
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wei-Wen Lim | -- | 2144 | 2022-08-19 01:08:15 | | | |
| 2 | Lindsay Dong | + 1 word(s) | 2145 | 2022-08-22 05:06:41 | | |
Video Upload Options
Interleukin-11 (IL11), a stromal-cell derived pleiotropic cytokine with profibrotic and cellular remodeling properties, as a potential biomarker in non-small cell lung cancer (NSCLC). IL11 is an important tumor-promoting cytokine that that has both diagnostic and prognostic value in patients with NSCLC. Multiple in vitro studies confirm that IL11 activates known tumor-promoting signaling pathways and clinical studies link increased IL11 expression to poorer prognosis.
1. Interleukin-11: Member of the IL6 Family of Cytokines
| Cytokine | Receptors | References |
|---|---|---|
| IL6 | IL6R, gp130/IL6ST | [7][8][9][10][11][12][13][14] |
| IL-31 | IL31Rα, OSMR | [15] |
| LIF | LIFR/LIFRα, gp130/IL6ST | [16] |
| OSM | OSMR/OSMRβ, gp130/IL6ST, LIFR | [17][18] |
| CLCF1 | CNTFR, LIFR, gp130/IL6ST | [14][19] |
2. Interleukin 11 Drives Pulmonary Fibrosis and Inflammation
3. Interleukin 11 Is a Tumor-Promoting Cytokine in NSCLC
4. Sources of Biomarkers in NSCLC
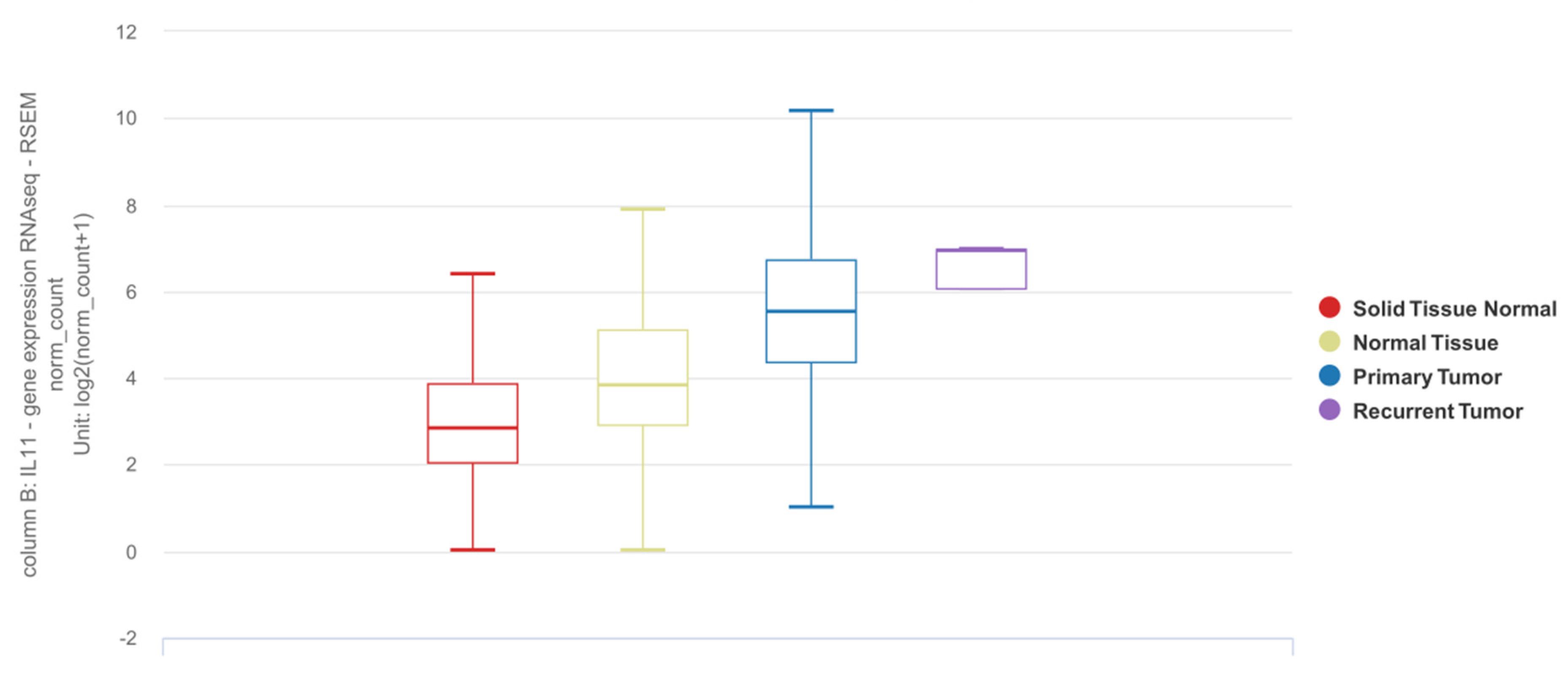
5. IL11 Differential Expression in NSCLC Tumor Tissue
6. Detection of IL11 for Diagnosis of NSCLC
To the best of the knowledge, there are two studies that describe the utility of IL11 as a diagnostic biomarker (Table 2). Pastor et al. recruited a prospective cohort of 369 patients for which use of diagnostic biomarkers in bronchoalveolar lavage fluid (BALF) can potentially facilitate early detection of lung cancer [52]. Among 80 cytokines and growth factors, inflammation-related protein array analyses identified IL11 expression to be differentially increased in LUAD BALF samples in an initial discovery cohort, and subsequently validated in two separate exploratory and diagnostic cohorts. This study demonstrated that BALF IL11 expression was largely restricted to patients with LUAD, with or without chronic obstructive pulmonary disease (COPD). Despite the eventual diagnosis at stage III or IV in the majority of the LUAD cohort, the diagnostic performance of BALF IL11 remains similar between the subgroups even for the early stages. Interestingly, BALF IL11 expression was not increased in squamous cell carcinoma (SCC), a type of NSCLC of epithelial origin, which may suggest cell type-selective IL11 expression in NSCLC. In another study, Wu et al. measured IL11 protein concentration in serum and exhaled breath condensate in NSCLC cases compared to healthy donors and found increased IL11 expression in NSCLC patients even at early-stage disease [53].
| Study | Recruited Population | Comparison | Sample Type | Diagnostic Biomarker | Assay | Receiver Operator Curve and Test Metrics | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) |
Cutoff (pg/mL) | Sensitivity (95% CI) |
Specificity (95% CI) | PPV (95% CI) |
NPV (95% CI) |
||||||
| Pastor et al. [52] | Age > 40 yrs, current or ex-smokers of 30 pack-years, evaluated for hemoptysis or pulmonary nodule or mass, excluding those with prior diagnosis of malignancy, active tuberculosis, history of drug abuse or other inflammatory disease apart from COPD | LUAD vs. non-LUAD—First validation cohort (n = 149) | BALF | IL11 protein | ELISA | 0.93 (0.90–0.97) | 42.0 | 90.2 (79–95.7) | 88.7 (90.6–93.5) | 80.7 (68.7–88.9) | 94.5 (87.8–97.6) |
| LUAD vs. non-LUAD—Second validation cohort (n = 160) | BALF | IL11 protein | ELISA | 0.95 (0.92–0.98) | 42.0 | 90.6 (79.7–95.9) | 83.0 (86.8–87.7) | 60.8 (49.7–70.8) | 96.8 (92.7–98.6) | ||
| Wu et al. [53] | NSCLC patients with no history of radiochemotherapy, immune-targeted therapy or surgery (n = 91 for serum, of which 63 have LUAD and 28 have SCC; 64 for EBC) | Healthy volunteers without acute or chronic infectious diseases, vital organ diseases, or genetic family tumor history (n = 72 for serum; 63 for EBC) | Serum | IL11 protein | ELISA | 0.93 (0.88–0.97) | 126.1 | 75.0 | 100.0 | NR | NR |
| EBC | IL11 protein | ELISA | 0.78 (0.69–0.86) | 21.5 | 78.1 | 79.4 | NR | NR | |||
7. Quantification of IL11 Expression for Prognostication of NSCLC
Accurate prognostication is important for identifying patients who may benefit from adjuvant or neoadjuvant therapy. Using molecular biomarkers in addition to clinical data can potentially allow patients to be stratified into risk groups with greater accuracy [54][55]. Numerous studies have identified IL11 mRNA in NSCLC lung tissue taken at the time of surgical resection to be prognostic of overall survival, both by itself and among other genes as part of a prognostic signature (summarized in Table 3).
| Study | Year | Training Cohort | Validation Cohort (s) | Cancer Type | Prognostic Signature | Findings |
|---|---|---|---|---|---|---|
| Kratz et al. [55] | 2012 | Non-squamous NSCLC (n = 361) | Stage I non-squamous NSCLC (n = 433), and stage I-III non-squamous NSCLC (n = 1006) | Non-squamous NSCLC | 11 Target genes (BAG1, BRCA1, CDC6, CDK2AP1, ERBB3, FUT3, IL11, LCK) and 3 reference genes (ESD, TBP, YAP1) |
|
| Watza et al. [56] | 2018 | NSCLC patients without history of bronchiectasis or cystic fibrosis (n = 280) | TCGA Lung SCC and TCGA LUAD datasets (n = 1026) | NSCLC | 23 genes involved in the interleukin signaling pathway, including IL11 |
|
| Wang et al. [57] | 2020 | TCGA LUAD dataset (497 LUAD tissues, 54 normal lung tissues) | n/a | LUAD | 6 genes (CRABP1, IGKV4-1, IL11, INHA, LGR4, VIPR1) |
|
| Fan et al. [58] | 2021 | TGCA LUAD dataset (n = 464)(majority stage I and II) | GSE13213 (n = 117), GSE30219 (n = 85), GSE31210 (n = 226), GSE72094 (n = 420)(majority stage I and II) | LUAD | 5 genes (IL7R, IL5RA, IL20RB, IL11, IL22RA1) |
|
| Chen et al. [59] | 2021 | TCGA LUAD (535 LUAD tissues, 59 normal lung tissues)GSE161116 (9 LUAD tissues, 9 LUAD brain metastasis tissues) | n/a | LUAD | 6 genes (TNFRSF11A, MS4A2, IL11, CAMP, MS4A1, F2RL1) |
|
| Peng et al. [60] | 2021 | GSE161116 (13 lung tumor tissues, 15 brain tissues), GSE747706 (18 lung tumor tissues, 18 normal tissues), GSE21933 (21 lung tumor tissues, 21 normal tissues) datasets | n/a | NSCLC andBrain tumor | n/a |
|
References
- Metcalfe, R.D.; Putoczki, T.L.; Griffin, M.D.W. Structural Understanding of Interleukin 6 Family Cytokine Signaling and Targeted Therapies: Focus on Interleukin 11. Front. Immunol. 2020, 11, 1424.
- Rose-John, S. Interleukin-6 Family Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028415.
- van Duijneveldt, G.; Griffin, M.D.W.; Putoczki, T.L. Emerging Roles for the IL-6 Family of Cytokines in Pancreatic Cancer. Clin. Sci. 2020, 134, 2091–2115.
- Felcher, C.M.; Bogni, E.S.; Kordon, E.C. IL-6 Cytokine Family: A Putative Target for Breast Cancer Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 1809.
- Unver, N.; McAllister, F. IL-6 Family Cytokines: Key Inflammatory Mediators as Biomarkers and Potential Therapeutic Targets. Cytokine Growth Factor Rev. 2018, 41, 10–17.
- Jones, S.A.; Jenkins, B.J. Recent Insights into Targeting the IL-6 Cytokine Family in Inflammatory Diseases and Cancer. Nat. Rev. Immunol. 2018, 18, 773–789.
- Xu, B.; Chen, Q.; Yue, C.; Lan, L.; Jiang, J.; Shen, Y.; Lu, B. Prognostic Value of IL-6R mRNA in Lung Adenocarcinoma and Squamous Cell Carcinoma. Oncol. Lett. 2018, 16, 2935–2948.
- Silva, E.M.; Mariano, V.S.; Pastrez, P.R.A.; Pinto, M.C.; Castro, A.G.; Syrjanen, K.J.; Longatto-Filho, A. High Systemic IL-6 Is Associated with Worse Prognosis in Patients with Non-Small Cell Lung Cancer. PLoS ONE 2017, 12, e0181125.
- Liao, C.; Yu, Z.; Guo, W.; Liu, Q.; Wu, Y.; Li, Y.; Bai, L. Prognostic Value of Circulating Inflammatory Factors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Cancer Biomark. 2014, 14, 469–481.
- De Vita, F.; Orditura, M.; Auriemma, A.; Infusino, S.; Roscigno, A.; Catalano, G. Serum Levels of Interleukin-6 as a Prognostic Factor in Advanced Non-Small Cell Lung Cancer. Oncol. Rep. 1998, 5, 649–652.
- Meaney, C.L.; Zingone, A.; Brown, D.; Yu, Y.; Cao, L.; Ryan, B.M. Identification of Serum Inflammatory Markers as Classifiers of Lung Cancer Mortality for Stage I Adenocarcinoma. Oncotarget 2017, 8, 40946–40957.
- Song, L.; Smith, M.A.; Doshi, P.; Sasser, K.; Fulp, W.; Altiok, S.; Haura, E.B. Antitumor Efficacy of the Anti-Interleukin-6 (IL-6) Antibody Siltuximab in Mouse Xenograft Models of Lung Cancer. J. Thorac. Oncol. 2014, 9, 974–982.
- Keegan, A.; Ricciuti, B.; Garden, P.; Cohen, L.; Nishihara, R.; Adeni, A.; Paweletz, C.; Supplee, J.; Jänne, P.A.; Severgnini, M.; et al. Plasma IL-6 Changes Correlate to PD-1 Inhibitor Responses in NSCLC. J. Immunother Cancer 2020, 8, e000678.
- Vicent, S.; Sayles, L.C.; Vaka, D.; Khatri, P.; Gevaert, O.; Chen, R.; Zheng, Y.; Gillespie, A.K.; Clarke, N.; Xu, Y.; et al. Cross-Species Functional Analysis of Cancer-Associated Fibroblasts Identifies a Critical Role for CLCF1 and IL-6 in Non-Small Cell Lung Cancer in Vivo. Cancer Res. 2012, 72, 5744–5756.
- Yang, Y.; Li, L.; Chen, F.; Zhang, L.; Bu, H. The Role of Interleukin-31 Polymorphisms in Non-Small Cell Lung Cancer Genetic Susceptibility and Clinical Outcome. Genet. Test. Mol. Biomark. 2018, 22, 314–319.
- Wang, H.; Si, S.N.; Jiang, M.; Chen, L.; Huang, K.; Yu, W. Leukemia Inhibitory Factor Is Involved in the Pathogenesis of NSCLC through Activation of the STAT3 Signaling Pathway. Oncol. Lett. 2021, 22, 663.
- Chen, D.; Chu, C.-Y.; Chen, C.-Y.; Yang, H.-C.; Chiang, Y.-Y.; Lin, T.-Y.; Chiang, I.-P.; Chuang, D.-Y.; Yu, C.-C.; Chow, K.-C. Expression of Short-Form Oncostatin M Receptor as a Decoy Receptor in Lung Adenocarcinomas. J. Pathol. 2008, 215, 290–299.
- Shien, K.; Papadimitrakopoulou, V.A.; Ruder, D.; Behrens, C.; Shen, L.; Kalhor, N.; Song, J.; Lee, J.J.; Wang, J.; Tang, X.; et al. JAK1/STAT3 Activation through a Proinflammatory Cytokine Pathway Leads to Resistance to Molecularly Targeted Therapy in Non-Small Cell Lung Cancer. Mol. Cancer Ther. 2017, 16, 2234–2245.
- Kim, J.W.; Marquez, C.P.; Kostyrko, K.; Koehne, A.L.; Marini, K.; Simpson, D.R.; Lee, A.G.; Leung, S.G.; Sayles, L.C.; Shrager, J.; et al. Antitumor Activity of an Engineered Decoy Receptor Targeting CLCF1-CNTFR Signaling in Lung Adenocarcinoma. Nat. Med. 2019, 25, 1783–1795.
- Ng, B.; Cook, S.A.; Schafer, S. Interleukin-11 Signaling Underlies Fibrosis, Parenchymal Dysfunction, and Chronic Inflammation of the Airway. Exp. Mol. Med. 2020, 52, 1871–1878.
- Ng, B.; Dong, J.; D’Agostino, G.; Viswanathan, S.; Widjaja, A.A.; Lim, W.-W.; Ko, N.S.J.; Tan, J.; Chothani, S.P.; Huang, B.; et al. Interleukin-11 Is a Therapeutic Target in Idiopathic Pulmonary Fibrosis. Sci. Transl. Med. 2019, 11, eaaw1237.
- Ng, B.; Dong, J.; Viswanathan, S.; Widjaja, A.A.; Paleja, B.S.; Adami, E.; Ko, N.S.J.; Wang, M.; Lim, S.; Tan, J.; et al. Fibroblast-Specific IL11 Signaling Drives Chronic Inflammation in Murine Fibrotic Lung Disease. FASEB J. 2020, 34, 11802–11815.
- Paul, S.R.; Bennett, F.; Calvetti, J.A.; Kelleher, K.; Wood, C.R.; O’Hara, R.M., Jr.; Leary, A.C.; Sibley, B.; Clark, S.C.; Williams, D.A. Molecular Cloning of a cDNA Encoding Interleukin 11, a Stromal Cell-Derived Lymphopoietic and Hematopoietic Cytokine. Proc. Natl. Acad. Sci. USA 1990, 87, 7512–7516.
- Tepler, I.; Elias, L.; Smith, J.W., 2nd; Hussein, M.; Rosen, G.; Chang, A.Y.; Moore, J.O.; Gordon, M.S.; Kuca, B.; Beach, K.J.; et al. A Randomized Placebo-Controlled Trial of Recombinant Human Interleukin-11 in Cancer Patients with Severe Thrombocytopenia due to Chemotherapy. Blood 1996, 87, 3607–3614.
- Xu, Y.; Song, X.; Du, F.; Zhao, Q.; Liu, L.; Ma, Z.; Lu, S. A Randomized Controlled Study of rhTPO and rhIL-11 for the Prophylactic Treatment of Chemotherapy-Induced Thrombocytopenia in Non-Small Cell Lung Cancer. J. Cancer 2018, 9, 4718–4725.
- Soda, H.; Raymond, E.; Sharma, S.; Lawrence, R.; Cerna, C.; Gomez, L.; Schaub, R.; Von Hoff, D.D.; Izbicka, E. Recombinant Human Interleukin-11 Is Unlikely to Stimulate the Growth of the Most Common Solid Tumors. Anticancer Drugs 1999, 10, 97–101.
- Saitoh, M.; Taguchi, K.; Momose, K.; Suga, K.; Yamazaki, N.; Ono, C.; Suzuki, T.; Takeuchi, O.; Yasuda, S.; Miyata, K. Recombinant Human Interleukin-11 Improved Carboplatin-Induced Thrombocytopenia without Affecting Antitumor Activities in Mice Bearing Lewis Lung Carcinoma Cells. Cancer Chemother. Pharmacol. 2002, 49, 161–166.
- Spence, M.J.; Streiff, R.; Day, D.; Ma, Y. Oncostatin M Induces Tissue-Type Plasminogen Activator and Plasminogen Activator Inhibitor-1 in Calu-1 Lung Carcinoma Cells. Cytokine 2002, 18, 26–34.
- Howlett, M.; Giraud, A.S.; Lescesen, H.; Jackson, C.B.; Kalantzis, A.; Van Driel, I.R.; Robb, L.; Van der Hoek, M.; Ernst, M.; Minamoto, T.; et al. The Interleukin-6 Family Cytokine Interleukin-11 Regulates Homeostatic Epithelial Cell Turnover and Promotes Gastric Tumor Development. Gastroenterology 2009, 136, 967–977.
- Wang, D.; Zheng, X.; Fu, B.; Nian, Z.; Qian, Y.; Sun, R.; Tian, Z.; Wei, H. Hepatectomy Promotes Recurrence of Liver Cancer by Enhancing IL-11-STAT3 Signaling. EBioMedicine 2019, 46, 119–132.
- Ma, J.; Song, X.; Xu, X.; Mou, Y. Cancer-Associated Fibroblasts Promote the Chemo-Resistance in Gastric Cancer through Secreting IL-11 Targeting JAK/STAT3/Bcl2 Pathway. Cancer Res. Treat. 2019, 51, 194–210.
- Wang, X.; Che, X.; Liu, C.; Fan, Y.; Bai, M.; Hou, K.; Shi, X.; Zhang, X.; Liu, B.; Zheng, C.; et al. Cancer-Associated Fibroblasts-Stimulated Interleukin-11 Promotes Metastasis of Gastric Cancer Cells Mediated by Upregulation of MUC1. Exp. Cell Res. 2018, 368, 184–193.
- Widjaja, A.A.; Viswanathan, S.; Jinrui, D.; Singh, B.K.; Tan, J.; Wei Ting, J.G.; Lamb, D.; Shekeran, S.G.; George, B.L.; Schafer, S.; et al. Molecular Dissection of Pro-Fibrotic IL11 Signaling in Cardiac and Pulmonary Fibroblasts. Front. Mol. Biosci. 2021, 8, 740650.
- Cook, S.A.; Schafer, S. Hiding in Plain Sight: Interleukin-11 Emerges as a Master Regulator of Fibrosis, Tissue Integrity, and Stromal Inflammation. Annu. Rev. Med. 2020, 71, 263–276.
- Peng, N.; Lu, M.; Kang, M.; Liu, X.; Li, B.; Dong, C. Recombinant Human IL-11 Promotes Lung Adenocarcinoma A549 Cell Growth and EMT through Activating STAT3/HIF-1α/EMT Signaling Pathway. Anticancer Agents Med. Chem. 2021, 21, 1996–2003.
- Zhao, M.; Liu, Y.; Liu, R.; Qi, J.; Hou, Y.; Chang, J.; Ren, L. Upregulation of IL-11, an IL-6 Family Cytokine, Promotes Tumor Progression and Correlates with Poor Prognosis in Non-Small Cell Lung Cancer. Cell. Physiol. Biochem. 2018, 45, 2213–2224.
- Cardó-Vila, M.; Marchiò, S.; Sato, M.; Staquicini, F.I.; Smith, T.L.; Bronk, J.K.; Yin, G.; Zurita, A.J.; Sun, M.; Behrens, C.; et al. Interleukin-11 Receptor Is a Candidate Target for Ligand-Directed Therapy in Lung Cancer: Analysis of Clinical Samples and BMTP-11 Preclinical Activity. Am. J. Pathol. 2016, 186, 2162–2170.
- Lokau, J.; Nitz, R.; Agthe, M.; Monhasery, N.; Aparicio-Siegmund, S.; Schumacher, N.; Wolf, J.; Möller-Hackbarth, K.; Waetzig, G.H.; Grötzinger, J.; et al. Proteolytic Cleavage Governs Interleukin-11 Trans-Signaling. Cell Rep. 2016, 14, 1761–1773.
- Brooks, G.D.; McLeod, L.; Alhayyani, S.; Miller, A.; Russell, P.A.; Ferlin, W.; Rose-John, S.; Ruwanpura, S.; Jenkins, B.J. IL6 Trans-Signaling Promotes KRAS-Driven Lung Carcinogenesis. Cancer Res. 2016, 76, 866–876.
- Ernst, M.; Najdovska, M.; Grail, D.; Lundgren-May, T.; Buchert, M.; Tye, H.; Matthews, V.B.; Armes, J.; Bhathal, P.S.; Hughes, N.R.; et al. STAT3 and STAT1 Mediate IL-11-Dependent and Inflammation-Associated Gastric Tumorigenesis in gp130 Receptor Mutant Mice. J. Clin. Investig. 2008, 118, 1727–1738.
- Ernst, M.; Putoczki, T.L. Molecular Pathways: IL11 as a Tumor-Promoting Cytokine-Translational Implications for Cancers. Clin. Cancer Res. 2014, 20, 5579–5588.
- Heavey, S.; O’Byrne, K.J.; Gately, K. Strategies for Co-Targeting the PI3K/AKT/mTOR Pathway in NSCLC. Cancer Treat. Rev. 2014, 40, 445–456.
- Al-Saad, S.; Donnem, T.; Al-Shibli, K.; Persson, M.; Bremnes, R.M.; Busund, L.-T. Diverse Prognostic Roles of Akt Isoforms, PTEN and PI3K in Tumor Epithelial Cells and Stromal Compartment in Non-Small Cell Lung Cancer. Anticancer Res. 2009, 29, 4175–4183.
- Tang, J.-M.; He, Q.-Y.; Guo, R.-X.; Chang, X.-J. Phosphorylated Akt Overexpression and Loss of PTEN Expression in Non-Small Cell Lung Cancer Confers Poor Prognosis. Lung Cancer 2006, 51, 181–191.
- Tong, M.; Wang, J.; Jiang, N.; Pan, H.; Li, D. Correlation between P-STAT3 Overexpression and Prognosis in Lung Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0182282.
- Yu, Y.; Zhao, Q.; He, X.-P.; Wang, Z.; Liu, X.-Y.; Zhang, Z.-P. Signal Transducer and Activator of Transcription 3 Overexpression Promotes Lymph Node Micrometastasis in Early-Stage Non-Small Cell Lung Cancer. Thorac. Cancer 2018, 9, 516–522.
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource; Food and Drug Administration (US): Silver Spring, MD, USA, 2016.
- Cancer Genome Atlas Research Network; Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.M.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer Analysis Project. Nat. Genet. 2013, 45, 1113–1120.
- GTEx Consortium The Genotype-Tissue Expression (GTEx) Project. Nat. Genet. 2013, 45, 580–585.
- Goldman, M.J.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and Interpreting Cancer Genomics Data via the Xena Platform. Nat. Biotechnol. 2020, 38, 675–678.
- Vivian, J.; Rao, A.A.; Nothaft, F.A.; Ketchum, C.; Armstrong, J.; Novak, A.; Pfeil, J.; Narkizian, J.; Deran, A.D.; Musselman-Brown, A.; et al. Toil Enables Reproducible, Open Source, Big Biomedical Data Analyses. Nat. Biotechnol. 2017, 35, 314–316.
- Pastor, M.D.; Nogal, A.; Molina-Pinelo, S.; Quintanal-Villalonga, Á.; Meléndez, R.; Ferrer, I.; Romero-Romero, B.; De Miguel, M.J.; López-Campos, J.L.; Corral, J.; et al. IL-11 and CCL-1: Novel Protein Diagnostic Biomarkers of Lung Adenocarcinoma in Bronchoalveolar Lavage Fluid (BALF). J. Thorac. Oncol. 2016, 11, 2183–2192.
- Wu, J.; Chen, J.; Lv, X.; Yang, Q.; Yao, S.; Zhang, D.; Chen, J. Clinical Value of Serum and Exhaled Breath Condensate Inflammatory Factor IL-11 Levels in Non-Small Cell Lung Cancer: Clinical Value of IL-11 in Non-Small Cell Lung Cancer. Int. J. Biol. Markers 2021, 36, 64–76.
- Haro, G.J.; Sheu, B.; Cook, N.R.; Woodard, G.A.; Mann, M.J.; Kratz, J.R. Comparison of Conventional TNM and Novel TNMB Staging Systems for Non-Small Cell Lung Cancer. JAMA Netw. Open 2019, 2, e1917062.
- Kratz, J.R.; He, J.; Van Den Eeden, S.K.; Zhu, Z.-H.; Gao, W.; Pham, P.T.; Mulvihill, M.S.; Ziaei, F.; Zhang, H.; Su, B.; et al. A Practical Molecular Assay to Predict Survival in Resected Non-Squamous, Non-Small-Cell Lung Cancer: Development and International Validation Studies. Lancet 2012, 379, 823–832.
- Watza, D.; Lusk, C.M.; Dyson, G.; Purrington, K.S.; Chen, K.; Wenzlaff, A.S.; Ratliff, V.; Neslund-Dudas, C.; Bepler, G.; Schwartz, A.G. Prognostic Modeling of the Immune-Centric Transcriptome Reveals Interleukin Signaling Candidates Contributing to Differential Patient Outcomes. Carcinogenesis 2018, 39, 1447–1454.
- Wang, H.; Wang, M.-S.; Wang, Y.; Huang, Y.-Q.; Shi, J.-P.; Ding, Z.-L.; Wang, W.-J. Prognostic Value of Immune Related Genes in Lung Adenocarcinoma. Oncol. Lett. 2020, 20, 259.
- Fan, T.; Pan, S.; Yang, S.; Hao, B.; Zhang, L.; Li, D.; Geng, Q. Clinical Significance and Immunologic Landscape of a Five-IL(R)-Based Signature in Lung Adenocarcinoma. Front. Immunol. 2021, 12, 693062.
- Chen, C.; Guo, Q.; Tang, Y.; Qu, W.; Zuo, J.; Ke, X.; Song, Y. Screening and Evaluation of the Role of Immune Genes of Brain Metastasis in Lung Adenocarcinoma Progression Based on the TCGA and GEO Databases. J. Thorac. Dis. 2021, 13, 5016–5034.
- Peng, L.; Tao, Y.; Wu, R.; Su, J.; Sun, M.; Cheng, Y.; Xie, Z.; Mao, J.; Zhan, X.; Liu, G. NFAT as a Biomarker and Therapeutic Target in Non-Small Cell Lung Cancer-Related Brain Metastasis. Front. Oncol. 2021, 11, 781150.




