Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alessandro Spirito | -- | 2040 | 2022-08-02 14:24:38 | | | |
| 2 | Camila Xu | + 4 word(s) | 2044 | 2022-08-03 02:43:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chiarito, M.; Spirito, A.; Nicolas, J.; Selberg, A.; Stefanini, G.; Colombo, A.; Reimers, B.; Kini, A.; Sharma, S.K.; Dangas, G.D.; et al. Types of Transcatheter Aortic Valve Replacement Devices. Encyclopedia. Available online: https://encyclopedia.pub/entry/25772 (accessed on 05 March 2026).
Chiarito M, Spirito A, Nicolas J, Selberg A, Stefanini G, Colombo A, et al. Types of Transcatheter Aortic Valve Replacement Devices. Encyclopedia. Available at: https://encyclopedia.pub/entry/25772. Accessed March 05, 2026.
Chiarito, Mauro, Alessandro Spirito, Johny Nicolas, Alexandra Selberg, Giulio Stefanini, Antonio Colombo, Bernhard Reimers, Annapoorna Kini, Samin K. Sharma, George D. Dangas, et al. "Types of Transcatheter Aortic Valve Replacement Devices" Encyclopedia, https://encyclopedia.pub/entry/25772 (accessed March 05, 2026).
Chiarito, M., Spirito, A., Nicolas, J., Selberg, A., Stefanini, G., Colombo, A., Reimers, B., Kini, A., Sharma, S.K., Dangas, G.D., & Mehran, R. (2022, August 02). Types of Transcatheter Aortic Valve Replacement Devices. In Encyclopedia. https://encyclopedia.pub/entry/25772
Chiarito, Mauro, et al. "Types of Transcatheter Aortic Valve Replacement Devices." Encyclopedia. Web. 02 August, 2022.
Copy Citation
Transcatheter aortic valve replacement (TAVR) was originally conceptualized in the early 1990s, largely inspired by the pioneering experiences in the field of percutaneous transluminal coronary angioplasty.
transcatheter aortic valve replacement
aortic stenosis
aortic regurgitation
1. Introduction
Aortic stenosis (AS) is the most common acquired heart valve disease in developed countries, affecting up to 10% of elderly patients [1]. The prevalence of AS is expected to increase over the next decades with the increasing life expectancy in most developed countries. Indeed, the global number of people older than 80 is foreseen to triple and surpass 400 million by 2050, with AS prevalence expected to be growing at a similar rate [2]. AS has a 50% mortality rate at 5 years from symptom onset if left untreated [3]. Until recently, surgical aortic valve replacement (SAVR) represented the only definitive treatment for patients with AS, as medical therapy can only mitigate symptoms. Nonetheless, considering the frailty and the relevant burden of comorbidities of many elderly patients with symptomatic AS, a considerable portion of this population was left untreated, due to high or prohibitive surgical risk. Transcatheter aortic valve replacement (TAVR) was originally conceptualized in the early 1990s [4], largely inspired by the pioneering experiences in the field of percutaneous transluminal coronary angioplasty. Following different studies in animal models [5], the first TAVR procedure was performed in 2002 for the treatment of AS in a patient with several comorbidities and cardiogenic shock. [6] Since then, TAVR has revolutionized the treatment of patients with severe, symptomatic AS, as randomized controlled trials have shown similar, if not superior, outcomes following TAVR as compared with SAVR in selected patients [7][8]. TAVR first emerged as a plausible treatment option for patients with AS at high or prohibitive surgical risk. Due to major advances in TAVR technologies, subsequent trials have shown that it is a safe and effective alternative to surgery for patients at intermediate-to-low surgical risk [8][9]. The number of TAVR procedures is rapidly increasing, and the continuous expansion of the population deemed suitable for TAVR [10][11] has corresponded with an impressive constant evolution in TAVR devices and materials. Indeed, these advances have significantly reduced periprocedural complications, making it safe to shorten hospital stay and improve long-term outcomes [12][13]. Moreover, these progresses have resulted in a wide armamentarium at researchers' disposal, including bioprostheses presenting different dimensions, designs, and deliverability, providing the opportunity to select a device based on each patient’s clinical and anatomic characteristics. In addition, as TAVR comes of age, clinical indications for TAVR are gradually expanding, from elderly and comorbid patients affected by calcific AS to younger patients, with bicuspid aortic valve, bioprosthesis degeneration, and/or aortic regurgitation (AR), all conditions that potentially require devices with specific features.
2. Types of Transcatheter Aortic Valve Replacement (TAVR) Devices
Since the first transcatheter implantation of the aortic Cribier–Edwards valve (Edwards Lifesciences, Irvine, CA, USA) in 2002 [6], several new transcatheter heart valves (THV) were introduced and approved for clinical use. The development of a safe and efficacious THV is technically challenging, as the valve must be crimped before implantation and then deployed over a heavily calcified aortic valve. Over time, improvements in valve design, materials, and delivery systems facilitated the implantation of the valve in the desired position, and decreased procedural and periprocedural complications [14]. For example, the sheath size for the delivery system was reduced from 24 to 12–14 Fr to enable implantation through a narrower vascular access and to reduce vascular complications. Sealing technologies, such as an outer skirt or a pericardial wrap, contributed to reduce the rates of paravalvular leak (PVL). Moreover, frame height was decreased, and the sizes of frames’ upper cells were enlarged to facilitate continued coronary access [15][16].
THV consist of a three-leaflet valve, made of bovine or porcine pericardium or polymeric material, mounted on a radiopaque metallic scaffold (frame), made of stainless steel, nitinol, or cobalt–chromium, and wrapped by an outer sheath (skirt or wrap)—in pericardial or polymeric material—to increase the surface area contact between the device and the native valve, and mitigate the risk of significant PVL (Figure 1). According to the position of the prosthetic leaflets relative to the native valve annulus, the THV is labeled as supra- or intra-annular. Supra-annular valves usually result in a larger effective orifice area (EOA) and lower transvalvular aortic mean gradients than intra-annular THV, which have a lower frame height that eases coronary access. Some THVs can be recaptured and repositioned after implantation, while other THV are non-repositionable after deployment. The delivery systems differ regarding the degree of flexion of the distal catheter and sheath diameter. The most common delivery approach is transfemoral, but other access routes (i.e., trans-subclavian, transaortic, transapical, transcarotid and transcaval) are used, as iliofemoral and aortic vessel diseases are commonly present in TAVR patients.
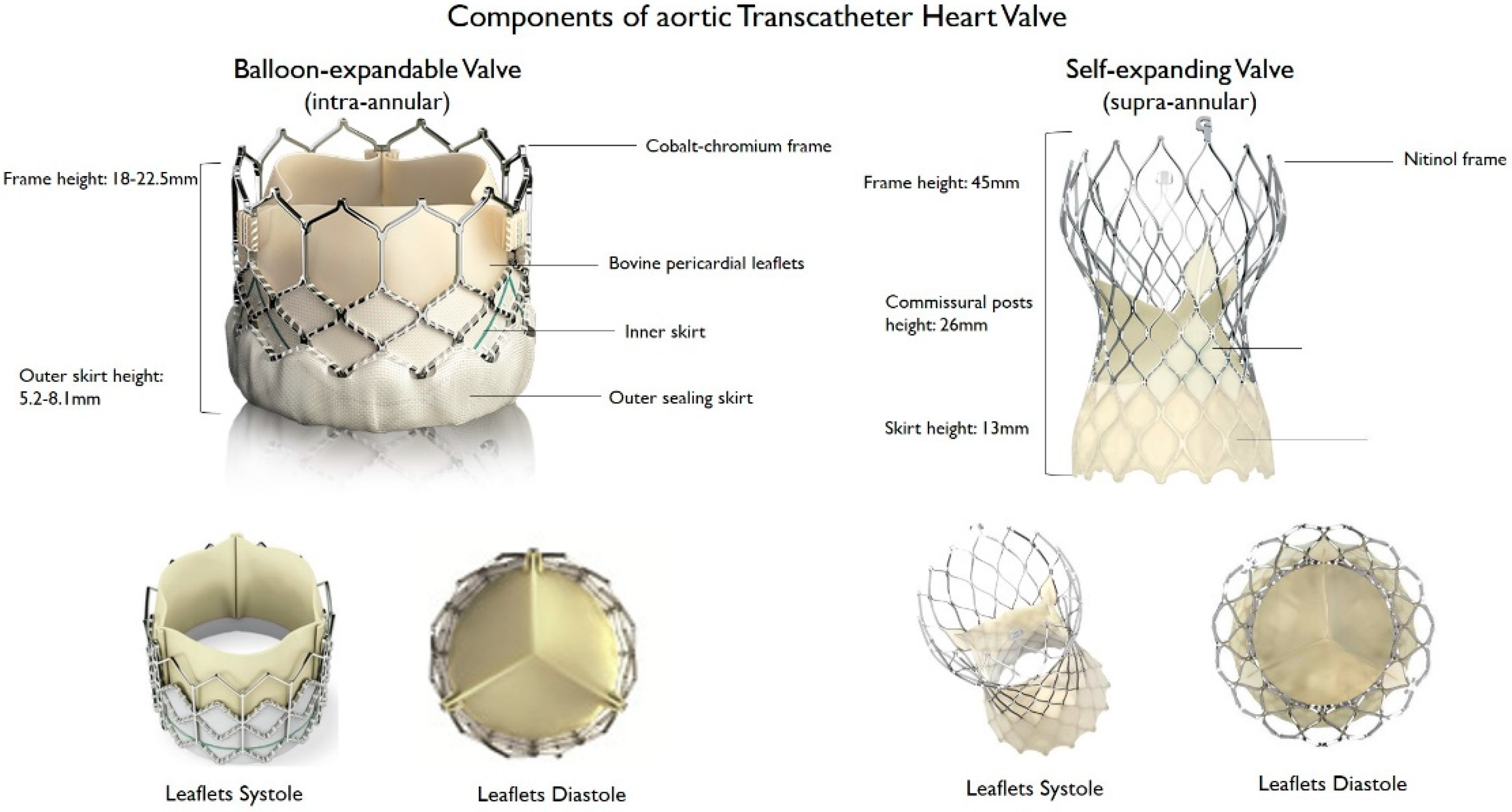 Figure 1. Main features of balloon–expandable and self–expanding aortic transcatheter heart valves.
Figure 1. Main features of balloon–expandable and self–expanding aortic transcatheter heart valves.Lastly, the most common classification of THVs is based on the mechanism of the valve frame expansion (Figure 2) as self-expandable (SE), balloon-expandable (BE) or mechanically expandable. Detailed information on each THV are provided in Table 1.
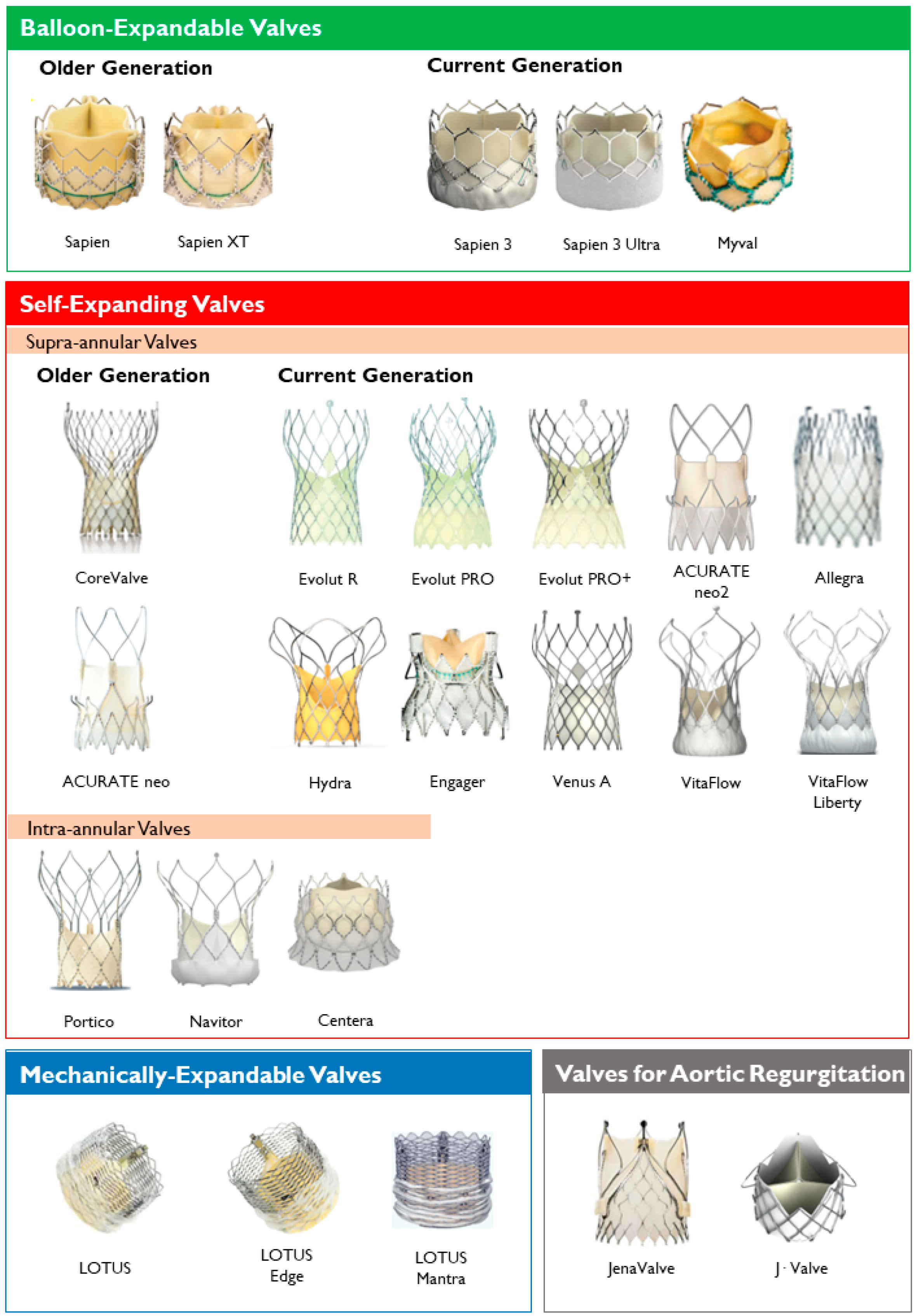 Figure 2. Transcatheter heart valves, stratified by mechanism of the valve frame expansion and leaflets position.
Figure 2. Transcatheter heart valves, stratified by mechanism of the valve frame expansion and leaflets position.Table 1. Overview of Transcatheter Aortic Valve Replacement (TAVR) Prostheses.
| Prosthesis | Frame Material | Leaflet Material | Valve Sizes (mm) | Sheath Sizes | Supra- or Intra- Annular |
Repositionable/Retrievable | Delivery Routes | FDA Approval | CE Mark Approval |
|---|---|---|---|---|---|---|---|---|---|
| Balloon-expandable | |||||||||
| Sapien | Stainless steel | Bovine pericardium | 23, 26 | 22F (23 mm), 24F (26 mm) | Intra-annular | No/No | TF, TA | ✓ | ✓ |
| Sapien XT | Cobalt-chromium | Bovine pericardium | 23, 26, 29 | 16F (23 mm), 18F (26 mm), 20F (29 mm) | Intra-annular | No/No | TF, TA, TAo | ✓ | ✓ |
| Sapien 3 | Cobalt-chromium | Bovine pericardium | 20, 23, 26, 29 | 14F (20, 23, 26 mm), 16F (29 mm) | Intra-annular | No/No | TF, TA, TAo | ✓ | ✓ |
| Sapien 3 Ultra | Cobalt-chromium | Bovine pericardium | 20, 23, 26, 29 | 14F | Intra-annular | No/No | TF | ✓ | ✓ |
| Myval THV | Nickel-cobalt | Bovine pericardium | 20, 23, 26, 29, 21.5, 24.5, 27.5, 30.5, 32 | 14F | Intra-annular | No/No | TF | ✓ | |
| Self-expanding | |||||||||
| CoreValve | Nitinol | Porcine pericardium | 23, 26, 29, 31 | 18F | Supra-annular | Yes/Yes | TF, TAo, SC | ✓ | ✓ |
| Evolut R | Nitinol | Porcine pericardium | 23, 26, 29, 34 | 14F (23, 26, 29 mm), 16F (34 mm) | Supra-annular | Yes/Yes | TF, TAo, SC | ✓ | ✓ |
| Evolut PRO | Nitinol | Porcine pericardium | 23, 26, 29, 34 | 16F | Supra-annular | Yes/Yes | TF, TAo, SC | ✓ | ✓ |
| Evolut PRO+ | Nitinol | Porcine pericardium | 23, 26, 29, 34 | 14F (23, 26, 29 mm), 16F (34 mm) | Supra-annular | Yes/Yes | TF, TAo, SC | ✓ | ✓ |
| ACURATE neo | Nitinol | Porcine pericardium | 23, 25, 27 | 18F | Supra-annular | No/No | TF, TA | ✓ | |
| ACURATE neo2 | Nitinol | Porcine pericardium | 23, 25, 27 | 14F | Supra-annular | No/No | TF, TA | ✓ | |
| Allegra | Nitinol | Bovine pericardium | 23, 27, 31 | 18F | Supra-annular | Yes/Yes | TF | ||
| Hydra | Nitinol | Bovine pericardium | 22, 26, 30 | 18F | Supra-annular | Yes/Yes | TF | ✓ | |
| Engager | Nitinol | Bovine pericardium | 23, 26 | 30F | Supra-annular | Yes/Yes | TA | ✓ | |
| Venus-A valve | Nitinol | Porcine pericardium | 23, 26, 29, 32 | Supra-annular | Yes/No | TF | |||
| VitaFlow | Nitinol | Bovine pericardium | 21, 24, 27, 30 | 16F (21, 24 mm), 18F (27, 30 mm) | Supra-annular | Yes/No | TF, TAo, CA | ||
| VitaFlow Liberty | Nitinol | Bovine pericardium | 21, 24, 27, 30 | 16F (21, 24 mm), 18F (27, 30 mm) | Supra-annular | Yes/No | TF, TAo, CA | ||
| Centera | Nitinol | Bovine pericardium | 23, 26 29 | 14F | Intra-annular | Yes/Yes | TF | ✓ | |
| Portico | Nitinol | Bovine pericardium | 23, 25, 27, 29 | 18F (23, 25 mm), 19F (27, 29 mm) | Intra-annular | Yes/Yes | TF, TAo, TAx, SC | ✓ | |
| Navitor | Nitinol | Bovine pericardium | 23, 25, 27, 29 | 14F (23, 25 mm), 15F (27, 29 mm) | Intra-annular | Yes/Yes | TF, TAo, TAx | ✓ | |
| Mechanically expandable | |||||||||
| Lotus | Nitinol | Bovine pericardium | 23, 25, 27 | 20F (23, 25 mm), 22F (27 mm) | Intra-annular | Yes/Yes | TF, TAo | ✓ | ✓ |
| Lotus Edge | Nitinol | Bovine pericardium | 23, 25, 27 | 15F | Intra-annular | Yes/Yes | TF, TAo | ✓ | ✓ |
| Lotus Mantra | Nitinol | Bovine pericardium | 23, 25, 27 | 12F | Intra-annular | Yes/Yes | TF, TAo | ✓ | ✓ |
| Aortic regurgitation | |||||||||
| JenaValve | Nitinol | Porcine pericardium | 23, 25, 27 | 19F | Intra-annular | Yes/Yes | TA | ✓ | |
| J·Valve | Nitinol | Bovine pericardium | 22, 25, 28 | 18F | Intra-annular | No/No | TA | ✓ |
TF-Transfemoral, TA-Transapical, TAo-Transaortic, TAx-Transaxillary, SC-Subclavian, CA-Carotid. FDA Approval–approved for use by the United States Food and Drug Administration. CE Mark Approval–approved for use across all EU member states, European Econamic Area, and Turkey by the European Commission. ✓ = approved.
2.1. Balloon-Expandable Valves
The first implanted THV (Cribier-Edwards) [6], SAPIEN devices (Edwards Lifesciences), and the Myval THV (Meril Life Sciences, Gujarat, India) belong to the BE group.
The expansion of these valves requires balloon inflation during rapid ventricular pacing, which may not be well tolerated by patients with reduced left ventricular ejection fraction (LVEF) or impaired renal function. All BE-THV are intra-annular, are not repositionable and have a lower stent frame profile, facilitating coronary access as compared with SE-THV. Furthermore, the delivery system allows for greater steerability than SE- or ME-THV, helping in valve implantation in patients with challenging vascular anatomy, such as in case of horizontal aorta, defined as an aortic angulation >60°.
The SAPIEN 3 consists of a trileaflet bovine pericardial valve mounted in a cobalt-chromium frame with an outer seal cuff to reduce PVL; in the SAPIEN 3 Ultra, frame height was increased to further reduce the rate of PVL [17]. The SAPIEN 3 and SAPIEN 3 Ultra are FDA approved [18].
The Myval THV (Meril Life Sciences, Gujarat, India) obtained the CE mark in 2019 but is not yet FDA-approved; it consists of a nickel–cobalt alloy (MP35N) frame, a trileaflet valve of bovine pericardium tissue and an external polymeric sealing cuff [19][20].
2.2. Self-Expanding Valves
The group of SE-THV includes a wider range of devices from different companies. The majority of SE-THV are supra-annular, resulting in a higher EOA, lower gradients, and lower rate of severe prosthesis–patient mismatch (PPM). Rapid ventricular pacing during implantation is not mandatory and most SE-THV are repositionable and/or retrievable, at the expense of limited steerability. Of note, the greater frame height of SE-THV may make coronary access more challenging.
The Medtronic Corevalve (Medtronic, Sunnyvale, CA, USA) was the first self-expanding THV developed, and together with its subsequent generations—Evolut R, Evolut PRO and Evolut PRO+—represents the most studied and commonly implanted SE-THV and has CE and FDA approval. The Evolut PRO+ added an outer porcine pericardial tissue wrap that increases surface area contact and tissue interaction between the THV and the native aortic annulus. Of note, the frame of Medtronic SE-THV, due to its higher radial force, exerts a higher pressure on the membranous septum and the conduction system than BE-THV, resulting in considerable risk of conduction abnormalities requiring PPI [21].
The ACURATE neo (older generation) [22] and ACURATE neo2 (newer generation) [23] produced by Boston Scientific, have similar characteristics to the Evolut THV, except for the fact that implant depth can be controlled due to its top-down deployment, it is non-repositionable, and that predilation of the aortic valve is strongly recommended.
Other SE-THV with CE but not FDA approval include: Allegra (NVT AG), whose grip uses a “squeeze-to-release” mechanism, avoiding any rotation during the entire implantation, performed in a stepwise manner [24][25]; Hydra (Vascular Innovations Co., Ltd., Nonthaburi, Thailand) which includes a mechanism for recapturing during release [14]; Engager (Medtronic), and Venus-A valve (Venus Medtech, Hangzhou, China) [26]. VitaFlow (Microport, Shanghai, China) and its subsequent iteration VitaFlow Liberty™ are novel THVs manufactured in China, for which the CE approval is ongoing [27][28].
Among SE-THV, some are intra-annular, such as Centera (Edwards) [29], Portico (Abbott Structural Heart, Westfield, IN, USA) and its iteration Navitor (Abbott Structural Heart). The Portico valve (Abbott) is a self-expanding, fully resheathable and retrievable valve with leaflet geometry designed to function in both round and elliptical configurations. The annular positioning facilitates the engagement of coronary ostia after implantation [15][30]. This valve is CE- and FDA- (September 2021) approved. The Navitor THV has as a key innovation in an active outer fabric cuff designed to reduce the PVL.
SE-THV with supra-annular designs are particularly indicated in patients with small or severely calcific annulus, TAVR-in-SAVR, and both supra- and intra-annular valves are indicated in patients at risk for poor tolerance to rapid pacing.
2.3. Mechanically-Expandable Valves
The expansion of mechanically expandable-THV is mediated by a mechanical controlled system and usually does not require rapid ventricular pacing. These valves are intra-annular, fully repositionable, and retrievable. This group included LOTUS (older generation), LOTUS Edge and LOTUS Mantra (newer generation, all produced by Boston Scientific, but currently recalled due to issues with the product delivery system) [31].
2.4. Valves with Active Fixation Mechanisms
In recent years, valves equipped with an active fixation mechanism were developed. The anchor mechanism enables fixation of the prosthesis onto the native valve leaflets, providing stability in the context of non-calcified native valves and allowing implantation in patients with aortic regurgitation.
The JenaValve (JenaValve Technology, München, Germany), a porcine pericardial valve in a low-profile nitinol frame with a paperclip-like fixation mechanism, is currently the only THV with a CE mark for use in patients with aortic regurgitation [32]. The J-Valve (JC Medical) is another device that can be employed for the treatment of native AR, as well as AS, and is currently being evaluated in an early feasibility study in the US [33]. Two further valves from China, the Venus-AVR valve (Venus Medtech) and VitaFlowVR (Microport), are at an advanced stage of development with high rates of procedural success in the challenging cohort of patients with a bicuspid aortic valve [15][26].
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011.
- Eveborn, G.W.; Schirmer, H.; Heggelund, G.; Lunde, P.; Rasmussen, K. The evolving epidemiology of valvular aortic stenosis. The Tromsø Study. Heart 2013, 99, 396–400.
- Schueler, R.; Hammerstingl, C.; Sinning, J.M.; Nickenig, G.; Omran, H. Prognosis of octogenarians with severe aortic valve stenosis at high risk for cardiovascular surgery. Heart 2010, 96, 1831–1836.
- Andersen, H.R. How Transcatheter Aortic Valve Implantation (TAVI) Was Born: The Struggle for a New Invention. Front. Cardiovasc. Med. 2021, 8, 1124.
- Andersen, H.R.; Knudsen, L.L.; Hasenkam, J.M. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur. Heart J. 1992, 13, 704–708.
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous Transcatheter Implantation of an Aortic Valve Prosthesis for Calcific Aortic Stenosis. Circulation 2002, 106, 3006–3008.
- Pagnesi, M.; Chiarito, M.; Stefanini, G.G.; Testa, L.; Reimers, B.; Colombo, A.; Latib, A. Is Transcatheter Aortic Valve Replacement Superior to Surgical Aortic Valve Replacement?: A Meta-Analysis of Randomized Controlled Trials. JACC Cardiovasc. Interv. 2017, 10, 1899–1901.
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715.
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705.
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791.
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 70, 252–289.
- Krishnaswamy, A.; Isogai, T.; Agrawal, A.; Shekhar, S.; Puri, R.; Reed, G.W.; Yun, J.J.; Unai, S.; Burns, D.J.P.; Vargo, P.R.; et al. Feasibility and Safety of Same-Day Discharge Following Transfemoral Transcatheter Aortic Valve Replacement. Cardiovasc. Interv. 2022, 15, 575–589.
- Zahid, S.; Rai, D.; Tanveer Ud Din, M.; Khan, M.Z.; Ullah, W.; Usman Khan, M.; Thakkar, S.; Hussein, A.; Baibhav, B.; Rao, M.; et al. Same-Day Discharge After Transcatheter Aortic Valve Implantation: Insights from the Nationwide Readmission Database 2015 to 2019. J. Am. Heart Assoc. 2022, 11, e024746.
- Rotman, O.M.; Bianchi, M.; Ghosh, R.P.; Kovarovic, B.; Bluestein, D. Principles of TAVR valve design, modelling, and testing. Expert Rev. Med. Devices 2018, 15, 771–791.
- Cahill, T.J.; Chen, M.; Hayashida, K.; Latib, A.; Modine, T.; Piazza, N.; Redwood, S.; Søndergaard, L.; Prendergast, B.D. Transcatheter aortic valve implantation: Current status and future perspectives. Eur. Heart J. 2018, 39, 2625–2634.
- Claessen, B.E.; Tang, G.H.L.; Kini, A.S.; Sharma, S.K. Considerations for Optimal Device Selection in Transcatheter Aortic Valve Replacement: A Review. JAMA Cardiol. 2021, 6, 102–112.
- Binder, R.K.; Rodés-Cabau, J.; Wood, D.A.; Webb, J.G. Edwards SAPIEN 3 valve. EuroIntervention 2012, 8, Q83–Q87.
- Nazif, T.M.; Cahill, T.J.; Daniels, D.; McCabe, J.M.; Reisman, M.; Chakravarty, T.; Makkar, R.; Krishnaswamy, A.; Kapadia, S.; Chehab, B.M.; et al. Real-World Experience with the SAPIEN 3 Ultra Transcatheter Heart Valve: A Propensity-Matched Analysis from the United States. Circ. Cardiovasc. Interv. 2021, 14, 948–957.
- Kawashima, H.; Soliman, O.; Wang, R.; Ono, M.; Hara, H.; Gao, C.; Zeller, E.; Thakkar, A.; Tamburino, C.; Bedogni, F.; et al. Rationale and design of a randomized clinical trial comparing safety and efficacy of myval transcatheter heart valve versus contemporary transcatheter heart valves in patients with severe symptomatic aortic valve stenosis: The LANDMARK trial. Am. Heart J. 2021, 232, 23–38.
- Sharma, S.K.; Rao, R.S.; Chandra, P.; Goel, P.K.; Bharadwaj, P.; Joseph, G.; Jose, J.; Mahajan, A.U.; Mehrotra, S.; Sengottovelu, G.; et al. First-in-human evaluation of a novel balloon-expandable transcatheter heart valve in patients with severe symptomatic native aortic stenosis: The MyVal-1 study. EuroIntervention 2020, 16, 421–429.
- Abdel-Wahab, M.; Mehilli, J.; Frerker, C.; Neumann, F.J.; Kurz, T.; Tölg, R.; Zachow, D.; Guerra, E.; Massberg, S.; Schäfer, U.; et al. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: The CHOICE randomized clinical trial. JAMA J. Am. Med. Assoc. 2014, 311, 1503–1514.
- Möllmann, H.; Diemert, P.; Grube, E.; Baldus, S.; Kempfert, J.; Abizaid, A. Symetis ACURATE TFTM aortic bioprosthesis. EuroIntervention 2013, 9, 107–110.
- Wong, I.; Bieliauskas, G.; De Backer, O.; Søndergaard, L. Technical Considerations for Transcatheter Aortic Valve Replacement with ACURATE neo2. JACC Cardiovasc. Interv. 2021, 14, 224–226.
- Cuevas, O.; Moreno, R.; Pascual-Tejerina, V.; Toggweiler, S.; Brinkert, M.; Baz, J.; Jimenez, V.; Molina, E.; Sánchez-Gila, J.; Taramasso, M.; et al. The Allegra transcatheter heart valve: European multicentre experience with a novel self-expanding transcatheter aortic valve. EuroIntervention 2019, 15, 71–73.
- Wenaweser, P.; Stortecky, S.; Schütz, T.; Praz, F.; Gloekler, S.; Windecker, S.; Elsässer, A. Transcatheter aortic valve implantation with the NVT Allegra transcatheter heart valve system: First-in-human experience with a novel self-expanding transcatheter heart valve. EuroIntervention 2016, 12, 71–77.
- Liao, Y.B.; Zhao, Z.G.; Wei, X.; Xu, Y.N.; Zuo, Z.L.; Li, Y.J.; Zheng, M.X.; Feng, Y.; Chen, M. Transcatheter aortic valve implantation with the self-expandable venus A-Valve and CoreValve devices: Preliminary Experiences in China. Catheter. Cardiovasc. Interv. 2017, 89, 528–533.
- Santangelo, G.; Ielasi, A.; Pellicano, M.; Latib, A.; Tespili, M.; Donatelli, F. An Update on New Generation Transcatheter Aortic Valves and Delivery Systems. J. Clin. Med. 2022, 11, 499.
- Zhou, D.; Pan, W.; Wang, J.; Wu, Y.; Chen, M.; Modine, T.; Mylotte, D.; Piazza, N.; Ge, J. VitaFlowTM transcatheter valve system in the treatment of severe aortic stenosis: One-year results of a multicenter study. Catheter. Cardiovasc. Interv. 2020, 95, 332–338.
- Ribeiro, H.B.; Urena, M.; Kuck, K.H.; Webb, J.G.; Rodés-Cabau, J. Edwards CENTERA valve. EuroIntervention 2012, 8, Q79–Q82.
- Manoharan, G.; Spence, M.S.; Rodés-Cabau, J.; Webb, J.G. St Jude Medical Portico valve. EuroIntervention 2012, 8, Q97–Q101.
- Meredith, I.T.; Hood, K.L.; Haratani, N.; Allocco, D.J.; Dawkins, K.D. Boston Scientific Lotus valve. EuroIntervention 2012, 8, Q70–Q74.
- Treede, H.; Mohr, F.W.; Baldus, S.; Rastan, A.; Ensminger, S.; Arnolde, M.; Kempfert, J.; Figulla, H.R. Transapical transcatheter aortic valve implantation using the JenaValveTM system: Acute and 30-day results of the multicentre CE-mark study. Eur. J. Cardiothorac. Surg. 2012, 41, B4556.
- Hensey, M.; Murdoch, D.J.; Sathananthan, J.; Alenezi, A.; Sathananthan, G.; Moss, R.; Blanke, P.; Leipsic, J.; Wood, D.A.; Cheung, A.; et al. First-in-human experience of a new-generation transfemoral transcatheter aortic valve for the treatment of severe aortic regurgitation: The J-Valve transfemoral system. EuroIntervention 2019, 14, E1553–E1555.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
11.8K
Entry Collection:
Hypertension and Cardiovascular Diseases
Revisions:
2 times
(View History)
Update Date:
03 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





