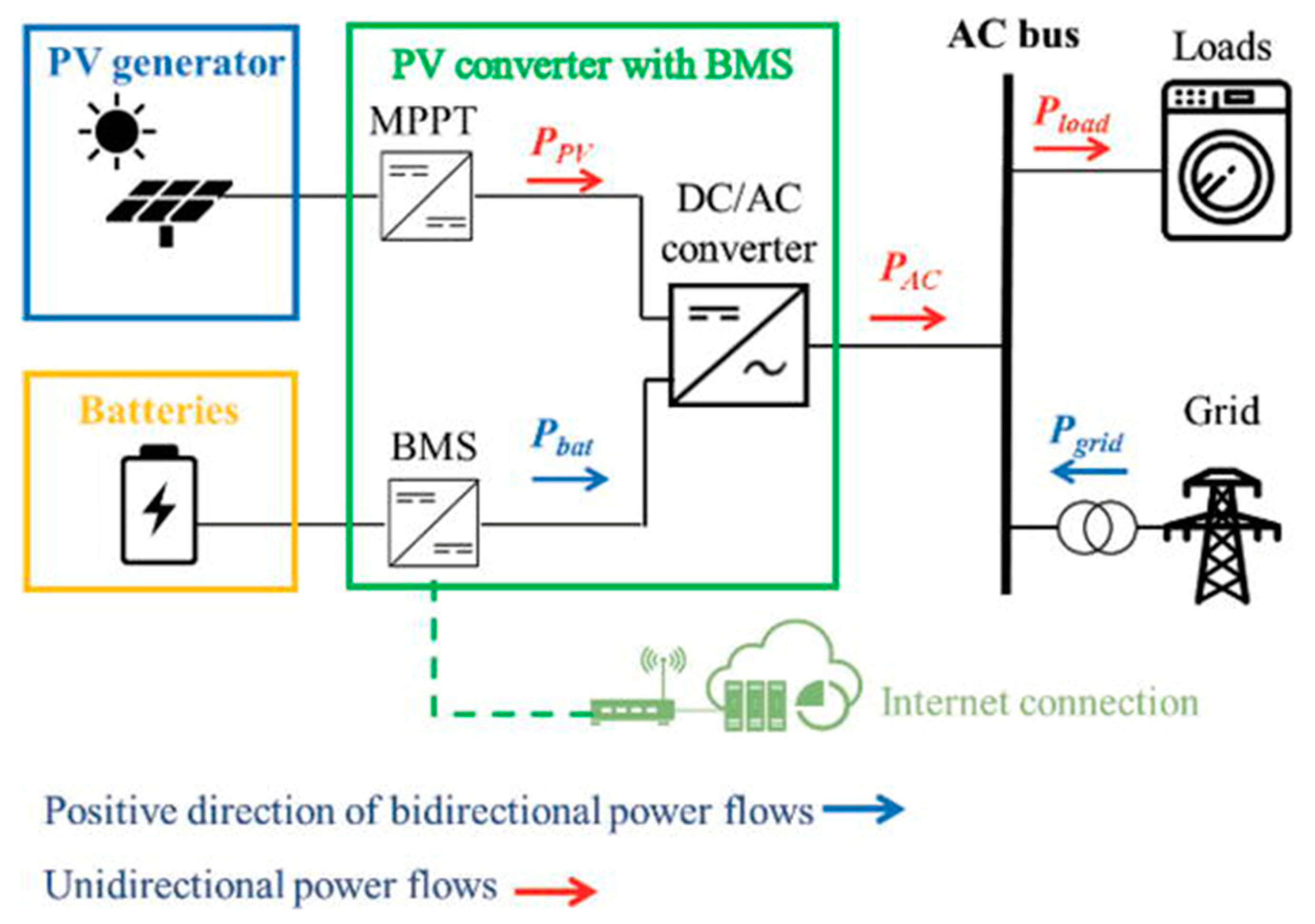
Figure 1. Solar PV-Battery Energy Storage System.
1.1. Selection and Deploying a Solar PV-Battery System
A variety of factors will significantly affect which solar PV-battery system is ideal for a household, including:
-
Amount of power and time of consumption;
-
Dimensions of available rooftop space;
-
Positioning and direction of solar PV panels.
Understanding the amount of energy consumption in a household may facilitate the evaluation of the impact of a solar PV system on the energy costs and establish whether battery storage is a cost-effective option. To realize the best options, licensed solar installers certified by South African PV GreenCard may further be consulted.
1.2. Emplacement of the Solar PV-Battery System
When solar PV panels are oriented directly toward effective solar irradiation between 09:00 a.m. and 15:00 p.m., they achieve the greatest power generation:
-
The PV panels must not be exposed to any shade. Even if a single PV cell is obscured by objects such as branches, roof vents, or satellite dishes, many other PV cells will lose power. Due to variations in the flow of energy through the panel, the latter will have a significant influence on the output of the panel.
-
The efficacy of batteries can be affected by the temperature in the surrounding environment.
-
Batteries necessitate a setting or housing that is well-insulated and well-ventilated. A battery enclosure should ideally be placed on the south or west side of a South African building.
1.3. Connecting Solar PV-Battery System to the Power Grid
Under its jurisdiction to administer electricity tariffs in South Africa, the National Energy Regulator of South Africa (NERSA) approved the establishment of a Renewable Energy Feed-in Tariff (REFIT) for the country. The feed-in tariff obligates the Renewable Energy Purchasing Agency (REPA), to purchase renewable energy from eligible producers at preset rates [2][3].
2. Battery Technologies
2.1. Lead–Acid Battery
The lead–acid battery, created in 1859, is the first kind of rechargeable battery ever developed [4]. Lead–acid batteries have a suboptimal energy density when compared to contemporary rechargeable batteries.
Lead–acid cells are composed of lead alloy grids (solid electrodes) that operate as current collectors and mechanically support the positive and negative active elements. The grids are interlaced with a permeable, electrically isolator and arrayed as positive and negative plates. The plate stack is embedded into an adequately contoured polymer housing to embody the cell elements and the electrolyte with the coupled positive and negative plates, terminals, a lid and venting arrangements. The construction of a lead–acid battery is shown in Figure 2.
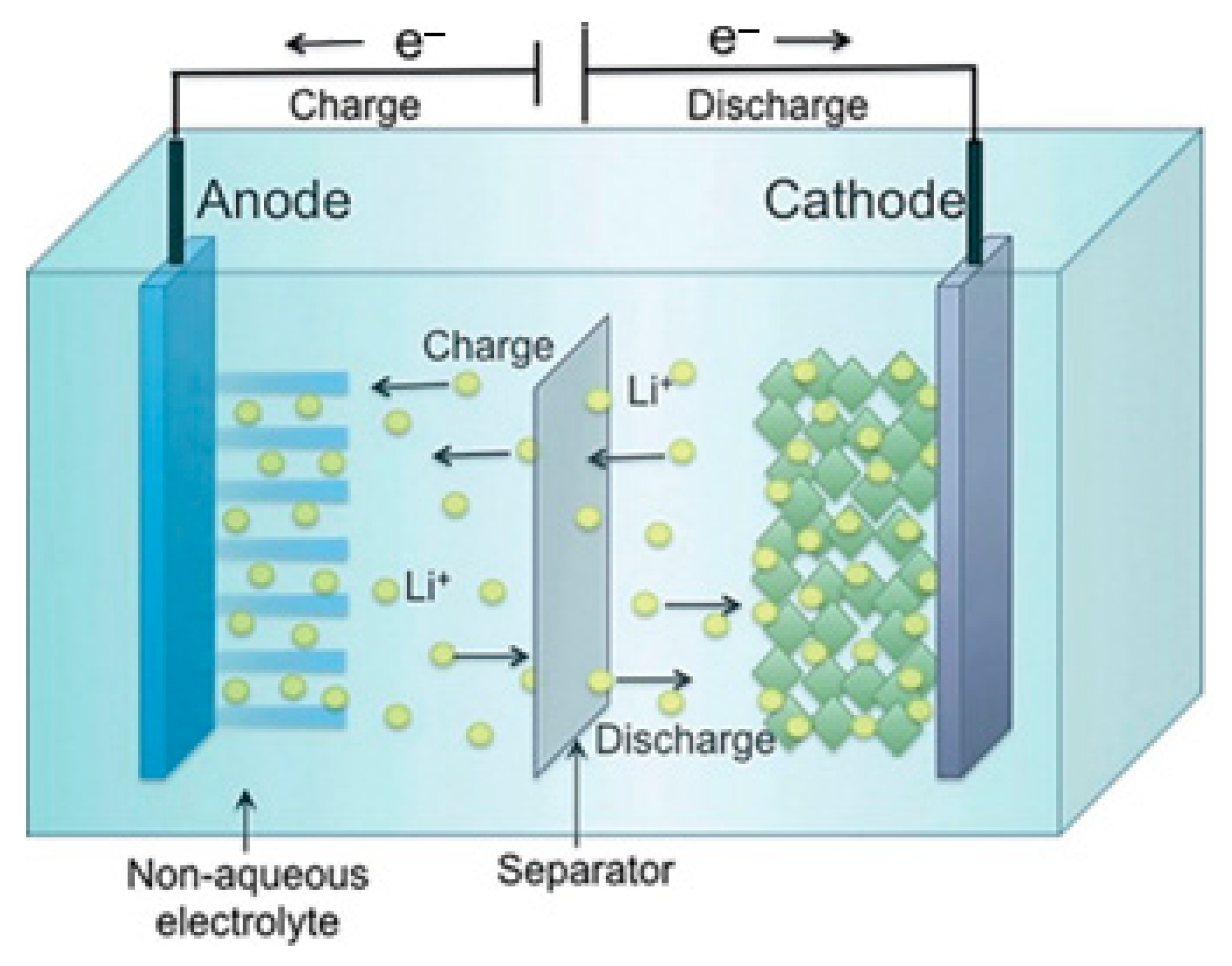
Figure 2. Lead–acid battery construction.
The operating voltage of the lead–acid cell is reasonably high at approximately 2.05 V. The positive active material (PAM) is considerably permeable lead dioxide (PbO₂) and the negative active material (NAM) is delicately isolated lead. The electrolyte utilized in the discharge process is thinned liquefied sulfuric acid (HSO4). HSO4 ions move to the negative electrode during discharge, producing H+ ions and lead sulfate (SO₄²−). Lead dioxide reacts with the electrolyte at the positive electrode to yield lead sulfate particles and water (H2O), as shown in Figure 3. Both electrodes are discharged to a feeble conductor, lead sulfate (PbSO4), and the electrolyte is incrementally diluted as the discharge progresses. On charging, the reactions are reversible [5][6].
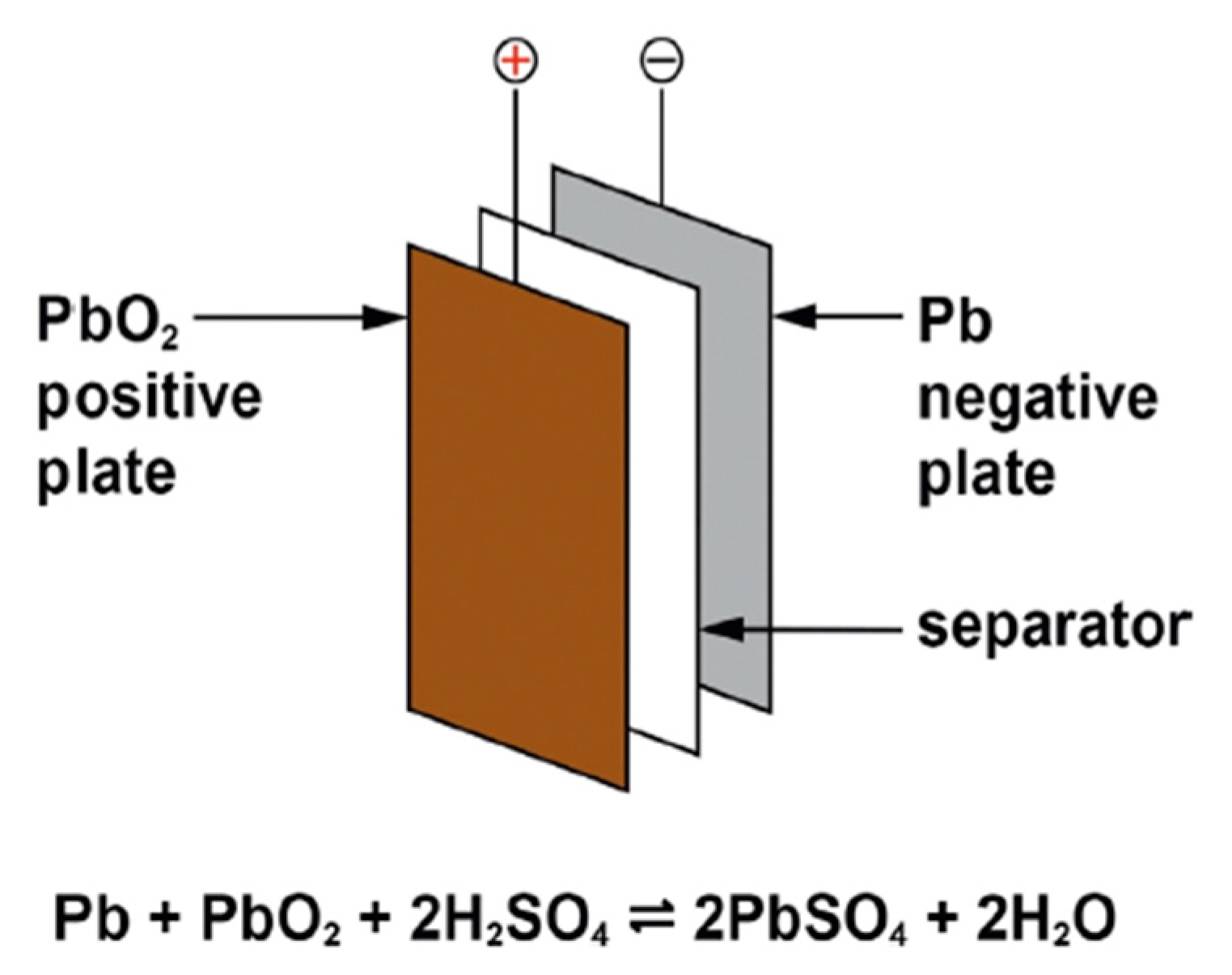
Figure 3. Chemistry of lead–acid battery.
The most recent innovations [7][8] have used enhanced lead batteries in a variety of grid-related plans as well as smaller-scale industrial and domestic energy storage applications. In recent years, systems with integrated super-capacitors have been described in addition to conventional lead–acid batteries; they are commonly referred to as carbon-enhanced (LC) lead batteries. These could have a negative electrode made of a mix of lead–acid and supercapacitor negatives made of carbon. The positive electrode is exactly like the one in a typical lead–acid battery in every way. The current tendency in operating renewable energy sources, especially solar PV sources, is for periodic discharges rather than a continuous restoration of the battery to a full state of charge (SOC). This partial state-of-charge (PSoC) behavior can be detrimental to lead–acid batteries since it induces permanent corrosion of the negative electrode, and sustainable development strategies are still being investigated [9][10].
2.2. Lithium-Ion Battery
Lithium-ion (Li-ion) batteries store energy in positive electrode materials composed of lithium extracts adequate for reversible physical adsorption of Li-ions and negative electrode materials made of carbon and can properly support Li in the solid state [11]. Since Li interacts severely with water, non-aqueous electrolytes are employed [12]. These are ionizable organic diluents, such as propylene carbonate, in solution with adequate lithium salts. To improve the safety of the cells, the separators are microporous plastic strips that may be covered with ceramic particles, as shown in Figure 4.
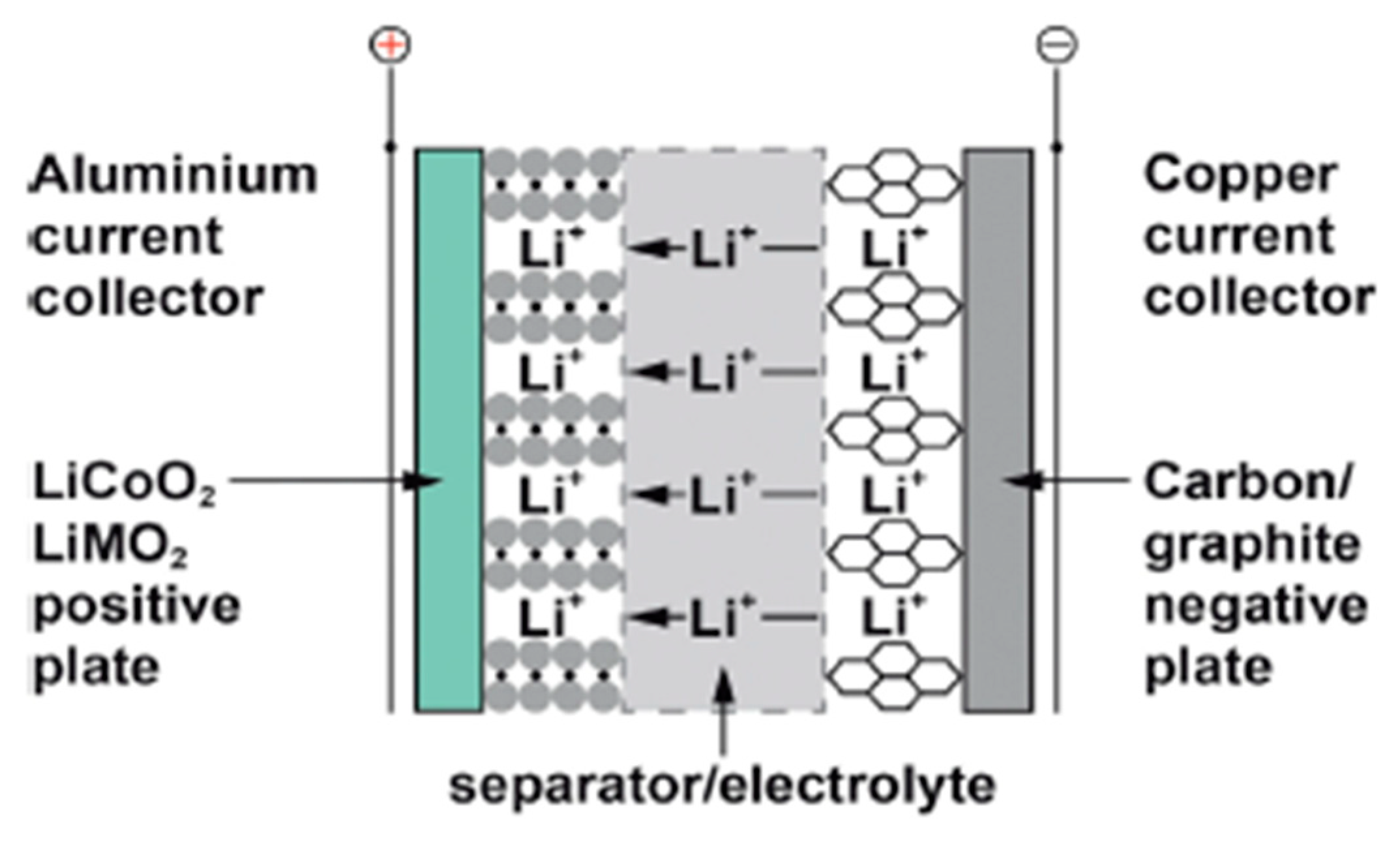
Figure 4. Chemistry and principal components of a lithium-ion battery.
Li-ion cells must be meticulously assessed in terms of safety. They have a high energy density and a volatile organic electrolyte.
2.3. Sodium–Sulfur Battery
The anodes of sodium–sulfur (Na-S) batteries are viscous liquid sodium and sulfur, and they run at hot temperatures, around 300 and 350 °C, to maintain the electrode’s liquid and to provide strong ionic conductivity in the electrolyte, which is a ceramic material [13][14]. At processing temperature, the electrolyte is beta-alumina (b-Al2O3), which transmits sodium ions. When sodium and sulfur are released, sodium polysulfide is produced [15]. They have a far superior energy density and durability over lead–acid batteries. To preclude cell defects from proliferating, security is essential, and careful design is essential. The chemistry and principal components of a sodium–sulfur battery are shown in Figure 5.
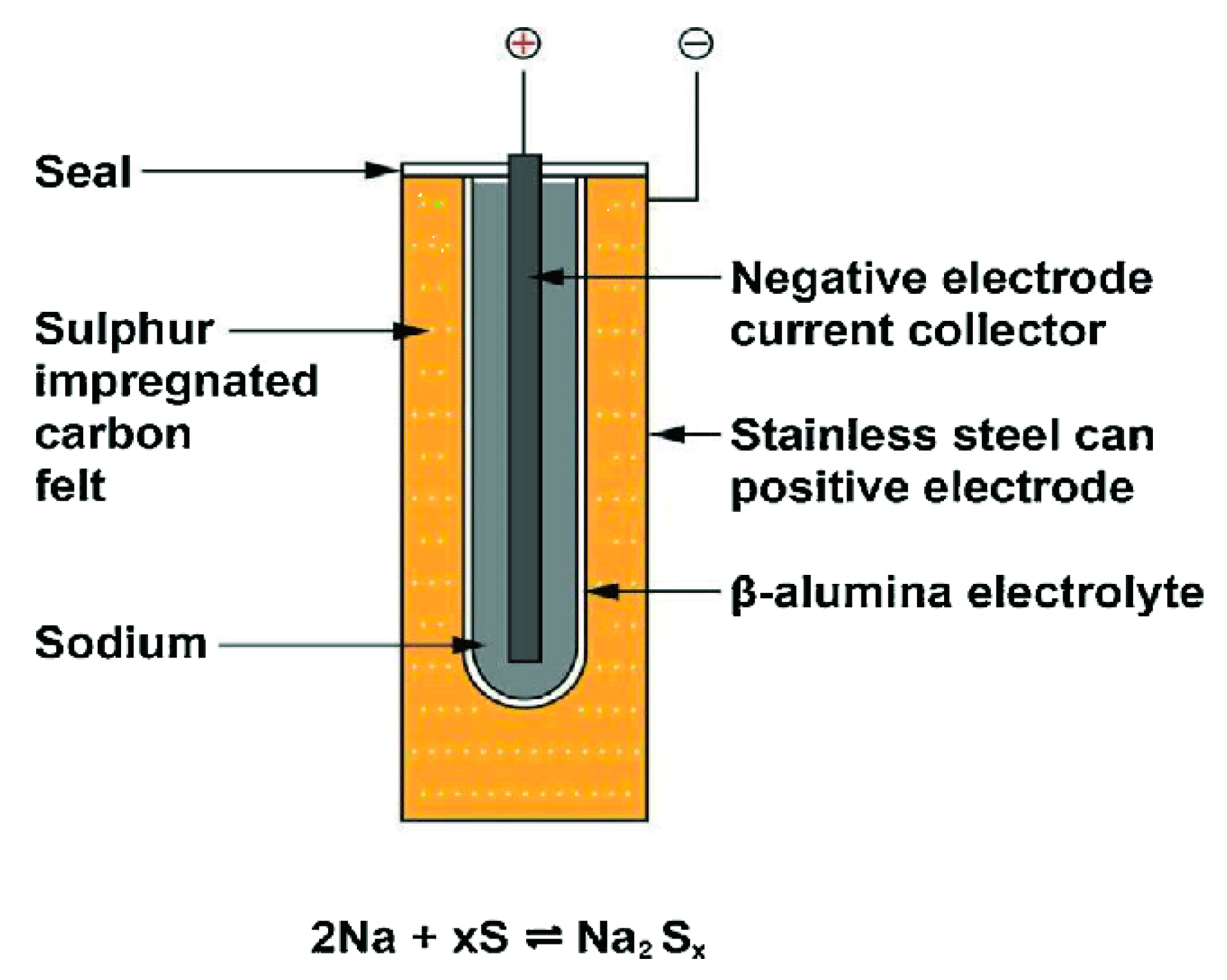
Figure 5. Chemistry and principal components of a sodium–sulfur battery.
Although Na-S batteries are made from abundant and inexpensive raw items, the production processes, as well as the necessity for insulation, cooling, and temperature control, make them fairly pricey. They are more cost-effective in large units since the thermal management of smaller batteries contributes to the cost relative to the battery’s volume. As a consequence, they are primarily employed for utility load levelling in big substations.
2.4. Sodium–Nickel Chloride Battery
In Na-NiCl2 batteries, a beta-alumina electrolyte is employed; however, the cathode is nickel chloride submerged in sodium aluminum chloride (NaAlCl4), a molten salt that conducts sodium ions, as opposed to a sulfur electrode [16]. Nickel metal and sodium chloride are created when sodium reacts with nickel chloride during discharge [16][17]. The system still needs heating, insulation, and temperature control even though it operates at a lower temperature than Na-S batteries (300 °C) [18]. Compared to Na-S batteries, the energy density is higher, but the battery life is longer. Figure 6 depicts the chemistry and main parts of a sodium–nickel chloride battery.

Figure 6. Chemistry and principal components of a sodium–nickel chloride battery.
2.5. Flow Batteries
Utility-scale energy storage has some promise thanks to flow batteries. There are many different compositions, but they all have energy-producing cells with electrode material stored remotely, making it possible for very large storage batteries to be made [19][20]. Vanadium redox batteries (VRB) are made up of cells with carbon composite electrodes submerged in a fluid containing aqueous acid and vanadium sulfate, with different valence states separated by an ion-selective membrane. At the positive electrode during discharge, V5+ is converted to V4+, while V2+ is converted to V3+ at the negative electrode. The volume of the vanadium sulfate solution, and hence the battery’s capacity, is potentially limitless because it is kept in a storage tank. Recharging causes reverse reactions, which replenish the materials. The batteries are complicated to use and made of heavy materials, but their expected lifespan is very lengthy. Only a few prototype systems have been implemented so far, and given the size of the battery, VRB batteries are only practical for utility energy storage. Figure 7 depicts the chemistry and main parts of a vanadium redox flow battery.
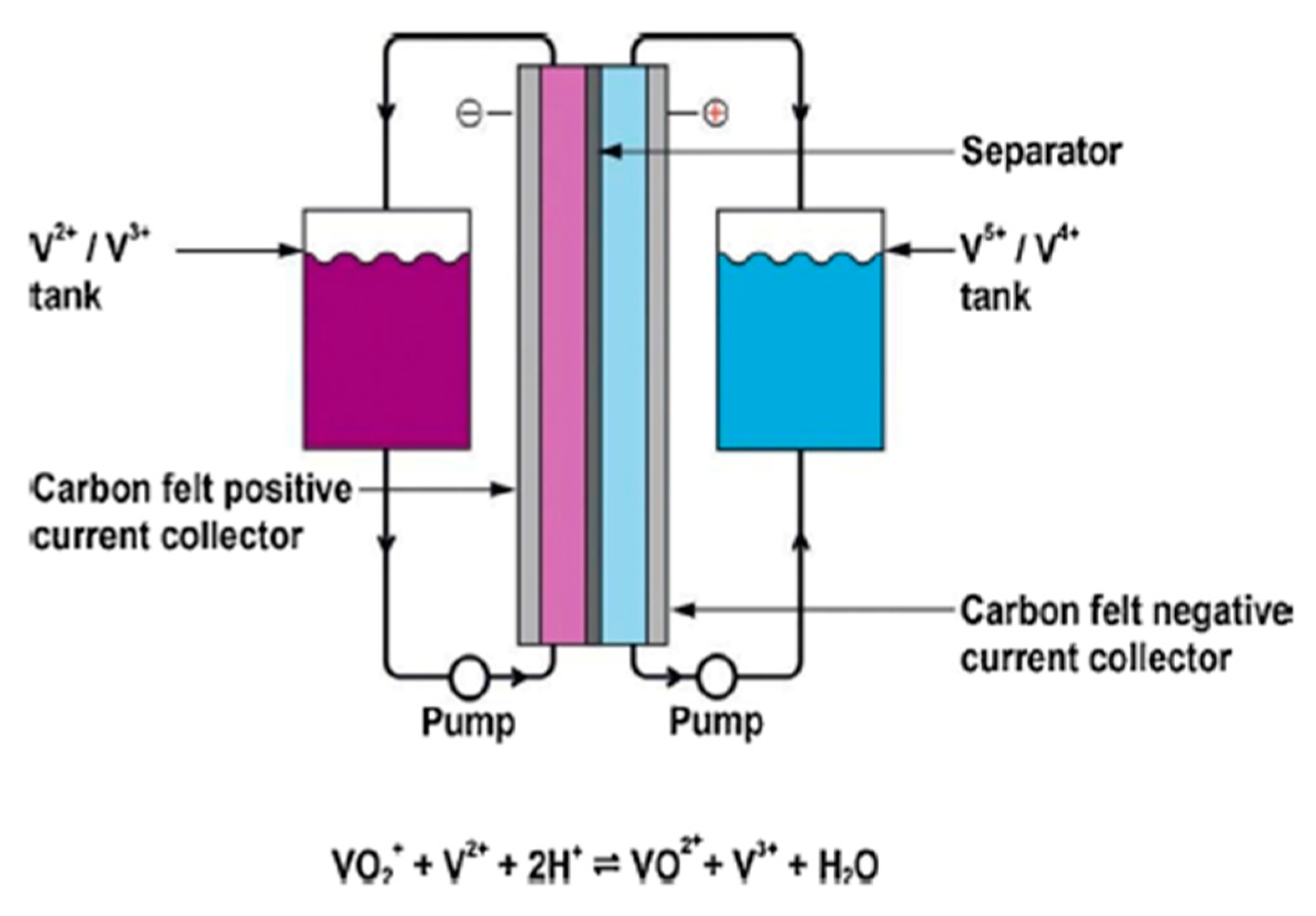
Figure 7. Chemistry and principal components of a vanadium redox flow battery.
Another kind of flow battery is the zinc–bromine (Zn-Br2) battery, as shown in Figure 8. Zinc bromide is synthesized when Zn reacts with Br2 within the cell. Br2 is injected into cells with carbon electrodes and a microporous plastic separator in an aqueous solution as an organic complexing agent [21][22].
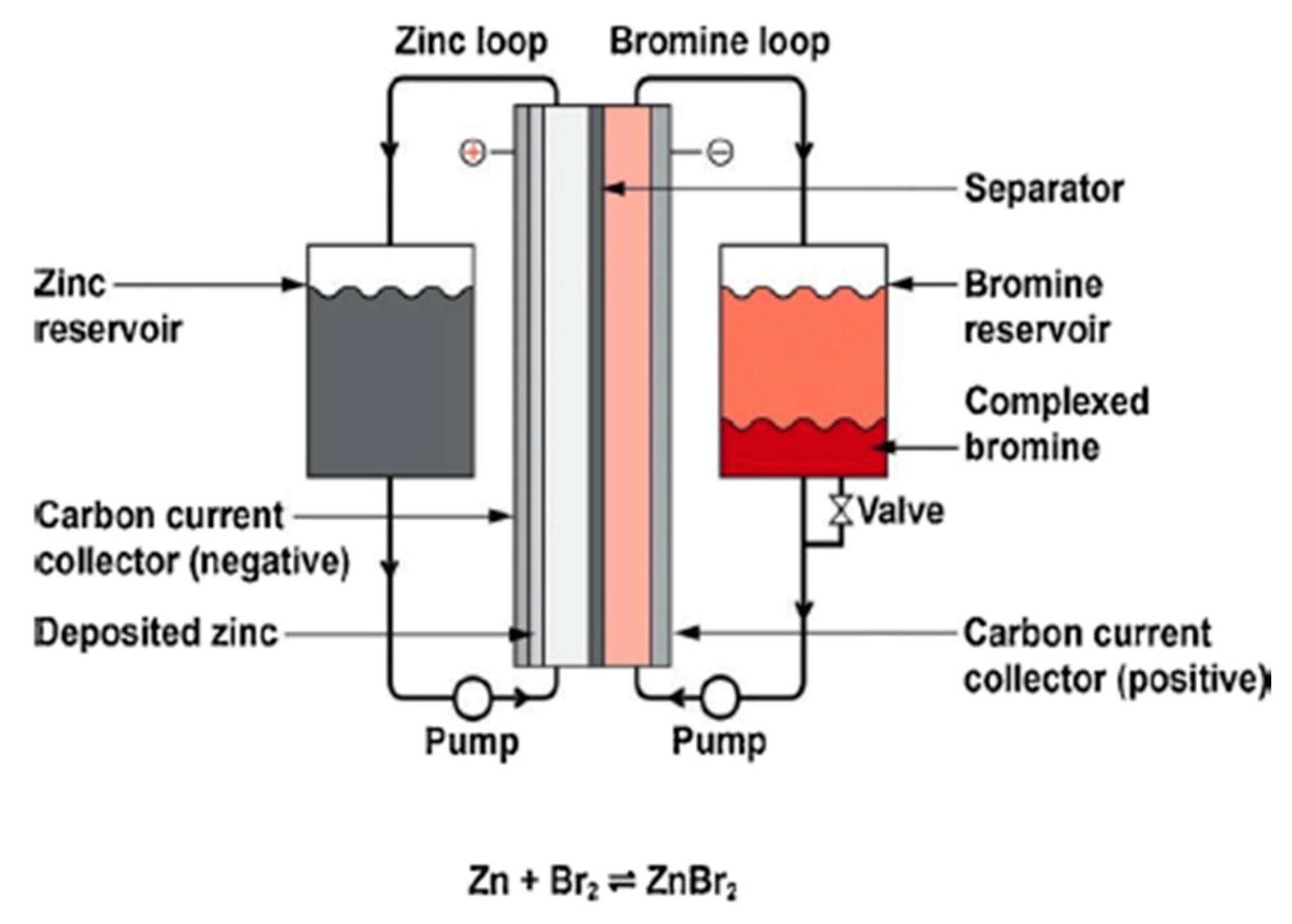
Figure 8. Chemistry and principal components of a zinc–bromine battery.
Metallic Zn is formed on charging, and while Br2 is housed in tanks, the Zn electrode enforces a limit on the capacity for any specific design. The price is cheaper than VRB batteries, but the average lifetime is less. The discharge of bromine is a perceived threat that must be avoided. Zn-Br2 batteries, like other flow batteries, have only been employed in moderate numbers for utility usage. There are a few similar types of flow batteries, such as iron–chromium batteries; however, they are not broadly utilized.
The technical comparison of the aforementioned battery technologies has been tabulated as demonstrated in Table 1.
Table 1. Technical comparison of battery technology in South Africa [4][5][6][7][8][9][10][11][12][13][14][15][16][17][18].
| Battery | Lead–Acid [4] | Lithium-Ion [5] | Sodium–Sulfur [6] | Sodium–Nickel Chloride [7] | Zinc–BROMINE [8] | Vanadium Redox [9] |
|---|---|---|---|---|---|---|
| Energy density (Wh/L) | 80–90 | 250–693 | 110 | 100–120 | 15–65 | 15–25 |
| Nominal cell voltage (V) | 2.1 | 3.6/3.7/3.8/3.85, LiFePO4 3.2 | 1.78–2.208 | 2.58 | 1.8 | 1.15–1.55 |
| Specific energy (Wh/kg) |
35–40 | 100–265 | 150 | 350 | 60–85 | 10–20 |
| Self-discharge rate | 1%/day | 5%/day | 20%/day | 5–20%/day | 10%/day | |
| Cycle durability | <350 | 400–1200 | 4500 | 4500 | >2000 | >12,000–14,000 |
| Charge/discharge efficiency | 50–95% | 80–90% | 80% | 85–95% | 75.9% | 75–80%< |
| Key Challenges and Limitations |
|
|
|
|
|
|
References
- Thirugnanam, K.; Kerk, S.K.; Yuen, C.; Liu, N.; Zhang, M. Energy Management for Renewable Microgrid in Reducing Diesel Generators Usage with Multiple Types of Battery. IEEE Trans. Ind. Electron. 2018, 65, 6772–6786.
- Venu, C.; Riffonneau, Y.; Bacha, S.; Baghzouz, Y. Battery Storage System sizing in distribution feeders with distributed photovoltaic systems. In Proceedings of the 2009 IEEE Bucharest PowerTech, Bucharest, Romania, 28 June–2 July 2009; pp. 1–5.
- Wang, Z.; Gu, C.; Li, F.; Bale, P.; Sun, H. Active Demand Response Using Shared Energy Storage for Household Energy Management. IEEE Trans. Smart Grid 2013, 4, 1888–1897.
- Kumar, D.; Zare, F.; Ghosh, A. DC Microgrid Technology: System Architectures, AC Grid Interfaces, Grounding Schemes, Power Quality, Communication Networks, Applications, and Standardizations Aspects. IEEE Access 2017, 5, 12230–12256.
- Lezhniuk, P.; Komar, V.; Rubanenko, O. Information Support for the Task of Estimation the Quality of Functioning of the Electricity Distribution Power Grids with Renewable Energy Source. In Proceedings of the 2020 IEEE 7th International Conference on Energy Smart Systems (ESS), Kyiv, Ukraine, 12–14 May 2020; pp. 168–171.
- 450-2020; IEEE Recommended Practice for Maintenance, Testing, and Replacement of Vented Lead-Acid Batteries for Stationary Applications—Redline. IEEE: Manhattan, NY, USA, 2021; pp. 1–115.
- P1361/D4; IEEE Draft Guide for Selection, Charging, Test and Evaluation of Lead-Acid Batteries Used in Stand-Alone Photovoltaic (PV) Systems. IEEE: Manhattan, NY, USA, 2013; pp. 1–41.
- 1188-2005; IEEE Recommended Practice for Maintenance, Testing, and Replacement of Valve-Regulated Lead-Acid (VRLA) Batteries for Stationary Applications—Redline. IEEE: Manhattan, NY, USA, 2006; pp. 1–49.
- Li, T.; Chen, Y.; Gou, H.Y.; Chen, X.Y.; Tang, M.G.; Lei, Y. A DC Voltage Swell Compensator Based on SMES Emulator and Lead-Acid Battery. IEEE Trans. Appl. Supercond. 2019, 29, 1–4.
- Freitas, D.C.C.; de Moraes, J.L.; Neto, E.C.; Sousa, J.R.B. Battery Charger Lead-Acid using IC BQ2031. IEEE Lat. Am. Trans. 2016, 14, 32–37.
- McKeon, B.B.; Furukawa, J.; Fenstermacher, S. Advanced Lead–Acid Batteries and the Development of Grid-Scale Energy Storage Systems. Proc. IEEE 2014, 102, 951–963.
- Chiu, H.; Lin, L.; Pan, P.; Tseng, M. A novel rapid charger for lead-acid batteries with energy recovery. IEEE Trans. Power Electron. 2006, 21, 640–647.
- Hannan, M.A.; Hoque, M.M.; Hussain, A.; Yusof, Y.; Ker, P.J. State-of-the-Art and Energy Management System of Lithium-Ion Batteries in Electric Vehicle Applications: Issues and Recommendations. IEEE Access 2018, 6, 19362–19378.
- Zhang, W.; Wang, L.; Wang, L.; Liao, C.; Zhang, Y. Joint State-of-Charge and State-of-Available-Power Estimation Based on the Online Parameter Identification of Lithium-Ion Battery Model. IEEE Trans. Ind. Electron. 2022, 69, 3677–3688.
- Al-Humaid, Y.M.; Khan, K.A.; Abdulgalil, M.A.; Khalid, M. Two-Stage Stochastic Optimization of Sodium-Sulfur Energy Storage Technology in Hybrid Renewable Power Systems. IEEE Access 2021, 9, 162962–162972.
- Saruta, K. Long-term performance of sodium sulfur battery. In Proceedings of the 2016 IEEE 2nd Annual Southern Power Electronics Conference (SPEC), Auckland, New Zealand, 5–8 December 2016; pp. 1–5.
- Hatta, T. Applications of sodium-sulfur batteries. In PES T&D 2012; IEEE: Manhattan, NY, USA, 2012; pp. 1–3.
- Manzoni, R. Sodium Nickel Chloride batteries in transportation applications. In Proceedings of the 2015 International Conference on Electrical Systems for Aircraft, Railway, Ship Propulsion and Road Vehicles (ESARS), Aachen, Germany, 3–5 March 2015; pp. 1–6.
- Marcondes, A.; Scherer, H.F.; Salgado, J.R.C.; de Freitas, R.L.B. Sodium-nickel chloride single cell battery electrical model—Discharge voltage behavior. In Proceedings of the 2019 Workshop on Communication Networks and Power Systems (WCNPS), Brasilia, Brazil, 3–4 October 2019; pp. 1–4.
- Benato, R.; Sessa, S.D.; Necci, A.; Palone, F. A general electric model of sodium-nickel chloride battery. In Proceedings of the 2016 AEIT International Annual Conference (AEIT), Capri, Italy, 5–7 October 2016; pp. 1–6.
- Challapuram, Y.R.; Quintero, G.M.; Bayne, S.B.; Subburaj, A.S.; Harral, M.A. Electrical Equivalent Model of Vanadium Redox Flow Battery. In Proceedings of the 2019 IEEE Green Technologies Conference (GreenTech), Lafayette, LA, USA, 3–6 April 2019; pp. 1–4.
- Vins, M.; Sirovy, M. Assessing Suitability of Various Battery Technologies for Energy Storages: Lithium-ion, Sodium-sulfur and Vanadium Redox Flow Batteries. In Proceedings of the 2020 International Conference on Applied Electronics (AE), Pilsen, Czech Republic, 8–9 September 2020; pp. 1–5.
More
Information
Subjects:
Engineering, Electrical & Electronic
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.6K
Revisions:
4 times
(View History)
Update Date:
01 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





