
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hien Dang | + 2315 word(s) | 2315 | 2020-09-21 06:09:24 | | | |
| 2 | Nicole Yin | Meta information modification | 2315 | 2020-10-12 06:30:43 | | | | |
| 3 | Lily Guo | Meta information modification | 2315 | 2021-01-07 10:49:06 | | |
Video Upload Options
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related morbidity and mortality worldwide. Most patients are diagnosed with advanced disease, limiting their options for treatment. While current treatments are adequate for lower staged disease, available systemic treatments are limited, with marginal benefit at best. The below section reviews the incidence, prevalence, healthcare associated cost, and etiologies of HCC. In addition, an overview of the classification, treatment algorithm, and treatment modalities are provided.
1. Introduction
Hepatocellular carcinoma (HCC) is the fourth most common cause of cancer-related mortality and the leading cause of cancer-related morbidity worldwide[1][2][3]. Relative to other malignancies, the incidence of HCC is fifth highest in men and ninth highest in women[4]. Risk factors for these patients are numerous including—hepatitis B virus (HBV), hepatitis C virus (HCV), non-alcoholic steatohepatitis (NASH), alcoholic liver disease, tobacco, aflatoxins, vinyl chloride and thorium dioxide, anabolic steroids, as well as other rare diseases affecting the liver and environmental toxins[5][6]. Advances in preventative measures against conditions leading to HCC, such as the HBV vaccine, have reduced its incidence, while antiviral therapy in HCV has been able to achieve sustained virologic responses and disease suppression. Despite these advances, in 2018, global mortality related to HCC still exceeded 600,000 deaths and is expected to rise to over 1 million by 2030[7][8].
2. Etiologies Predisposing to HCC
While HCC is defined as a singular entity, the liver pathologies resulting in HCC carcinogenesis are highly variable depending on the inciting injuries. Each insult utilizes a different process to promote genomic instability, ongoing inflammation, cellular damage and neoplastic proliferation. Here, we will provide an overview of each of the main disease processes in the liver that may progress to HCC.
Most cases of HCC are due to viral causes, namely hepatitis B virus (HBV). HBV is a partially double-stranded DNA virus and can either exist as an acute infection with longstanding immunity or a chronic infection with the potential for reactivation[9]. HBV causes HCC by directly damaging hepatocytes leading to deranged regeneration as well as by integrating into the hepatocyte genome and altering transcription, translation and regulation[10]. The direct insult, acute viral infection of hepatocytes, activates the highly immunogenic liver immunoanatomy to recruit immune response cells (i.e., T cells, B cells, macrophages, etc.). The result is inflammation, degeneration and regeneration of the hepatocytes[10]. In chronic infection, on the other hand, integration of the HBV viral genome results in immune tolerance of HBV and HBV related molecules in the liver, possibly via inhibition or decreased expression of a co-inhibitory receptors on T cells that mitigate the CD8 T cell response[11]. Likely this process is carried out via multiple immune- and genome-modulating pathways yet to be discovered. Interestingly, the specific immunoanatomy even within HBV can be augmented and/or modified from other etiologies. For example, aflatoxin exposure has been shown to have a synergistic effect when combined with HBV, leading to a higher incidence of HCC in patients exposed to both[12]. In the setting of inflammatory regeneration leading to fibrosis and immune tolerance, the liver becomes vulnerable to HCC neoplastic proliferation.
Alternatively, HCV, which is an RNA virus, cannot integrate into the host hepatocyte genome and thus requires continued replication[13]. This continuous replication causes chronic inflammation leading to the degeneration and fibrosis as described above. However, evidence of differing tumorigenesis and immunoanatomy based on immune cell types present between HBV and HCV continues to evolve as our understanding of HCC increases[13]. Overall, the chronic, immune-driven inflammation characteristic of these viral etiologies leads to or creates the milieu that results in the majority of HCC cases.
Another etiology, non-alcoholic steatohepatitis (NASH), is a growing epidemiologic burden with the rising prevalence of obesity in the US and worldwide. NASH causes inflammation in hepatocytes by depositing excess circulating fat globules within the cells. These fatty hepatocytes become distressed and compete for oxygen leading to inflammation, loss of function and an immune response that results in subsequent fibrosis[14]. NASH also causes “multiple-parallel” hits whereby fatty deposits damage and inflame visceral tissues which cause increased metabolic stress in the already weakened fatty hepatocytes tasked with filtering the molecules of inflammation combined with a globally poor nutritional state[15]. Specifically, Kupffer cells, which are specialized macrophages located within the liver sinusoids and part of the normal healthy liver immunoanatomy, are activated, cytokine production is increased and an environment of fibrosis, regeneration and deranged proliferation is created[16].
Alcohol is a well-known carcinogen for a variety of cancers and contributes to their development in a multimodal fashion. Acetaldehyde, the metabolite of ethanol, impairs the cell’s DNA repair mechanisms through direct cross-linking[17]. Alcohol also leads to the production of reactive oxygen species, pro-inflammatory cytokines and the downregulation of normal immunosuppression during metabolism, a process that takes place within hepatocytes[18][19][20]. These processes combine to damage and alter hepatocytes, predisposing these cells to mutagenesis and subsequent carcinogenesis.
While each etiology shares the overall theme of insult leading to fibrosis, degeneration, regeneration and finally mutagenesis ending in HCC tumor cells, the process by which these stages are carried out is clearly quite heterogeneous. As such, it becomes extremely important to strive to tailor treatment options based on the specific causative etiology, a task which, as we will see, has yet to be achieved.
3. Current Management of HCC
3.1. BCLC Staging and Treatment Algorithm
Current HCC treatment pathways depend on the stage at which a patient is diagnosed. The most commonly used staging system in HCC is the Barcelona clinic liver cancer (BCLC) system. The BCLC system classifies patients into five prognostic groups in order of advancing disease stage—0, A, B, C and D. The staging system incorporates liver function (Child-Pugh class), tumor burden (number, size, vascular invasion, metastases) and patient performance status (using the Eastern Cooperative Oncology Group [ECOG] status)[21]. Patients classified as BCLC stage 0 or A are eligible for curative therapies, namely surgical resection, liver transplantation and percutaneous radiofrequency ablation. Five-year survival rates are quoted at greater than 70%. BCLC stage B patients are eligible for locoregional therapy, such as trans-arterial chemoembolization (TACE) or trans-arterial radioembolization (TARE). Median survival is quoted at 2 years. BCLC stage C patients have advanced disease defined as either having portal vein invasion or extrahepatic spread. These patients can be offered systemic treatment only. BCLC stage D, also called terminal stage, is currently treated symptomatically with best supportive care[22] . Figure 1 displays the BCLC staging system with a graphic representation of the associated anatomy, tumor burden liver and recommended treatment. Noticeably, none of the available treatment options addresses the etiology of HCC tumor development. In addition, currently no immunotherapy is considered standard of care for any stage.
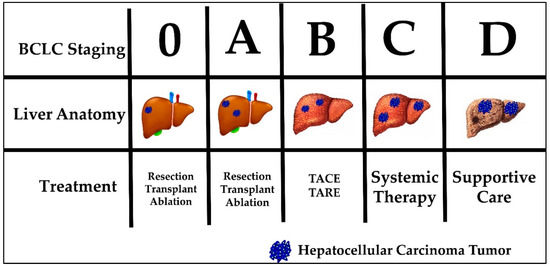
Figure 1. Barcelona Clinic Liver Cancer (BCLC) staging, liver anatomy, tumor burden and treatment.
3.2. Surgical Management
Surgical management of HCC is divided into two categories, resection or surgical removal of part of the liver based on anatomic segments or transplant, the complete removal of the native liver and replacement from deceased- or live-donor tissue. The decision to resect depends on tumor size, location and degree of underlying hepatic decompensation. Patients eligible for resection often have preserved liver function and tumors which are localized anatomically without any vascular invasion, falling into BCLC categories 0 and A[23][24][25]. A resection is potentially curative if tumors are solitary and generally < 5 cm in diameter[23]. Diminished liver function can negate eligibility for resection of HCC tumors, even those that fit the size, location and invasion criteria. Though defined by overriding guidelines, decisions to resect can vary between institutions. Recovery from resection is dependent on the volume of remnant liver and its underlying function[24]. Resection for HCC has a role in the treatment algorithm but does not offer a solution to patients with high tumor burden, advanced HCC, and/or diminished underlying liver function.
Transplant for HCC is usually reserved for patients in BCLC categories 0 and A who do not meet the above resection criteria. In the US, the Milan criteria is often used to determine transplant eligibility for HCC patients. The Milan criteria states that patients who have—1) a single tumor with diameter less than 5 cm OR 2) not more than three foci of tumor, each one not exceeding 3 cm AND 3) no angioinvasion AND 4) no extrahepatic involvement are eligible for liver transplantation[26][27][28]. In the US, liver transplant is considered the gold standard for treatment of HCC that falls within the Milan criteria[24]. Additional, more liberal criteria proposed by the University of San Francisco expanded the Milan criteria to—1) a single tumor with diameter less than 6.5 cm OR 2) not more than three foci of tumor, each one not exceeding 4.5 cm OR 3) total tumor diameter not exceeding 8.5 cm AND 4) no angioinvasion[23][29]. Survival at 1, 3 and 5-years were predictably worse in the UCSF group compared to Milan, although only late-stage T4 tumors were shown to have inferior outcomes compared to Milan criteria on multivariable analysis[30]. The use of the Milan criteria, namely in the US, is likely here to stay and is driven partially by ongoing organ shortages and lack of the robust living-donor liver programs which exist in other countries. This limits the number of patients who receive liver transplants. In addition, surgical intervention is invasive and exposes patients to the risks of general anesthesia as well as a gamut of surgical complications highlighting the need for less invasive, more specific treatments.
Radiofrequency ablation (RFA) is being increasingly utilized to treat small, early stage BCLC 0 and A tumors as well as to treat patients who are at risk to progress while waiting on the transplant list[31]. RFA is the superheating of HCC tumor tissue via radio wave transmission through a probe inserted through the skin guided by ultrasound or cross-sectional imaging[31]. A recent study, however, has shown that, in HCC patients who meet the resection criteria treated with resection versus RFA, the overall 5-year survival and recurrence-free survival were lower in the RFA group (75 v 54% and 51 v 28%, respectively)[32]. This modality, in its current state, is clearly reserved for patients with small tumors who cannot tolerate surgical intervention or those on the transplant waiting list who are at risk of tumor progression but does not offer a solution to patients with advanced HCC, nor is it superior to current surgical options available.
3.3. Locoregional Therapy
As HCC BCLC stage progresses to B, the patient is often no longer eligible for surgical resection, transplant or ablation. Instead, reducing tumor burden becomes the goal of treatment. This scenario is also the case for patients who may be in lower BCLC stages but their diminished overall health renders them ineligible for safe surgical intervention. Treatments designed for this purpose include the direct administration of either intra-arterial chemotherapy (trans-arterial chemoembolization (TACE)) or radiotherapy (trans-arterial radioembolization (TARE)) and are collectively referred to as locoregional therapy[33][34]. Specifically, TACE involves direct delivery of doxorubicin followed by the blocking of blood flow via selective hepatic artery branch embolization to ensure chemotherapy dwell time. Recent advances allow TACE treatment of both early, non-surgical and some late stage patients (BCLC 0, A, B, & some C) with a solitary nodule or up to 3 nodules under 3 cm[33]. One trial, stopped early, showed that mean survival was significantly longer with TACE compared to symptomatic treatment alone (28.6 months v 17.9 months)[34].
TARE, on the other hand, utilizes selective intra-arterial injection of radioactive yttrium-90 (Y-90), iodine-131 (131I) or rhenium-188 (188Re) to cause radiation-induced cell necrosis[35]. Similar to TACE, TARE is indicated for non-surgical BCLC Stage 0, A, B & some C patients[36]. One study reported median survival of 16.9 months in BCLC Stage B patients and another retrospective analysis demonstrated a median survival of 14 months when TARE was used as first-line therapy compared to 8 months in patients receiving standard therapy[36][37]. Most complications of TARE stem from radiation damage causing bile duct stricture and cholangitis.
These locoregional methods of treatment for advanced disease, while showing some benefit to patients, ultimately do not lead to a sustained response. Residual tumor and local recurrence often necessitate additional TARE or TACE procedures and progression of disease sometimes renders these locoregional therapies ineffective with no response seen[34] [42]. Also, these procedures are provided only at selected centers equipped with skilled providers able to perform both the procedure and post-procedural care. This results in limited availability and a significant healthcare cost. While some believe there is the potential for locoregional therapy to “downstage” cancers, making the patient eligible for resection or transplant, this concept has not yet been accepted into standard practice.
3.4. Systemic Therapy
For advanced staged HCC tumors (BCLC B & C), surgical and locoregional therapies are not recommended. Instead, the disease is so advanced only systemic therapies are applicable in this patient population. Sorafenib, one of the medications which is FDA approved for advanced HCC, is a systemic oral therapy which inactivates multiple kinase proteins, including vascular endothelial growth factor receptor (VEGFR), platelet derived growth factor receptor (PDGFR) and rapidly accelerated fibrosarcoma (RAF) kinases, halting pathways responsible for angiogenesis and cell growth[38][39]. It is approved for use in the treatment of kidney and thyroid cancer. Sorafenib was tested in late stage HCC (BCLC B & C) treatment in the US & European Sorafenib in patients with advanced hepatocellular carcinoma (SHARP) trial and showed an increased survival versus placebo (10.7 months v 7.9 months)[40]. This finding was corroborated in a repeat trial done in the Asia-Pacific (6.5 months v 4.2 months)[41]. Subsequently, other systemic therapies have been marketed as non-inferior to sorafenib including regorafenib (RESOURCE trial)[42][43] , cabozantinib (CELESTIAL trial)[44] and lenvatinib[42]. Although these medications were the first to offer treatment to late stage HCC patients, at best they offered a modest 3 month median survival benefit. The shortcomings of sorafenib, beyond its marginal benefit to the patient, include the frequent intolerance of side effects associated with treatment. Other systemic medications have more recently been approved for HCC, however further studies will be required to elucidate their efficacy. No current standard therapeutic modality offers favorable survival outcomes to patients with advanced stage HCC. However, given the unique nature of the liver and HCC tumors, immunotherapy may be the answer.
References
- Li, S.; Yang, F.; Ren, X. Immunotherapy for hepatocellular carcinoma. Drug Discov. Ther. 2015, 9, 363–371.
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global epidemiology of hepatocellular carcinoma: An emphasis on demographic and regional variability. Clin. Liver Dis. 2015, 19, 223–238.
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386.
- Hao-Wen Sim; Jennifer Knox; Hepatocellular carcinoma in the era of immunotherapy. Current Problems in Cancer 2018, 42, 40-48, 10.1016/j.currproblcancer.2017.10.007.
- Bosetti, C.; Turati, F.; la Vecchia, C. Hepatocellular carcinoma epidemiology. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 753–770.
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576.
- WHO. Projections of Mortality and Causes of Death, 2016 to 2060. Available online: http://www.who.int/healthinfo/global_burden_disease/projections/en/ (accessed on 20 July 2020).
- Cronin, K.A.; Lake, A.J.; Scott, S.; Sherman, R.L.; Noone, A.M.; Howlader, N.; Henley, S.J.; Anderson, R.N.; Firth, A.U.; Ma, J.; et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018, 124, 2785–2800.
- Yongyan Chen; Zhigang Tian; HBV-Induced Immune Imbalance in the Development of HCC. Frontiers in Immunology 2019, 10, 2048, 10.3389/fimmu.2019.02048.
- Michielsen, P.; Ho, E.; Viral hepatitis B and hepatocellular carcinoma. Acta Gastroenterol. Belg. 2011, 74, 4–8.
- Lu Zong; Hui Peng; Cheng Sun; Fenglei Li; Meijuan Zheng; Yongyan Chen; Haiming Wei; Rui Sun; Zhigang Tian; Breakdown of adaptive immunotolerance induces hepatocellular carcinoma in HBsAg-tg mice. Nature Communications 2019, 10, 221, 10.1038/s41467-018-08096-8.
- Michael Kew; Aflatoxins as a cause of hepatocellular carcinoma.. Journal of Gastrointestinal and Liver Diseases 2013, 22, 305–310.
- Yang Luo; Yue Zhang; Di Wang; Di Shen; Yi-Qun Che; Eradication of Hepatitis C Virus (HCV) Improves Survival of Hepatocellular Carcinoma Patients with Active HCV Infection - A Real-World Cohort Study.. null 2020, 12, 5323-5330.
- Yousef Fazel; Aaron B. Koenig; Mehmet Sayiner; Zachary D. Goodman; Zobair M. Younossi; Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism 2016, 65, 1017-1025, 10.1016/j.metabol.2016.01.012.
- Herbert Tilg; Alexander R. Moschen; Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836-1846, 10.1002/hep.24001.
- Kook Hwan Kim; M.-S. Lee; Pathogenesis of Nonalcoholic Steatohepatitis and Hormone-Based Therapeutic Approaches. Frontiers in Endocrinology 2018, 9, 485, 10.3389/fendo.2018.00485.
- Vicki L. Dellarco; A mutagenicity assessment of acetaldehyde. Mutation Research/Reviews in Genetic Toxicology 1988, 195, 1-20, 10.1016/0165-1110(88)90013-9.
- an, G.; Wang, X.; Sun, C.; Zheng, X.; Wei, H.; Tian, Z.; Sun, R. Chronic Alcohol Consumption Promotes Diethylnitrosamine-Induced Hepatocarcinogenesis via Immune Disturbances. Sci. Rep. 2017, 7, 2567.
- Seitz, H.K.; Stickel, F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol. Chem. 2006, 387, 349–360.
- McClain, C.J.; Barve, S.; Deaciuc, I.; Kugelmas, M.; Hill, D. Cytokines in alcoholic liver disease. Semin. Liver Dis. 1999, 19, 205–219.
- Josep M. Llovet; Concepció Brú; Jordi Bruix; Prognosis of Hepatocellular Carcinoma: The BCLC Staging Classification. Seminars in Liver Disease 1999, 19, 329-338, 10.1055/s-2007-1007122.
- Singal, A.G.; Parikh, N.D.; Rich, N.E.; John, B.V.; Pillai, A. Hepatocellular Carcinoma Surveillance and Staging. In Hepatocellular Carcinoma: Translational Precision Medicine Approaches; Hoshida, Y., Ed.; Humana: Cham, Switzerland, 2019; pp. 27–51.
- Attwa, M.H.; El-Etreby, S.A. Guide for diagnosis and treatment of hepatocellular carcinoma. World J. Hepatol. 2015, 7, 1632–1651.
- Orcutt, S.T.; Anaya, D.A. Liver Resection and Surgical Strategies for Management of Primary Liver Cancer. Cancer Control 2018, 25, 1073274817744621.
- Cauchy, F.; Soubrane, O.; Belghiti, J. Liver resection for HCC: Patient’s selection and controversial scenarios. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 881–896
- Sapisochin, G.; Bruix, J. Liver transplantation for hepatocellular carcinoma: Outcomes and novel surgical approaches. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 203–217.
- Bhardwaj, N.; Perera, M.T.; Silva, M.A. Current Treatment Approaches to HCC with a Special Consideration to Transplantation. J. Transplant. 2016, 2016, 7926264.
- Vitale, A.; Volk, M.; Cillo, U. Transplant benefit for patients with hepatocellular carcinoma. World J. Gastroenterol. 2013, 19, 9183–9188.
- Francis Y. Yao; Linda Ferrell; Nathan M. Bass; Jessica J. Watson; Peter Bacchetti; Alan Venook; Nancy L. Ascher; John P. Roberts; Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology 2001, 33, 1394-1403, 10.1053/jhep.2001.24563.
- Tarkan Unek; Comparison of Milan and UCSF criteria for liver transplantation to treat hepatocellular carcinoma. World Journal of Gastroenterology 2011, 17, 4206-4212, 10.3748/wjg.v17.i37.4206.
- Debra A. Gervais; Shaunagh McDermott; Radiofrequency Ablation of Liver Tumors. Seminars in Interventional Radiology 2013, 30, 49-055, 10.1055/s-0033-1333653.
- Jiwei Huang; Lvnan Yan; Zheyu Cheng; Hong Wu; Liang Du; Jinzhou Wang; Yinglong Xu; Yong Zeng; A Randomized Trial Comparing Radiofrequency Ablation and Surgical Resection for HCC Conforming to the Milan Criteria. Annals of Surgery 2010, 252, 903-912, 10.1097/sla.0b013e3181efc656.
- Raoul, J.L.; Forner, A.; Bolondi, L.; Cheung, T.T.; Kloeckner, R.; de Baere, T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat. Rev. 2019, 72, 28–36.
- Llovet, J.M.; Real, M.I.; Montana, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Sola, R.; et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet 2002, 359, 1734–1739.
- Bruno Sangro; Riad Salem; A.R. Kennedy; Douglas Coldwell; Harpreet Wasan; Radioembolization for Hepatocellular Carcinoma. American Journal of Clinical Oncology 2011, 34, 422-431, 10.1097/coc.0b013e3181df0a50.
- Fatih Boyvat; Interventional radiological treatment of hepatocellular carcinoma.. Exp Clin Transplant . 2017, 15(Suppl 2), 25-30, 10.6002/ect.TOND16.L8.
- Thomas Couri; Anjana Pillai; Goals and targets for personalized therapy for HCC. Hepatology International 2019, 13, 125-137, 10.1007/s12072-018-9919-1.
- Wilhelm, S.M.; Adnane, L.; Newell, P.; Villanueva, A.; Llovet, J.M.; Lynch, M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 2008, 7, 3129–3140.
- Keating, G.M.; Santoro, A. Sorafenib: A review of its use in advanced hepatocellular carcinoma. Drugs 2009, 69, 223–240
- Giancarlo Spinzi; Silvia Paggi; Sorafenib in Advanced Hepatocellular Carcinoma. New England Journal of Medicine 2008, 359, 2497-2499, 10.1056/nejmc081780.
- Ann-Lii Cheng; Yoon-Koo Kang; Zhendong Chen; Chao-Jung Tsao; Shukui Qin; Jun Suk Kim; Rongcheng Luo; Jifeng Feng; Shenglong Ye; Tsai-Sheng Yang; et al.Jianming XuYan SunHoujie LiangJiwei LiuJiejun WangWon Young TakHongming PanKarin BurockJessie ZouDimitris VoliotisZhongzhen Guan Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. The Lancet Oncology 2009, 10, 25-34, 10.1016/s1470-2045(08)70285-7.
- Personeni, N.; Pressiani, T.; Bozzarelli, S.; Rimassa, L. Targeted agents for second-line treatment of advanced hepatocellular carcinoma. World J. Gastrointest. Oncol. 2019, 11, 788–803.
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66.
- Ghassan K. Abou-Alfa; Tim Meyer; Ann-Lii Cheng; Anthony B. El-Khoueiry; Lorenza Rimassa; Baek-Yeol Ryoo; Irfan Cicin; Philippe Merle; YenHsun Chen; Joong-Won Park; et al.Jean-Frederic BlancLuigi BolondiHeinz-Josef KlümpenStephen L. ChanVittorina ZagonelTiziana PressianiMin-Hee RyuAlan P. VenookColin HesselAnne E. Borgman-HageyGisela SchwabRobin K. Kelley Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. New England Journal of Medicine 2018, 379, 54-63, 10.1056/nejmoa1717002.
- Ghassan K. Abou-Alfa; Tim Meyer; Ann-Lii Cheng; Anthony B. El-Khoueiry; Lorenza Rimassa; Baek-Yeol Ryoo; Irfan Cicin; Philippe Merle; YenHsun Chen; Joong-Won Park; et al.Jean-Frederic BlancLuigi BolondiHeinz-Josef KlümpenStephen L. ChanVittorina ZagonelTiziana PressianiMin-Hee RyuAlan P. VenookColin HesselAnne E. Borgman-HageyGisela SchwabRobin K. Kelley Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. New England Journal of Medicine 2018, 379, 54-63, 10.1056/nejmoa1717002.




