Chemotherapy has an essential role not only in advanced solid tumor therapy intervention but also in society’s health at large. Chemoresistance, however, seriously restricts the efficiency and sensitivity of chemotherapeutic agents, representing a significant threat to patients’ quality of life and life expectancy. How to reverse chemoresistance, improve efficacy sensitization response, and reduce adverse side effects need to be tackled urgently. Consequently, studies on the effect of ultrasonic microbubble cavitation on enhanced permeability and retention (EPR) have attracted the attention of researchers. Compared with the traditional targeted drug delivery regimen, the microbubble cavitation effect, which can be used to enhance the EPR effect, has the advantages of less trauma, low cost, and good sensitization effect, and has significant application prospects.
- ultrasound
- microbubbles
- cavitation effect
- EPR effect
- tumor therapy
1. A Brief Overview of Ultrasonic Microbubble Cavitation
2. Ultrasonic Microbubble Cavitation Promoting Tumor Therapy by Enhancing the EPR Effect
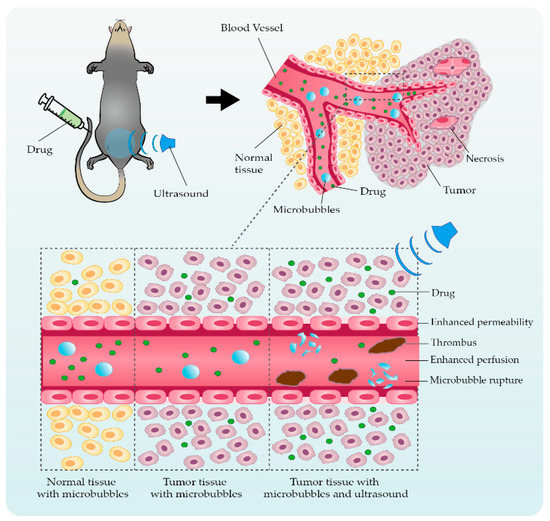
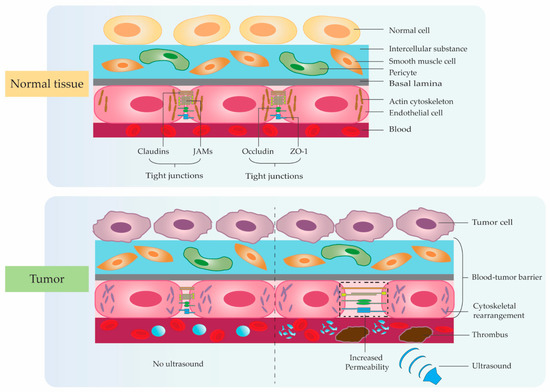
3. Application Studies Using Ultrasonic Microbubble Cavitation on Tumor Therapy
| Cavitation Mechanism | Authors | Cell Type | Component | Intervention | Outcomes after Cavitation Effect |
|---|---|---|---|---|---|
| Enhanced permeability | Tinkov et al. [48] | Renal carcinoma cell | Doxorubicin | Group 1: DOX Group 2: DOX + MBs |
Approximately two-fold enhanced anti-proliferative effect in DOX-loaded MBs. DOX-loaded MBs with high affinity to the nucleus. |
| Enhanced permeability Promoted drug diffusion |
Li F. et al. [49] | Renal carcinoma cell | rAAV | Group 1: rAAV Group 2: rAAV + MBs Group 3: rAAV + US Group 4: rAAV + UTMD |
US-mediated MBs inhibit tumor cell proliferation and induce apoptosis. US-mediated MBs promote viral transfection approximately two-fold. |
| Enhanced permeability Promoted drug diffusion |
Haag P. et al. [50] | Prostate tumor cell | ODNs | Group 1: MBs Group 2: ODNs Group 3: ODNs + MBs Group 4: ODNs + US Group 5: ODNs + MBs + US |
Best US frequency: 1.75 MHz; best MI: 1.9. US-mediated MBs inhibit AR protein levels by 36.23%. US-mediated MBs promote viral transfection approximately 40-fold. |
| Promoted drug uptake | Yan F. et al. [51] | Breast cancer cell | Paclitaxel and LyP-1 Peptide | Group 1: MBs Group 2: PTX-loaded MBs Group 3: Targeted PTX-loaded MBs |
Targeted ultrasonic MBs encapsulation rate: 63%. US for 2 min increased cell uptake approximately seven-fold. |
| Enhanced permeability Promoted drug diffusion |
Cochran M.C. et al. [52] | Breast cancer cell | Doxorubicin and paclitaxel | Group 1: MBs Group 2: MBs + US Group 3: Drug-loaded MBs Group 4: Drug-loaded MBs + US |
The encapsulation efficiency of PTX and DOX: 72%, 20.5%. The payload of PTX-loaded MBs is 20 times DOX. The anti-tumor effect increased by 80.1%. |
| Promoted drug diffusion | Wang D.S. et al. [53] | Vascular endothelial tumor cell | DNA | Group 1: Cationic MBs + US Group 2: Neutral MBs + US Group 3: US Group 4: Cationic MBs |
Cationic MBs protect plasmid DNA from degradation. Cationic MBs promote gene transfection approximately two-fold. |
| Enhanced permeability | Ren S.T. et al. [54] | Colon adenocarcinoma cell | Docetaxel | Group 1: DOC Group 2: DOC + US Group 3: MBs + US Group 4: DOC + MBs + US |
The maximum encapsulation rate: 54.9%. The anti-tumor effect increased approximately two-fold. |
| Enhanced permeability Promoted drug diffusion |
Escoffre J.M. et al. [55] | Glioblastoma cell | Doxorubicin | Group 1: MBs + US Group 2: DOX + MBs Group 3: DOX + MBs + US |
US-mediated MBs significantly increased drug uptake. Tumor cell death with US-mediated MBs was enhanced approximately three-fold. |
| Cavitation Mechanism | Authors | Cell Type | Component | Intervention | Outcomes after Cavitation Effect |
|---|---|---|---|---|---|
| Enhanced permeability | Wang G. et al. [56] | Hepatic cancer | Evans Blue | Group 1: EB Group 2: EB + MBs Group 3: EB + US Group 4: EB + MBs + US |
US-mediated MBs cavitation can increase tumor vascular permeability. The cavitation effect promotes drug release approximately three-fold. |
| Enhanced permeability | Tang Q. et al. [57] | Hepatic cancer | HSV-TK/GCV | Group 1: pEGFP-KDR-TK + pEGFP-C1-AFP-TK Group 2: pEGFP-KDR-TK + pEGFP-C1-AFP-TK + US Group 3: pEGFP-KDR-TK + pEGFP-C1-AFP-TK + MBs + US |
US-mediated MBs can increase killing effect of HSV-TK/GCV and CD/5-FC systems on vascular and hepatoma cells. US-mediated MBs can increase tumor vascular permeability and gene transfection efficiency. |
| Enhanced permeability Induced tumor necrosis |
Li P. et al. [58] | Subcutaneous VX2 cancer | Evans Blue | Group 1: EB Group 2: EB + MBs Group 3: EB + US Group 4: EB + MBs + US |
US-mediated MBs can induce tumor microvasculature disruption resulting in hemorrhage, edema, and thrombosis to cause necrosis. |
| Enhanced permeability | Cool S.K. et al. [43] | No tumor | ICG-liposomes | Group 1: Drug-MBs + US Group 2: Drug + MBs + US Group 3: MBs + US Group 4: Drug + US |
MBs can increase ICG-liposomes loaded approximately three-fold. US-mediated MBs increase drug release two times more. US-mediated MBs can cause skin lesions due during microbubble collapse. |
| Enhanced permeability Promoted drug diffusion Enhanced perfusion |
Lin C.Y. et al. [59] | Colon cancer | DOX | Group 1: DOX Group 2: DOX + MBs Group 3: DOX + US Group 4: DOX + MBs + US |
US-mediated MBs cavitation can increase tissue permeability and destroy tumor vessels. US-mediated MBs can increase tumor drug uptake and inhibit growth. US intervention time should be less than 2 min. |
| Enhanced permeability Promoted drug diffusion |
Fokong S. et al. [60] | Colon cancer | Rhodamine-B Coumarin-6 |
Group 1: MBs-Rhodamine-B Group 2: MBs-Coumarin-6 Group 3: MBs-Rhodamine-B + US Group 4: MBs-Coumarin-6 + US |
The polymer-based MBs are highly suitable for image-guided, targeted, and triggered drug delivery to tumors and blood vessels. |
| Enhanced permeability Induced tumor necrosis |
Huang P. et al. [7] | Colon cancer | No drug | Group 1: MBs Group 2: US Group 3: MBs + US |
US-mediated MBs inhibit tumor growth and metastasis. US-mediated MBs destroy tumor cell nucleus and microvascular. US-mediated MBs decreases the expression of CD31. |
Although applications of acoustic cavitation have realized significant progress, there are still many difficulties and challenges which require further efforts to explore more suitable delivery systems and effective efficacy. Firstly, the particle size of microbubbles remains a key factor affecting localized drug accumulation and cavitation effects in tumors. Therefore, we need to develop a new process to solve the situation that the cavitation effect is weakened due to the low accumulation of microbubbles around the tumor tissue caused by the unsuitable particle size of microbubbles. Secondly, with the emergence of multifunctional drug delivery systems, the structure with membrane shells continues to present complex trends. We need to reduce the adverse effects of membrane shell material, particle size, and targeted modification type on the cavitation effect in the complex and variable TME for achieving the controllability and stability of the targeted microbubble-loading system between different individuals with finally attaining the standardized application in clinical intervention. Of course, due to the different research directions of each scholar, there are also differences in the parameters they used. We need more efforts to verify the effects of ultrasound intervention time, irradiation time interval, ultrasound frequency, sound intensity, microbubble size, drug dose, and concentration on the therapeutic efficacy of the disease. Meanwhile, the integration of multiple technologies needs to be carried out, including protein-membrane-targeted modification, photothermal, magnetic field, radiation, free radicals, gene interference, immunotherapy, etc., to comprehensively enhance the anti-tumor efficacy. Absolutely, the safety of the delivery vehicle is also an issue that closer attention should be paid to. Although many studies have confirmed the high histocompatibility of microbubbles, more research is still needed in the future to further confirm the potential harm caused by long-term accumulation in the body.
At present, there are still many problems to be solved in the treatment of tumors with low-frequency ultrasound combined with microbubbles, but it is undeniable that this technology has shown great clinical application value as a safe, effective, easy-to-operate, and targeted non-invasive treatment method. With the development of technology, this promising non-invasive tumor treatment method will be widely used in clinical practice.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14081642
