Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mirzokhid Ibrokhimjon ugli Abdirakhimov | -- | 8890 | 2022-08-02 10:10:04 | | | |
| 2 | Mirzokhid Ibrokhimjon ugli Abdirakhimov | -5338 word(s) | 3552 | 2022-08-11 13:52:24 | | | | |
| 3 | Sirius Huang | Meta information modification | 3552 | 2022-08-12 06:12:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Abdirakhimov, M.; Al-Rashed, M.H.; Wójcik, J. H2S Removal from Gas Mixtures Using Zeolites. Encyclopedia. Available online: https://encyclopedia.pub/entry/26042 (accessed on 08 February 2026).
Abdirakhimov M, Al-Rashed MH, Wójcik J. H2S Removal from Gas Mixtures Using Zeolites. Encyclopedia. Available at: https://encyclopedia.pub/entry/26042. Accessed February 08, 2026.
Abdirakhimov, Mirzokhid, Mohsen H. Al-Rashed, Janusz Wójcik. "H2S Removal from Gas Mixtures Using Zeolites" Encyclopedia, https://encyclopedia.pub/entry/26042 (accessed February 08, 2026).
Abdirakhimov, M., Al-Rashed, M.H., & Wójcik, J. (2022, August 10). H2S Removal from Gas Mixtures Using Zeolites. In Encyclopedia. https://encyclopedia.pub/entry/26042
Abdirakhimov, Mirzokhid, et al. "H2S Removal from Gas Mixtures Using Zeolites." Encyclopedia. Web. 10 August, 2022.
Copy Citation
Natural gas, biogas, and refinery gas all include H2S, which has adverse effects not only on the environment and human health but also on the equipment and catalysts that are employed in the relevant processes. H2S is removed from the aforementioned gases using a variety of techniques in order to fulfill the necessary sales criteria and for reasons of safety. The adsorption method stands out among various other approaches due to its straightforward operation, high level of efficiency, and low overall cost. This technique makes use of a variety of adsorbents, such as metal-organic frameworks (MOFs), activated carbon, and zeolites. The use of zeolite-based adsorbents is by far the most common of these various types.
H2S removal
separation
zeolites
adsorption
1. Introduction
Crude oil, natural gas, coal, and biogas are the primary forms of energy resources on the planet. There is an ever-increasing demand for these fuels, and governments are implementing policies to reach zero emissions. The presence of various toxic substances in these fuels leads to the release of harmful substances into the environment during their use. Therefore, fuels are tested to meet certain standards before use. According to the International Energy Agency, natural gas accounts for more than 23% of the world’s fuel supply. Natural gas contains a number of various gases that are toxic, such as H2S, mercaptans, and disulfides. Natural gas and biogas both contain varying amounts of hydrogen sulfide, and because of its corrosive and toxic qualities, they both need to be purified. Natural gas may have varying quantities of H2S in order to satisfy regulatory criteria. For instance, the standards for a pipeline allow for up to 5 ppm in European countries[1], while in the United States it is only 4 ppm [2]
Techniques such as adsorption, absorption, cryogenic distillation, and membrane processes are utilized most frequently in order to bring the amount of H2S to an acceptable level. Each approach comes with its own set of benefits and drawbacks; the one chosen will depend primarily on the concentration of H2S present in the mixture as well as the price of the adsorbent or absorbent used in the process. Because of its low cost, high level of safety, and numerous other advantages, adsorption is by far the most popular approach. It makes use of a wide variety of adsorbents, including metal-organic frameworks (MOFs), activated carbon, and zeolites, and new combinations of these adsorbents are continually being produced and examined.
2. Measurement of Hydrogen Sulfide Concentration
The concentration of H2S is one of the most crucial quality standards for natural gas and biogas. Therefore, it is determined using a variety of techniques. Cost, application complexity, and measurement precision all vary among them. As a result, several techniques are employed for various goals. Standards for accurate measurements have been developed and registered by international organizations such as ASTM, UOP, and GPA [3]. The following approaches have been employed in lab settings in numerous research studies because they are straightforward to use despite the complexity of these basic test methods.
2.1. Gas Chromatography
One of the most accurate methods for identifying the precise concentration of the individual components that comprise a mixture is the use of gas chromatography. Measurement of the signals that are produced by the components of the sample that are separated in the column is the basis for this method. Porous polymers, such as Poropack [4], Hayesep-Q column, and sulfur chemiluminescence detector (SCD)[5], are utilized as GC column materials. This method is utilized for doing exhaustive compositional analysis [3].
2.2. Analyzers
Analyzers are readily accessible for purchase in the marketplace and have found widespread application [6][7] to monitor or identify certain gases for a number of applications. The following are some of the most prevalent H2S measurement methods that were utilized in the development of the analyzers. These devices are based on a number of different methodologies.
Electrochemical
Detectors are utilized largely for the purposes of personnel protection and providing information regarding pollution in the workplace. Nevertheless, as can be seen from the numerous studies that have been reported in the literature [8][9], detectors were utilized to measure the concentration of H2S for the sake of research as well. In the electrochemical detectors, the release of free electrons and a decrease in the resistance between the two electrodes occur when H2S molecules take the place of oxygen molecules. This change, which may be detected immediately, is directly proportional to the amount of H2S that is present in the gas. On a lab scale, the H2S concentration can be measured in a variety of ranges, from 0 to 1000 parts per million (ppm), using electrochemical sensors. Although this kind of instrumentation is readily available in both portable and fixed-point solutions and has the potential to be useful in measuring H2S, it is not without drawbacks. Electrochemical cell-based sensors need to be calibrated frequently. They are also susceptible to high temperatures, high humidity, low oxygen levels, and other conditions that might cause drift and cell degradation.
Lead Acetate
H2S tape analyzers make use of tape that has been coated with lead acetate in order to detect the presence of H2S in a gas sample. This detection method is predicated on the reaction that takes place between H2S and lead acetate, which results in the formation of lead sulfide. There is a direct correlation between the amount of H2S present and the darkness of the stain on the type. These tape analyzers have the capability of accurately measuring low levels of H2S, despite the considerable initial investment required to purchase one. Despite having portable possibilities, this kind of analyzer is often stationary. The interference from SO2 and both low and high humidity can affect the lead acetate tape.
Tunable Diode Lasers
Tunable diode lasers (TDL) are frequently utilized in the process of measuring the concentrations of the constituents of the gas. The absorption properties of different gases are utilized by the technology so that the concentration of the target gas can be accurately measured. After travelling through the gas sample, the laser light’s intensity is measured by a detector so that the target gas concentration can be derived from this information. Measurements that are exact and exceptionally dependable can be produced by a correctly built TDL analyzer even when the concentration of H2S present is very low.
3. Physicochemical Properties of the H2S
Hydrogen sulfide is a colorless and flammable gas that has a strong and distinctive odor of rotten eggs. It is also known as hydrosulfuric acid, sewer gas, stink damp, dihydrogen monosulfide, dihydrogen sulfide, sulfane, sulfurated hydrogen, and sulfur hydride. It has a greater density than air and may settle on the ground. The characteristics of H2S that are most important to know are outlined in Table 1.
Table 1. Chemical and physical properties of H2S [10].
| Properties | Value |
|---|---|
| Molar mass | 34.081 g/mol |
| Specific gravity | 1.2 |
| Boiling point | −60.33 °C |
| Melting point | −85.49 °C |
| Smell | Rotten eggs |
| Color | Colorless |
| Density | 1.5392 g/L at STP |
| Solubility in water | 4 g dm−3 (at 20 °C) |
| Lower Explosive Limit | 4.3% |
| Upper Explosive Limit (UEL): | 45% |
| Autoignition Temperature: | 270 °C |
| Vapor Pressure: | 1880 kPa at 20 °C |
| Vapor Density (Relative to Air): | 1.189 |
| Kinetic diameter | 0.36 nm |
4. Adsorption of H2S
The term “adsorption” refers to a process in which specific compounds are selectively retained by the formation of a physical bond with a solid surface known as an “adsorbent.” Certain gases are drawn to solid surfaces due to the attraction exerted by Van der Waals forces and hydrophobic interactions. The bonding energies involved in adsorption fall somewhere in the range of 10 to 70 kJ/mol [11]. If the adsorbent can be regenerated, adsorption is an energy-efficient process because it allows the adsorbent to be reused multiple times during the adsorption-desorption cycle.
A number of different adsorbents, including metal oxides [12] [13] [14], metals [15], metal-organic frameworks [16], zeolites, and carbon-based materials [17], are utilized in the process of removing hydrogen sulfide from a gas mixture that also contains CH4, CO2, N2, and H2. For an adsorbent to be effective in the process, it needs to have a high adsorption capacity, the ability to be regenerated, the ability to maintain its stability at high temperatures, and high selectivity for the molecule that needs to be removed. Adsorption of H2S is often performed in a fixed bed adsorption column when it is done on a small scale, such as in a laboratory (see Figure 1).
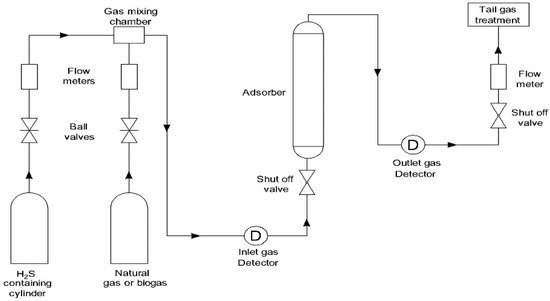
Figure 1. Typical experimental laboratory setup for the removal of H2S.
5. Zeolites
Zeolites are crystalline solids formed by the systematic interaction of tetrahedral molecules of AlO4 and SiO4. The following empirical formula can be used to represent them:
M2/nO·Al2O3·xSiO2·yH2O
Because AlO4 tetrahedra are only linked to SiO4 tetrahedra, x in this oxide formula is usually equal to or greater than 2, and n is the cation valence. The cation and water molecules are accommodated in the framework’s channels and linked spaces. The cations are very mobile and may generally be exchanged with other cations to variable degrees.
According to the International Zeolite Association [18], there are more than 40 natural and more than 280 zeolites that have been artificially synthesized. These zeolites are distinguished from one another by their unique crystal structure and the ratios of Al and Si atoms. In the zeolite molecule, the Al atom is negatively charged and can be balanced with cations such as Na+, K+, and Ca2+. Zeolites have pores that allow molecules of a lower diameter to be adsorbed, whereas molecules of a larger diameter are unable to pass through these pores. This is an attractive adsorptive property for removing impurities such as hydrogen sulfide, carbon dioxide, and moisture from natural gas. To this day, a large number of zeolites have been synthesized and evaluated for the separation of H2S from a wide variety of mixed gases, and the findings have been very encouraging.
The zeolites known as Faujasite (FAU) and Linde Type A (LTA) are utilized rather frequently in the separation processes. They are composed of sodalite (SOD) structures that are organized in a pattern that varies depending on the amount of aluminium to silicon (Figure 2).
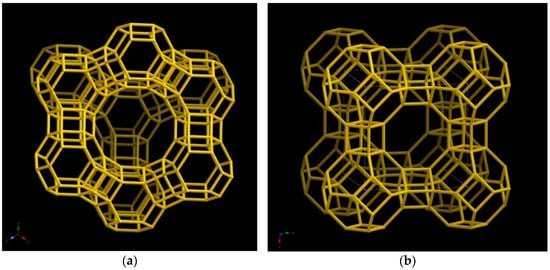
Figure 2. Structure of (a) Faujasite (FAU) and (b) Linde Type A (LTA) zeolites [18].
6. The Synthesis of Zeolites
Natural zeolites can be found in large quantities all over the world, but they are not commercially available because of their low purity, high cleaning cost, and inability to be used in certain processes. Synthetic zeolites, on the other hand, are produced on an industrial scale. These zeolites serve as adsorbents, catalysts, and ion-exchangers, among other functions, and are utilized for a variety of applications. Milton [19] developed the first low-silica zeolites in the late 1940s by hydrothermally crystallizing alkali aluminosilicate gels under low temperatures and pressures. Synthesizing zeolites can be done in a number of different ways, including hydrothermal [7], alkali-fusion [20], and microwave-assisted [21] methods. The synthesis of an appropriate zeolite can be accomplished using any one of the aforementioned approaches. As raw materials, a variety of salts and minerals containing the elements aluminium and silicon are utilized. The elements aluminium and silicon can be obtained from a variety of sources, including sodium aluminate, aluminium sulphate, aluminate nitrate, sodium, or alkaline silicate, and minerals such as clay and kaolin [22]. The synthesis of an appropriate zeolite can be accomplished using any one of the aforementioned approaches. As raw materials, a variety of salts and minerals containing the elements aluminium and silicon are utilized. The elements aluminium and silicon can be obtained from a variety of sources, including sodium aluminate, aluminium sulphate, aluminate nitrate, sodium, or alkaline silicate; minerals such clay and kaolin [23]; and from iron ore tailings, rice husk ash, coal fly ash, lithium slag, and paper sludge ash.
7. H2S Adsorption Mechanism on Zeolites
There are two primary explanations for why H2S can be absorbed physically by zeolites:
-
One of the things that set zeolites apart from other minerals is the fact that their chemical structure remains relatively unchanged throughout time. These particular chemical structures have pores that are on the nanometer scale, and they appear to function as molecular sieves. Even molecules that are significantly smaller than the pores found in zeolites have the potential to become “trapped” within these pores. In light of this, it is possible to estimate the zeolite that can be employed [24] by first determining the kinetic diameter of the molecule that needs to be separated. Zeolites with pore sizes larger than 0.36 nm, which is the kinetic diameter of H2S, have the ability to adsorb H2S. The presence of impurities such as CO2, which has a kinetic diameter of 0.33 nm, causes a low separation efficiency due to competitive adsorption. Table 2 provides the kinetic diameters of several molecules that could be present in the mixture with H2S and induce competitive adsorption.
Table 2. Kinetic diameter for various molecules [24].
| Molecule | Kinetic Diameter (nm) |
|---|---|
| NH3 | 0.26 |
| H2O | 0.28 |
| CO2 | 0.33 |
| N2 | 0.36 |
| O2 | 0.36 |
| SO2 | 0.36 |
| CH4 | 0.38 |
-
The second explanation is that zeolites are able to adsorb H2S as a result of contact with the polar structure of the zeolite [25]. When the intracrystalline pores and channels of zeolites are filled with H2S molecules, the zeolites are unable to adsorb, and there are no more adsorptions that take place. It is possible to restore the adsorption capacity of zeolite by modifying the conditions of the adsorption equilibrium [26] in terms of temperature, pressure, or both. This process is referred to as “regeneration”. The contact between the molecules being adsorbed and the active sites (sites placed over the external surface of the zeolites, or close to the micropore openings) of the zeolite becomes less strong with time, which results in the molecules being desorbed. During the adsorption process, the permanent structure of the zeolites is maintained since the phase of H2S that is adsorbed onto the zeolites does not change any of the zeolites’ atoms. Temperature, pressure, and polarity are the three most important factors that determine how well H2S can be adsorbed.
Sometimes, chemical bonds such as covalent or ionic are formed between the surface of the zeolite and H2S. This process is known as chemisorption, and its interaction is much stronger than physisorption. Chemisorption can, in fact, meet the demand for the selective capture of H2S; however, one drawback is that it results in the development of irreversible bonds, which reduces the potential for regeneration. Therefore, for H2S separation in industrial processes, physisorption is preferred, as the reversible process take place by weak van der Waals forces and electrostatic interaction.
8. The Effect of Si/Al Ratio on H2S Adsorption
The ratio of silicon to aluminium, which is known as the Si/Al ratio, is the most significant factor in determining the structures and properties of zeolites. Zeolites can be categorized as low, medium, high, or silica, depending on this ratio [27]. The thermal stability, acidity, and structural makeup of zeolites are all influenced by the Si/Al ratio. When the ratio of silicon to aluminium increases, some properties, such as thermal stability, increase. The amount of silica found in hydrophilic zeolites is quite low, whereas the amount of silica found in hydrophobic zeolites is relatively considerable . Ion exchange capacity and cation concentration are both influenced by the amount of aluminium present, and both tend to decrease as the ratio of silicon to aluminium rises. For the purpose of separating hydrogen sulfide from a variety of streams, hydrophilic zeolites with a high level of surface selectivity are recommended. In this case, zeolites of types A and X that have a low silica content and demonstrate strong adsorption capabilities are preferable candidates. Karge and Rasko [28] investigated how the ratio of silicon to aluminium influences the mechanism of hydrogen sulfide adsorption by the FAU zeolites (X and Y). When the ratio of silicon to aluminium was larger than 2.5 (Y), they discovered that physical adsorption of H2S occurred. Due to the fact that low silica zeolites (Si/Al = 1–1.5) are aluminium saturated and have the largest cation concentration, it may be deduced that Na+ cations play an important role in the dissociative adsorption. Consequently, an increase in the Si/Al ratio causes a decrease in the amount of Na+ ions, which in turn causes a decreased dissociation of H2S into HS- and H+, indicating an increase in physical adsorption. Moreover, by replacing the Na+ cations in a 4A zeolite with Ca2+, a 5A zeolite is produced, resulting in an increase in physisorption [29]. The Si/Al ratio of zeolites, which are frequently employed in separation processes, is shown in Table 3 below:
Table 3. The Si/Al ratio of zeolites [24].
| Type of the Zeolites | Si/Al Ratio |
|---|---|
| Linde type A (LTA) | 1–6 |
| Faujasite X type | 1–1.5 |
| Faujasite Y type | higher than 1.5 |
| Chabazite (CHA) | 1-to infinity |
| MFI framework (ZSM-5) | 10-to infinity |
| Mordenite (MOR) | 4–12 |
9. H2S Selectivity
The ability of an adsorbent to selectively adsorb a component in a gas mixture is affected by the presence of many components in the mixture. Adsorbents may have the capability to adsorb multiple components at the same time [30][31]. As a result, the selectivity of adsorbents is an essential factor to consider while making your selection. In order to choose and evaluate zeolites that are capable of selectively adsorbing hydrogen sulfide from gas streams, it is vital to take into consideration the zeolites’ capacity to absorb other contaminants, such as carbon dioxide and water. The polarity of zeolites depends on the Si/Al ratio [32] and is a significant factor in determining their selectivity. They are well attracted to zeolites due to the fact that methane and its homologues in natural gas are non-polar and polar molecules such as H2S, CO2, and water. As was said earlier, selecting the appropriate zeolite is essential to decrease the adsorption of CO2 and H2O molecules while simultaneously enhancing affinity toward H2S molecules.
10. Adsorption Capacity
The number of contaminations that the adsorbent needs to remove is an essential factor in determining how successful the operation will be. A decrease in the length of the adsorption cycle results from an increase in the adsorption capacity, which improves the separation of the targeted molecule from the mixture. The adsorption capacity can be determined by calculating the ratio of the amount of the adsorbed molecule to the mass of the adsorbent, and is often expressed in the unit of mmol/g or mg/g.
The following equation can be used to determine the maximum adsorption capacity of a zeolite:
where, C0 and Ce represent the initial and equilibrium concentration (ppm) of the H2S, respectively; V (L) represents the total volume of gas mixture used in each experimental run; and m (g) represents the dry mass of the adsorbent that was used for the adsorption process. All concentrations are expressed in mg/g.
The adsorption capacity for continuous adsorption can be calculated from the breakthrough curves using the following equation [33]:
-
Qtot = total gas flow rate (Nl/h).
-
MW = molecular weight of H2S.
-
Cin = inlet H2S concentration (ppmv).
-
t1= breakthrough time when the outlet concentration is 1 ppmv (h).
-
t0= breakthrough time at the last detection of 0 ppmv (h).
-
Vm = molar volume (24,414 Nl/mol). m = mass of adsorbent material (g).
11. Applications of Natural and Synthetic Zeolites
Although natural zeolites have adsorption properties that are several times higher than those of synthetic zeolites, very few natural zeolites have been studied in the context of the removal of hydrogen sulfide from a variety of gases. According to the research, natural zeolites are also good at separating H2S. Various procedures, including high-temperature calcination, the addition of various metals, and acid processing, can be employed to improve their efficacy in H2S removal. Because natural zeolites are insufficient for commercial use as catalysts and adsorbents, synthesized zeolites are utilized instead. It is necessary to improve the characteristics of zeolites. To that end, metals can be added, calcination temperatures can be changed, and the composition of zeolites can be altered in other ways. Metal ions are introduced into the zeolite structure by two methods: ion exchange and impregnation. In recent years, zeolite-based adsorbents used in the separation of H2S from gas mixtures have been synthesized from various cheap raw materials because they are widely available and have a low production cost.
12. Conclusions
In the separation of H2S gas from gas mixtures, the presence of other gases in the mixture, such as CO2, H2O, and CO, complicates the process because of competitive adsorption. Therefore, it is very important to find and synthesize efficient zeolites with a high adsorption capacity, selectivity, and regenerability that can separate H2S under a variety of conditions. Natural and synthetic zeolites, and their modifications, are widely used in the separation of H2S from various gaseous mixtures. It is difficult to find the most effective adsorbent because there are so many different structural types of zeolites, modification possibilities, and conditions to consider when removing H2S. However, by understanding the properties of zeolites and the gas to be removed, the proposed zeolite or its modification can be assumed for the screening in the separation processes. Linde Type A (4A and 5A) and Faujasite (13X) zeolites with a low silica content, and their modifications with Cu and Ag, which have good adsorption properties, are very good candidates for the removal of H2S.
References
- Conference Paper
- Mokhatab, Saeid Poe, William A.. Handbook of Natural Gas; Gulf Professional Publishing: 225 Wyman Street, Waltham, MA 02451, USA, 2012; pp. 1689-1699.
- Moore, P.J.; Spitler, R.W. .; Hydrogen Sulfide Measurement and Detection. Proceedings 2003, 1, 118–123.
- Sittichai Kulawong; Ritchard Artkla; Preecha Sriprapakhan; Pisit Maneechot; Biogas purification by adsorption of hydrogen sulphide on NaX and Ag-exchanged NaX zeolites. Biomass and Bioenergy 2022, 159, 106417, 10.1016/j.biombioe.2022.106417.
- Parveen Kumar; Chun-Yi Sung; Oki Muraza; Matteo Cococcioni; Saleh Al Hashimi; Alon McCormick; Michael Tsapatsis; H2S adsorption by Ag and Cu ion exchanged faujasites. Microporous and Mesoporous Materials 2011, 146, 127-133, 10.1016/j.micromeso.2011.05.014.
- Davide Papurello; Andrea Lanzini; Maurizio Bressan; Massimo Santarelli; H2S Removal with Sorbent Obtained from Sewage Sludges. Processes 2020, 8, 130, 10.3390/pr8020130.
- Farideh Bandarchian; Mansoor Anbia; Conventional hydrothermal synthesis of nanoporous molecular sieve 13X for selective adsorption of trace amount of hydrogen sulfide from mixture with propane. Journal of Natural Gas Science and Engineering 2015, 26, 1380-1387, 10.1016/j.jngse.2015.08.019.
- Linda Barelli; Gianni Bidini; Luca Micoli; Elena Sisani; Maria Turco; 13X Ex-Cu zeolite performance characterization towards H2S removal for biogas use in molten carbonate fuel cells. Energy 2018, 160, 44-53, 10.1016/j.energy.2018.05.057.
- Chunguang Liu; Rui Zhang; Song Wei; Jing Wang; Yang Liu; Man Li; Rutao Liu; Selective removal of H 2 S from biogas using a regenerable hybrid TiO 2 /zeolite composite. Fuel 2015, 157, 183-190, 10.1016/j.fuel.2015.05.003.
- Hydrogen sulfide . National Library of Medicine. Retrieved 2022-8-10
- Gabelman, A.; Adsorption Basics: Part 1.. Chem. Eng. Prog. 2017, 113, 48–53. .
- Lee D. Gasper-Galvin; Aysel T. Atimtay; Raghubir P. Gupta; Zeolite-Supported Metal Oxide Sorbents for Hot-Gas Desulfurization. Industrial & Engineering Chemistry Research 1998, 37, 4157-4166, 10.1021/ie930439i.
- Laure Neveux; David Chiche; Javier Pérez-Pellitero; Loïc Favergeon; Anne-Sophie Gay; Michèle Pijolat; New insight into the ZnO sulfidation reaction: mechanism and kinetics modeling of the ZnS outward growth. Physical Chemistry Chemical Physics 2012, 15, 1532-1545, 10.1039/c2cp42988h.
- W.F. Elseviers; H. Verelst; Transition metal oxides for hot gas desulphurisation. Fuel 1999, 78, 601-612, 10.1016/s0016-2361(98)00185-9.
- Phillip R. Westmoreland; Douglas P. Harrison; Evaluation of candidate solids for high-temperature desulfurization of low-Btu gases. Environmental Science & Technology 1976, 10, 659-661, 10.1021/es60118a010.
- Youssef Belmabkhout; Prashant M. Bhatt; Karim Adil; Renjith S. Pillai; Amandine Cadiau; Aleksander Shkurenko; Guillaume Maurin; Gongping Liu; William J. Koros; Mohamed Eddaoudi; et al. Natural gas upgrading using a fluorinated MOF with tuned H2S and CO2 adsorption selectivity. Nature Energy 2018, 3, 1059-1066, 10.1038/s41560-018-0267-0.
- L. H. De Oliveira; J. G. Meneguin; M. V. Pereira; J. F. Do Nascimento; Pedro Arroyo; Adsorption of hydrogen sulfide, carbon dioxide, methane, and their mixtures on activated carbon. Chemical Engineering Communications 2019, 206, 1533-1553, 10.1080/00986445.2019.1601627.
- Database of Zeolite Structures . Database of Zeolite Structures. Retrieved 2022-8-10
- Mark E. Davis; Raul F. Lobo; Zeolite and molecular sieve synthesis. Chemistry of Materials 1992, 4, 756-768, 10.1021/cm00022a005.
- Yafei Zhao; Bing Zhang; Xiang Zhang; Jinhua Wang; Jindun Liu; Rongfeng Chen; Preparation of highly ordered cubic NaA zeolite from halloysite mineral for adsorption of ammonium ions. Journal of Hazardous Materials 2010, 178, 658-664, 10.1016/j.jhazmat.2010.01.136.
- Jalil R. Ugal Sameer H. Kareem Sahar S. Hassan; Microwave-Assisted Synthesis of Zeolites A From Iraqi Cheap Raw Materials as Adsorbents for H2S Gas. J. of University of Anbar for pure science 2014, 8, 23-32.
- Robert L. Bedard; Synthesis of Zeolites and Manufacture of Zeolitic Catalysts and Adsorbents. null 2010, 1, 61-83, 10.1002/9783527629565.ch3.
- Abdul Khaleque; Masruck Alam; Mozammel Hoque; Shuvodip Mondal; Jahid Bin Haider; Bentuo Xu; M.A.H. Johir; Aneek Krishna Karmakar; J.L. Zhou; Mohammad Boshir Ahmed; et al.Mohammad Ali Moni Zeolite synthesis from low-cost materials and environmental applications: A review. Environmental Advances 2020, 2, 100019, 10.1016/j.envadv.2020.100019.
- Robert W. Broach; Zeolite Types and Structures. null 2010, 1, 27-59, 10.1002/9783527629565.ch2.
- Hafez Maghsoudi; Mohammad Soltanieh; Simultaneous separation of H 2 S and CO 2 from CH 4 by a high silica CHA-type zeolite membrane. Journal of Membrane Science 2014, 470, 159-165, 10.1016/j.memsci.2014.07.025.
- Stephen R. Dunne; Industrial Gas Phase Adsorptive Separations. null 2010, 1, 273-305, 10.1002/9783527629565.ch9.
- Kulprathipanja, S. . Introduction; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 1-26.
- Hellmut G Karge; János Raskó; Hydrogen sulfide adsorption on faujasite-type zeolites with systematically varied Si-Al ratios. Journal of Colloid and Interface Science 1978, 64, 522-532, 10.1016/0021-9797(78)90394-6.
- Annika Starke; Christoph Pasel; Christian Bläker; Tobias Eckardt; Jens Zimmermann; Dieter Bathen; Impact of Na+ and Ca2+ Cations on the Adsorption of H2S on Binder-Free LTA Zeolites. Adsorption Science & Technology 2021, 2021, 1-12, 10.1155/2021/5531974.
- Manolis M. Tomadakis; Howell H. Heck; Marwan E. Jubran; Khalid Al-Harthi; Pressure-Swing Adsorption Separation of H2S from CO2with Molecular Sieves 4A, 5A, and 13X. Separation Science and Technology 2011, 46, 428-433, 10.1080/01496395.2010.520292.
- Roghaye Dehghan; Mansoor Anbia; Zeolites for adsorptive desulfurization from fuels: A review. Fuel Processing Technology 2017, 167, 99-116, 10.1016/j.fuproc.2017.06.015.
- Y. Yokogawa; M. Sakanishi; N. Morikawa; A. Nakamura; I. Kishida; H.K. Varma; VSC Adsorptive Properties in Ion Exchanged Zeolite Materials in Gaseous and Aqueous Medium. Procedia Engineering 2012, 36, 168-172, 10.1016/j.proeng.2012.03.026.
- Elena Sisani; Giovanni Cinti; Gabriele Discepoli; Daniele Penchini; Umberto Desideri; Fabio Marmottini; Adsorptive removal of H 2 S in biogas conditions for high temperature fuel cell systems. International Journal of Hydrogen Energy 2014, 39, 21753-21766, 10.1016/j.ijhydene.2014.07.173.
More
Information
Subjects:
Engineering, Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.5K
Revisions:
3 times
(View History)
Update Date:
12 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




