
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jian Lu | -- | 2687 | 2022-08-11 19:11:27 | | | |
| 2 | Sirius Huang | Meta information modification | 2687 | 2022-08-15 11:02:17 | | |
Video Upload Options
Chemotherapy has an essential role not only in advanced solid tumor therapy intervention but also in society’s health at large. Chemoresistance, however, seriously restricts the efficiency and sensitivity of chemotherapeutic agents, representing a significant threat to patients’ quality of life and life expectancy. How to reverse chemoresistance, improve efficacy sensitization response, and reduce adverse side effects need to be tackled urgently. Consequently, studies on the effect of ultrasonic microbubble cavitation on enhanced permeability and retention (EPR) have attracted the attention of researchers. Compared with the traditional targeted drug delivery regimen, the microbubble cavitation effect, which can be used to enhance the EPR effect, has the advantages of less trauma, low cost, and good sensitization effect, and has significant application prospects.
1. A Brief Overview of Ultrasonic Microbubble Cavitation
2. Ultrasonic Microbubble Cavitation Promoting Tumor Therapy by Enhancing the EPR Effect
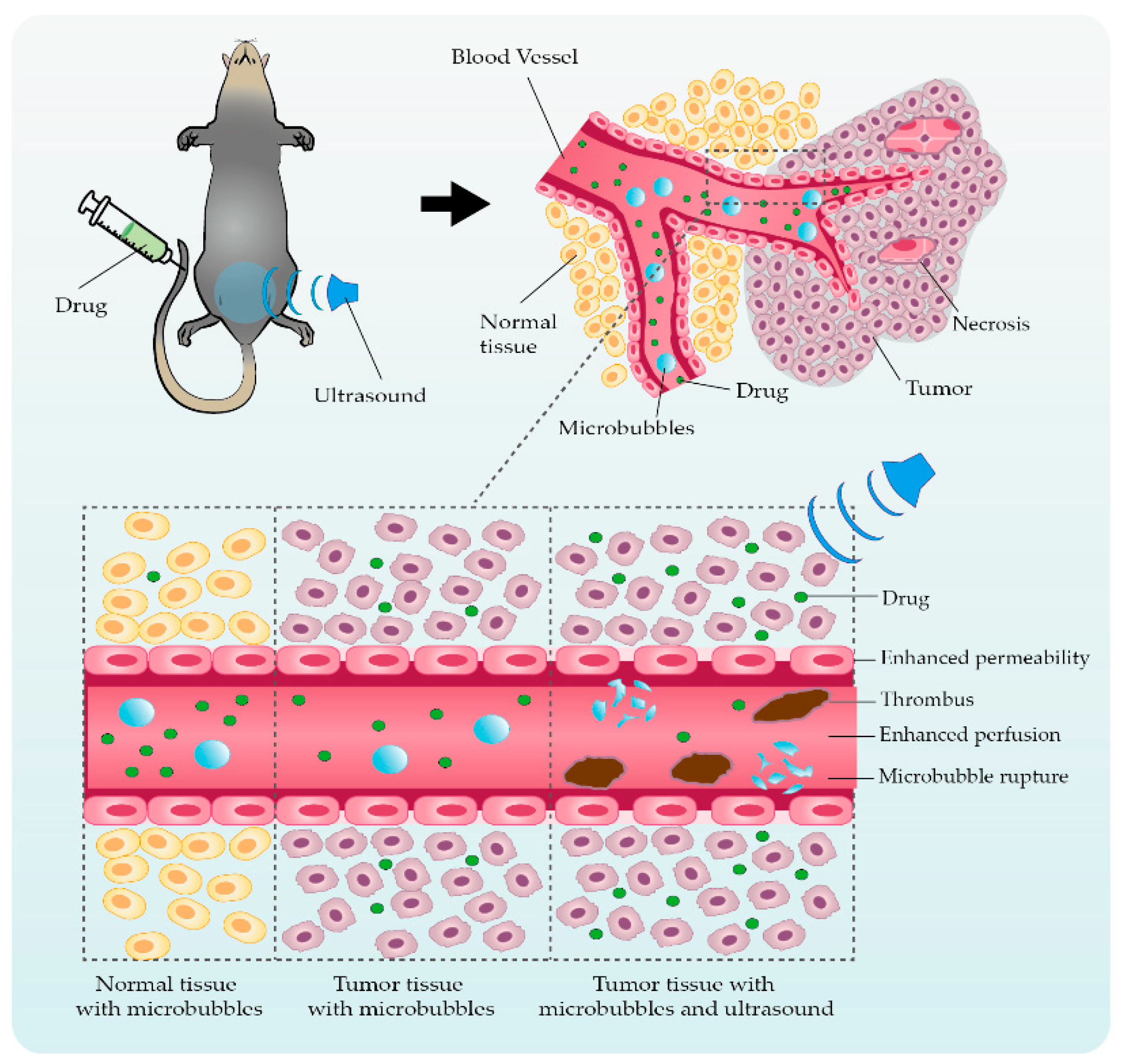
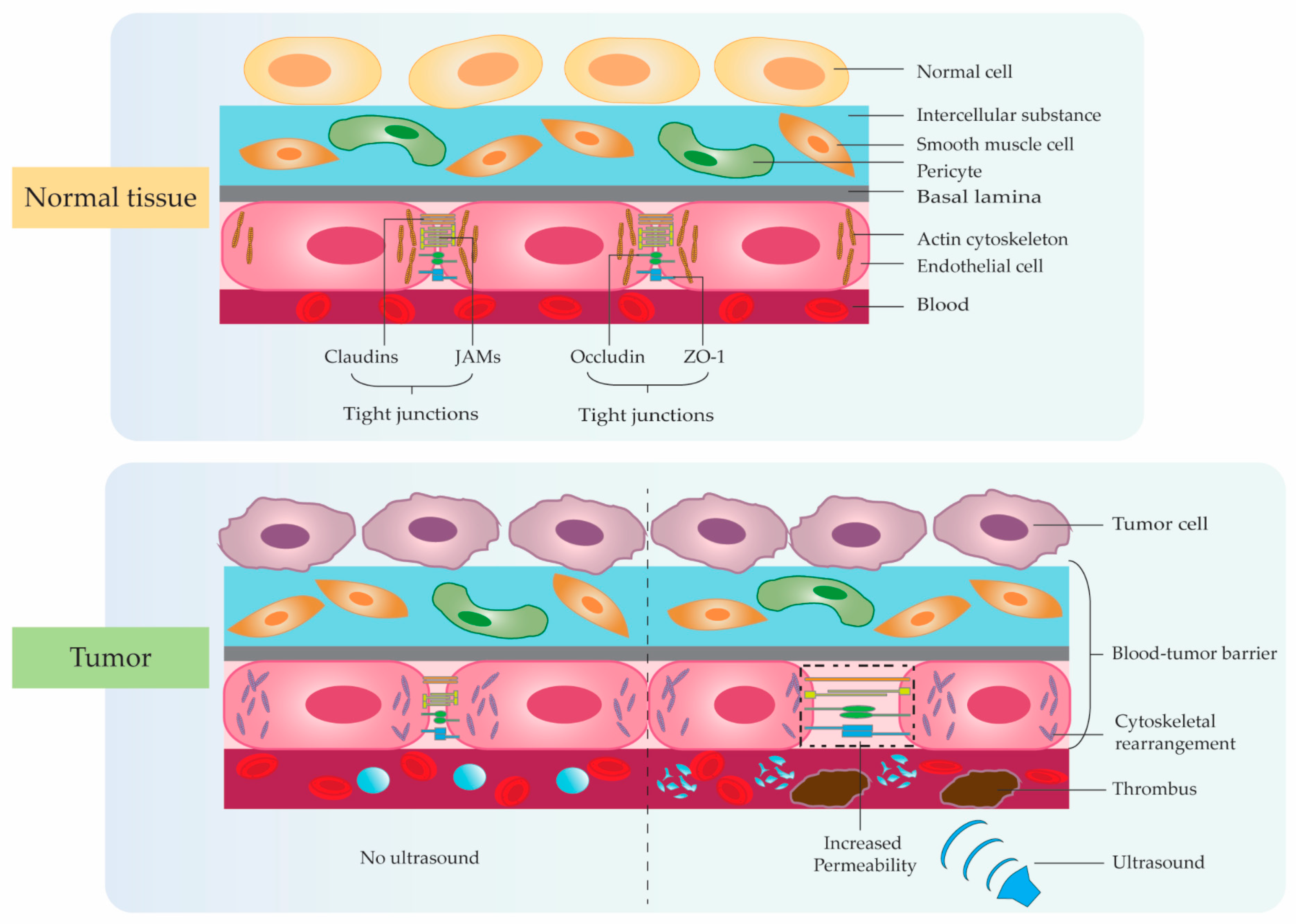
3. Application Studies Using Ultrasonic Microbubble Cavitation on Tumor Therapy
| Cavitation Mechanism | Authors | Cell Type | Component | Intervention | Outcomes after Cavitation Effect |
|---|---|---|---|---|---|
| Enhanced permeability | Tinkov et al. [47] | Renal carcinoma cell | Doxorubicin | Group 1: DOX Group 2: DOX + MBs |
Approximately two-fold enhanced anti-proliferative effect in DOX-loaded MBs. DOX-loaded MBs with high affinity to the nucleus. |
| Enhanced permeability Promoted drug diffusion |
Li F. et al. [48] | Renal carcinoma cell | rAAV | Group 1: rAAV Group 2: rAAV + MBs Group 3: rAAV + US Group 4: rAAV + UTMD |
US-mediated MBs inhibit tumor cell proliferation and induce apoptosis. US-mediated MBs promote viral transfection approximately two-fold. |
| Enhanced permeability Promoted drug diffusion |
Haag P. et al. [49] | Prostate tumor cell | ODNs | Group 1: MBs Group 2: ODNs Group 3: ODNs + MBs Group 4: ODNs + US Group 5: ODNs + MBs + US |
Best US frequency: 1.75 MHz; best MI: 1.9. US-mediated MBs inhibit AR protein levels by 36.23%. US-mediated MBs promote viral transfection approximately 40-fold. |
| Promoted drug uptake | Yan F. et al. [50] | Breast cancer cell | Paclitaxel and LyP-1 Peptide | Group 1: MBs Group 2: PTX-loaded MBs Group 3: Targeted PTX-loaded MBs |
Targeted ultrasonic MBs encapsulation rate: 63%. US for 2 min increased cell uptake approximately seven-fold. |
| Enhanced permeability Promoted drug diffusion |
Cochran M.C. et al. [51] | Breast cancer cell | Doxorubicin and paclitaxel | Group 1: MBs Group 2: MBs + US Group 3: Drug-loaded MBs Group 4: Drug-loaded MBs + US |
The encapsulation efficiency of PTX and DOX: 72%, 20.5%. The payload of PTX-loaded MBs is 20 times DOX. The anti-tumor effect increased by 80.1%. |
| Promoted drug diffusion | Wang D.S. et al. [52] | Vascular endothelial tumor cell | DNA | Group 1: Cationic MBs + US Group 2: Neutral MBs + US Group 3: US Group 4: Cationic MBs |
Cationic MBs protect plasmid DNA from degradation. Cationic MBs promote gene transfection approximately two-fold. |
| Enhanced permeability | Ren S.T. et al. [53] | Colon adenocarcinoma cell | Docetaxel | Group 1: DOC Group 2: DOC + US Group 3: MBs + US Group 4: DOC + MBs + US |
The maximum encapsulation rate: 54.9%. The anti-tumor effect increased approximately two-fold. |
| Enhanced permeability Promoted drug diffusion |
Escoffre J.M. et al. [54] | Glioblastoma cell | Doxorubicin | Group 1: MBs + US Group 2: DOX + MBs Group 3: DOX + MBs + US |
US-mediated MBs significantly increased drug uptake. Tumor cell death with US-mediated MBs was enhanced approximately three-fold. |
| Cavitation Mechanism | Authors | Cell Type | Component | Intervention | Outcomes after Cavitation Effect |
|---|---|---|---|---|---|
| Enhanced permeability | Wang G. et al. [55] | Hepatic cancer | Evans Blue | Group 1: EB Group 2: EB + MBs Group 3: EB + US Group 4: EB + MBs + US |
US-mediated MBs cavitation can increase tumor vascular permeability. The cavitation effect promotes drug release approximately three-fold. |
| Enhanced permeability | Tang Q. et al. [56] | Hepatic cancer | HSV-TK/GCV | Group 1: pEGFP-KDR-TK + pEGFP-C1-AFP-TK Group 2: pEGFP-KDR-TK + pEGFP-C1-AFP-TK + US Group 3: pEGFP-KDR-TK + pEGFP-C1-AFP-TK + MBs + US |
US-mediated MBs can increase killing effect of HSV-TK/GCV and CD/5-FC systems on vascular and hepatoma cells. US-mediated MBs can increase tumor vascular permeability and gene transfection efficiency. |
| Enhanced permeability Induced tumor necrosis |
Li P. et al. [57] | Subcutaneous VX2 cancer | Evans Blue | Group 1: EB Group 2: EB + MBs Group 3: EB + US Group 4: EB + MBs + US |
US-mediated MBs can induce tumor microvasculature disruption resulting in hemorrhage, edema, and thrombosis to cause necrosis. |
| Enhanced permeability | Cool S.K. et al. [42] | No tumor | ICG-liposomes | Group 1: Drug-MBs + US Group 2: Drug + MBs + US Group 3: MBs + US Group 4: Drug + US |
MBs can increase ICG-liposomes loaded approximately three-fold. US-mediated MBs increase drug release two times more. US-mediated MBs can cause skin lesions due during microbubble collapse. |
| Enhanced permeability Promoted drug diffusion Enhanced perfusion |
Lin C.Y. et al. [58] | Colon cancer | DOX | Group 1: DOX Group 2: DOX + MBs Group 3: DOX + US Group 4: DOX + MBs + US |
US-mediated MBs cavitation can increase tissue permeability and destroy tumor vessels. US-mediated MBs can increase tumor drug uptake and inhibit growth. US intervention time should be less than 2 min. |
| Enhanced permeability Promoted drug diffusion |
Fokong S. et al. [59] | Colon cancer | Rhodamine-B Coumarin-6 |
Group 1: MBs-Rhodamine-B Group 2: MBs-Coumarin-6 Group 3: MBs-Rhodamine-B + US Group 4: MBs-Coumarin-6 + US |
The polymer-based MBs are highly suitable for image-guided, targeted, and triggered drug delivery to tumors and blood vessels. |
| Enhanced permeability Induced tumor necrosis |
Huang P. et al. [7] | Colon cancer | No drug | Group 1: MBs Group 2: US Group 3: MBs + US |
US-mediated MBs inhibit tumor growth and metastasis. US-mediated MBs destroy tumor cell nucleus and microvascular. US-mediated MBs decreases the expression of CD31. |
Although applications of acoustic cavitation have realized significant progress, there are still many difficulties and challenges which require further efforts to explore more suitable delivery systems and effective efficacy. Firstly, the particle size of microbubbles remains a key factor affecting localized drug accumulation and cavitation effects in tumors. Therefore, we need to develop a new process to solve the situation that the cavitation effect is weakened due to the low accumulation of microbubbles around the tumor tissue caused by the unsuitable particle size of microbubbles. Secondly, with the emergence of multifunctional drug delivery systems, the structure with membrane shells continues to present complex trends. We need to reduce the adverse effects of membrane shell material, particle size, and targeted modification type on the cavitation effect in the complex and variable TME for achieving the controllability and stability of the targeted microbubble-loading system between different individuals with finally attaining the standardized application in clinical intervention. Of course, due to the different research directions of each scholar, there are also differences in the parameters they used. We need more efforts to verify the effects of ultrasound intervention time, irradiation time interval, ultrasound frequency, sound intensity, microbubble size, drug dose, and concentration on the therapeutic efficacy of the disease. Meanwhile, the integration of multiple technologies needs to be carried out, including protein-membrane-targeted modification, photothermal, magnetic field, radiation, free radicals, gene interference, immunotherapy, etc., to comprehensively enhance the anti-tumor efficacy. Absolutely, the safety of the delivery vehicle is also an issue that closer attention should be paid to. Although many studies have confirmed the high histocompatibility of microbubbles, more research is still needed in the future to further confirm the potential harm caused by long-term accumulation in the body.
At present, there are still many problems to be solved in the treatment of tumors with low-frequency ultrasound combined with microbubbles, but it is undeniable that this technology has shown great clinical application value as a safe, effective, easy-to-operate, and targeted non-invasive treatment method. With the development of technology, this promising non-invasive tumor treatment method will be widely used in clinical practice.
References
- Inui, A.; Honda, A.; Yamanaka, S.; Ikeno, T.; Yamamoto, K. Effect of ultrasonic frequency and surfactant addition on microcapsule destruction. Ultrason. Sonochem. 2021, 70, 105308.
- Li, M.; Lan, B.; Sankin, G.; Zhou, Y.; Liu, W.; Xia, J.; Wang, D.; Trahey, G.; Zhong, P.; Yao, J. Simultaneous Photoacoustic Imaging and Cavitation Mapping in Shockwave Lithotripsy. IEEE Trans. Med. Imaging 2020, 39, 468–477.
- Suslick, K.S.; Eddingsaas, N.C.; Flannigan, D.J.; Hopkins, S.D.; Xu, H. The Chemical History of a Bubble. Acc. Chem. Res. 2018, 51, 2169–2178.
- Yildirim, A.; Blum, N.T.; Goodwin, A.P. Colloids, nanoparticles, and materials for imaging, delivery, ablation, and theranostics by focused ultrasound (FUS). Theranostics 2019, 9, 2572–2594.
- Ye, L.; Zhu, X.; He, Y.; Wei, X. Ultrasonic cavitation damage characteristics of materials and a prediction model of cavitation impact load based on size effect. Ultrason. Sonochem. 2020, 66, 105115.
- Daecher, A.; Stanczak, M.; Liu, J.B.; Zhang, J.; Du, S.; Forsberg, F.; Leeper, D.B.; Eisenbrey, J.R. Localized microbubble cavitation-based antivascular therapy for improving HCC treatment response to radiotherapy. Cancer Lett. 2017, 411, 100–105.
- Huang, P.T.; You, X.D.; Pan, M.Q.; Li, S.Y.; Zhang, Y.; Zhao, Y.Z.; Wang, M.H.; Hong, Y.R.; Pu, Z.X.; Chen, L.R.; et al. A novel therapeutic strategy using ultrasound mediated microbubbles destruction to treat colon cancer in a mouse model. Cancer Lett. 2013, 335, 183–190.
- Wang, F.; Dong, L.; Liang, S.M.; Wei, X.X.; Wang, Y.L.; Chang, L.S.; Guo, K.; Wu, H.W.; Chang, Y.Q.; Yin, Y.L.; et al. Ultrasound-triggered drug delivery for glioma therapy through gambogic acid-loaded nanobubble-microbubble complexes. Biomed. Pharm. 2022, 150, 113042.
- Liu, T.; Li, M.; Tang, J.; Li, J.; Zhou, Y.; Liu, Y.; Yang, F.; Gu, N. An acoustic strategy for gold nanoparticle loading in platelets as biomimetic multifunctional carriers. J. Mater. Chem. B 2019, 7, 2138–2144.
- Stride, E.; Coussios, C. Nucleation, mapping and control of cavitation for drug delivery. Nat. Rev. Phys. 2019, 1, 495–509.
- Athanassiadis, A.G.; Ma, Z.C.; Moreno-Gomez, N.; Melde, K.; Choi, E.; Goyal, R.; Fischer, P. Ultrasound-Responsive Systems as Components for Smart Materials. Chem. Rev. 2022, 122, 5165–5208.
- Rapoport, N. Ultrasound-mediated micellar drug delivery. Int. J. Hyperth. 2012, 28, 374–385.
- Sankin, G.N.; Simmons, W.N.; Zhu, S.L.; Zhong, P. Shock wave interaction with laser-generated single bubbles. Phys. Rev. Lett. 2005, 95, 034501.
- Chen, H.; Kreider, W.; Brayman, A.A.; Bailey, M.R.; Matula, T.J. Blood vessel deformations on microsecond time scales by ultrasonic cavitation. Phys. Rev. Lett. 2011, 106, 034301.
- Bang, J.H.; Suslick, K.S. Applications of ultrasound to the synthesis of nanostructured materials. Adv. Mater. 2010, 22, 1039–1059.
- Fernandez Rivas, D.; Prosperetti, A.; Zijlstra, A.G.; Lohse, D.; Gardeniers, H.J. Efficient sonochemistry through microbubbles generated with micromachined surfaces. Angew. Chem. Int. Ed. Engl. 2010, 49, 9699–9701.
- Kwan, J.J.; Graham, S.; Myers, R.; Carlisle, R.; Stride, E.; Coussios, C.C. Ultrasound-induced inertial cavitation from gas-stabilizing nanoparticles. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2015, 92, 023019.
- Escoffre, J.M.; Piron, J.; Novell, A.; Bouakaz, A. Doxorubicin delivery into tumor cells with ultrasound and microbubbles. Mol. Pharm. 2011, 8, 799–806.
- Grainger, S.J.; Serna, J.V.; Sunny, S.; Zhou, Y.; Deng, C.X.; El-Sayed, M.E. Pulsed ultrasound enhances nanoparticle penetration into breast cancer spheroids. Mol. Pharm. 2010, 7, 2006–2019.
- Marmottant, P.; Bouakaz, A.; de Jong, N.; Quilliet, C. Buckling resistance of solid shell bubbles under ultrasound. J. Acoust. Soc. Am. 2011, 129, 1231–1239.
- van Wamel, A.; Kooiman, K.; Harteveld, M.; Emmer, M.; ten Cate, F.J.; Versluis, M.; de Jong, N. Vibrating microbubbles poking individual cells: Drug transfer into cells via sonoporation. J. Control. Release 2006, 112, 149–155.
- Mehier-Humbert, S.; Bettinger, T.; Yan, F.; Guy, R.H. Plasma membrane poration induced by ultrasound exposure: Implication for drug delivery. J. Control. Release 2005, 104, 213–222.
- Xia, H.; Yang, D.; He, W.; Zhu, X.; Yan, Y.; Liu, Z.; Liu, T.; Yang, J.; Tan, S.; Jiang, J.; et al. Ultrasound-mediated microbubbles cavitation enhanced chemotherapy of advanced prostate cancer by increasing the permeability of blood-prostate barrier. Transl. Oncol. 2021, 14, 101177.
- Park, J.; Fan, Z.; Deng, C.X. Effects of shear stress cultivation on cell membrane disruption and intracellular calcium concentration in sonoporation of endothelial cells. J. Biomech. 2011, 44, 164–169.
- Qin, P.; Han, T.; Yu, A.C.H.; Xu, L. Mechanistic understanding the bioeffects of ultrasound-driven microbubbles to enhance macromolecule delivery. J. Control. Release 2018, 272, 169–181.
- Eggen, S.; Afadzi, M.; Nilssen, E.A.; Haugstad, S.B.; Angelsen, B.; Davies Cde, L. Ultrasound improves the uptake and distribution of liposomal Doxorubicin in prostate cancer xenografts. Ultrasound Med. Biol. 2013, 39, 1255–1266.
- Qiu, S.F.; Li, D.X.; Wang, Y.G.; Xiu, J.C.; Lyu, C.Y.; Kutty, S.; Zha, D.G.; Wu, J.F. Ultrasound-Mediated Microbubble Cavitation Transiently Reverses Acute Hindlimb Tissue Ischemia through Augmentation of Microcirculation Perfusion Via the Enos/No Pathway. Ultrasound Med. Biol. 2021, 47, 1014–1023.
- Ji, C.; Wang, L.; Dai, R.; Shan, L.; Yang, H.; Zhu, H.; Meng, Q. Hyperthermia exacerbates the effects of cathepsin L on claudin-1 in a blood-brain barrier model in vitro. Brain Res. 2016, 1631, 72–79.
- Cai, H.; Liu, W.; Xue, Y.; Shang, X.; Liu, J.; Li, Z.; Wang, P.; Liu, L.; Hu, Y.; Liu, Y. Roundabout 4 regulates blood-tumor barrier permeability through the modulation of ZO-1, Occludin, and Claudin-5 expression. J. Neuropathol. Exp. Neurol. 2015, 74, 25–37.
- Ma, J.; Wang, P.; Liu, Y.; Zhao, L.; Li, Z.; Xue, Y. Kruppel-like factor 4 regulates blood-tumor barrier permeability via ZO-1, occludin and claudin-5. J. Cell Physiol. 2014, 229, 916–926.
- Wang, F.; Song, X.; Zhou, M.; Wei, L.; Dai, Q.; Li, Z.; Lu, N.; Guo, Q. Wogonin inhibits H2O2-induced vascular permeability through suppressing the phosphorylation of caveolin-1. Toxicology 2013, 305, 10–19.
- Martin, T.A.; Mason, M.D.; Jiang, W.G. HGF and the regulation of tight junctions in human prostate cancer cells. Oncol. Rep. 2014, 32, 213–224.
- Tang, L.; Zhang, C.Y.; Yang, Q.; Xie, H.; Liu, D.D.; Tian, H.B.; Lu, L.X.; Xu, J.Y.; Li, W.Y.; Xu, G.X.; et al. Melatonin maintains inner blood-retinal barrier via inhibition of p38/TXNIP/NF-kappa B pathway in diabetic retinopathy. J. Cell. Physiol. 2021, 236, 5848–5864.
- Liao, J.Z.; Li, Q.W.; Lei, C.Q.; Yu, W.L.; Deng, J.C.; Guo, J.Y.; Han, Q.Y.; Hu, L.M.; Li, Y.; Pan, J.Q.; et al. Toxic effects of copper on the jejunum and colon of pigs: Mechanisms related to gut barrier dysfunction and inflammation influenced by the gut microbiota. Food Funct. 2021, 12, 9642–9657.
- Poplawska, M.; Dutta, D.; Jayaram, M.; Chong, N.S.; Salifu, M.; Lim, S.H. Genes modulating intestinal permeability and microbial community are dysregulated in sickle cell disease. Ann. Hematol. 2022, 101, 1009–1013.
- Cheng, C.Y.; Mruk, D.D. The Blood-Testis Barrier and Its Implications for Male Contraception. Pharmacol. Rev. 2012, 64, 16–64.
- Rose, E.C.; Odle, J.; Blikslager, A.T.; Ziegler, A.L. Probiotics, Prebiotics and Epithelial Tight Junctions: A Promising Approach to Modulate Intestinal Barrier Function. Int. J. Mol. Sci. 2021, 22, 6729.
- Kaminsky, L.W.; Al-Sadi, R.; Ma, O.M. IL-1 beta and the Intestinal Epithelial Tight Junction Barrier. Front. Immunol. 2021, 12, 767456.
- Sasson, E.; Anzi, S.; Bell, B.; Yakovian, O.; Zorsky, M.; Deutsch, U.; Engelhardt, B.; Sherman, E.; Vatine, G.; Dzikowski, R.; et al. Nano-scale architecture of blood-brain barrier tight-junctions. Elife 2021, 10, e63253.
- Bae, M.J.; Lee, Y.M.; Kim, Y.H.; Han, H.S.; Lee, H.J. Utilizing Ultrasound to Transiently Increase Blood-Brain Barrier Permeability, Modulate of the Tight Junction Proteins, and Alter Cytoskeletal Structure. Curr. Neurovasc. Res. 2015, 12, 375–383.
- Shang, X.; Wang, P.; Liu, Y.; Zhang, Z.; Xue, Y. Mechanism of low-frequency ultrasound in opening blood-tumor barrier by tight junction. J. Mol. Neurosci. 2011, 43, 364–369.
- Cool, S.K.; Geers, B.; Roels, S.; Stremersch, S.; Vanderperren, K.; Saunders, J.H.; De Smedt, S.C.; Demeester, J.; Sanders, N.N. Coupling of drug containing liposomes to microbubbles improves ultrasound triggered drug delivery in mice. J. Control. Release 2013, 172, 885–893.
- Goertz, D.E. An overview of the influence of therapeutic ultrasound exposures on the vasculature: High intensity ultrasound and microbubble-mediated bioeffects. Int. J. Hyperth. 2015, 31, 134–144.
- Kuijpers, M.J.; Gilio, K.; Reitsma, S.; Nergiz-Unal, R.; Prinzen, L.; Heeneman, S.; Lutgens, E.; van Zandvoort, M.A.; Nieswandt, B.; Egbrink, M.G.; et al. Complementary roles of platelets and coagulation in thrombus formation on plaques acutely ruptured by targeted ultrasound treatment: A novel intravital model. J. Thromb. Haemost 2009, 7, 152–161.
- Song, F.Y.; Gao, H.; Li, D.Y.; Petrov, A.V.; Petrov, V.V.; Wen, D.S.; Sukhorukov, G.B. Low intensity focused ultrasound responsive microcapsules for non-ablative ultrafast intracellular release of small molecules. J. Mater. Chem. B 2021, 9, 2384–2393.
- Tu, L.; Liao, Z.; Luo, Z.; Wu, Y.L.; Herrmann, A.; Huo, S. Ultrasound-controlled drug release and drug activation for cancer therapy. Exploration 2021, 1, 20210023.
- Tinkov, S.; Winter, G.; Coester, C.; Bekeredjian, R. New doxorubicin-loaded phospholipid microbubbles for targeted tumor therapy: Part I--Formulation development and in-vitro characterization. J. Control. Release 2010, 143, 143–150.
- Li, F.; Jin, L.; Wang, H.; Wei, F.; Bai, M.; Shi, Q.; Du, L. The dual effect of ultrasound-targeted microbubble destruction in mediating recombinant adeno-associated virus delivery in renal cell carcinoma: Transfection enhancement and tumor inhibition. J. Gene Med. 2014, 16, 28–39.
- Haag, P.; Frauscher, F.; Gradl, J.; Seitz, A.; Schafer, G.; Lindner, J.R.; Klibanov, A.L.; Bartsch, G.; Klocker, H.; Eder, I.E. Microbubble-enhanced ultrasound to deliver an antisense oligodeoxynucleotide targeting the human androgen receptor into prostate tumours. J. Steroid. Biochem. Mol. Biol. 2006, 102, 103–113.
- Yan, F.; Li, X.; Jin, Q.; Jiang, C.; Zhang, Z.; Ling, T.; Qiu, B.; Zheng, H. Therapeutic ultrasonic microbubbles carrying paclitaxel and LyP-1 peptide: Preparation, characterization and application to ultrasound-assisted chemotherapy in breast cancer cells. Ultrasound Med. Biol. 2011, 37, 768–779.
- Cochran, M.C.; Eisenbrey, J.; Ouma, R.O.; Soulen, M.; Wheatley, M.A. Doxorubicin and paclitaxel loaded microbubbles for ultrasound triggered drug delivery. Int. J. Pharm. 2011, 414, 161–170.
- Wang, D.S.; Panje, C.; Pysz, M.A.; Paulmurugan, R.; Rosenberg, J.; Gambhir, S.S.; Schneider, M.; Willmann, J.K. Cationic versus neutral microbubbles for ultrasound-mediated gene delivery in cancer. Radiology 2012, 264, 721–732.
- Ren, S.T.; Liao, Y.R.; Kang, X.N.; Li, Y.P.; Zhang, H.; Ai, H.; Sun, Q.; Jing, J.; Zhao, X.H.; Tan, L.F.; et al. The antitumor effect of a new docetaxel-loaded microbubble combined with low-frequency ultrasound in vitro: Preparation and parameter analysis. Pharm. Res. 2013, 30, 1574–1585.
- Escoffre, J.M.; Mannaris, C.; Geers, B.; Novell, A.; Lentacker, I.; Averkiou, M.; Bouakaz, A. Doxorubicin liposome-loaded microbubbles for contrast imaging and ultrasound-triggered drug delivery. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 78–87.
- Wang, G.; Zhuo, Z.; Xia, H.; Zhang, Y.; He, Y.; Tan, W.; Gao, Y. Investigation into the impact of diagnostic ultrasound with microbubbles on the capillary permeability of rat hepatomas. Ultrasound Med. Biol. 2013, 39, 628–637.
- Tang, Q.; He, X.; Liao, H.; He, L.; Wang, Y.; Zhou, D.; Ye, S.; Chen, Q. Ultrasound microbubble contrast agent-mediated suicide gene transfection in the treatment of hepatic cancer. Oncol. Lett. 2012, 4, 970–972.
- Li, P.; Zhu, M.; Xu, Y.; Zhao, Y.; Gao, S.; Liu, Z.; Gao, Y.H. Impact of microbubble enhanced, pulsed, focused ultrasound on tumor circulation of subcutaneous VX2 cancer. Chin. Med. J. 2014, 127, 2605–2611.
- Lin, C.Y.; Li, J.R.; Tseng, H.C.; Wu, M.F.; Lin, W.L. Enhancement of focused ultrasound with microbubbles on the treatments of anticancer nanodrug in mouse tumors. Nanomedicine 2012, 8, 900–907.
- Fokong, S.; Theek, B.; Wu, Z.; Koczera, P.; Appold, L.; Jorge, S.; Resch-Genger, U.; van Zandvoort, M.; Storm, G.; Kiessling, F.; et al. Image-guided, targeted and triggered drug delivery to tumors using polymer-based microbubbles. J. Control. Release 2012, 163, 75–81.





