Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Alessandro Spirito and Version 2 by Camila Xu.
Transcatheter aortic valve replacement (TAVR) was originally conceptualized in the early 1990s, largely inspired by the pioneering experiences in the field of percutaneous transluminal coronary angioplasty.
- transcatheter aortic valve replacement
- aortic stenosis
- aortic regurgitation
1. Introduction
Aortic stenosis (AS) is the most common acquired heart valve disease in developed countries, affecting up to 10% of elderly patients [1]. The prevalence of AS is expected to increase over the next decades with the increasing life expectancy in most developed countries. Indeed, the global number of people older than 80 is foreseen to triple and surpass 400 million by 2050, with AS prevalence expected to be growing at a similar rate [2]. AS has a 50% mortality rate at 5 years from symptom onset if left untreated [3]. Until recently, surgical aortic valve replacement (SAVR) represented the only definitive treatment for patients with AS, as medical therapy can only mitigate symptoms. Nonetheless, considering the frailty and the relevant burden of comorbidities of many elderly patients with symptomatic AS, a considerable portion of this population was left untreated, due to high or prohibitive surgical risk. Transcatheter aortic valve replacement (TAVR) was originally conceptualized in the early 1990s [4], largely inspired by the pioneering experiences in the field of percutaneous transluminal coronary angioplasty. Following different studies in animal models [5], the first TAVR procedure was performed in 2002 for the treatment of AS in a patient with several comorbidities and cardiogenic shock. [6] Since then, TAVR has revolutionized the treatment of patients with severe, symptomatic AS, as randomized controlled trials have shown similar, if not superior, outcomes following TAVR as compared with SAVR in selected patients [7][8][7,8]. TAVR first emerged as a plausible treatment option for patients with AS at high or prohibitive surgical risk. Due to major advances in TAVR technologies, subsequent trials have shown that it is a safe and effective alternative to surgery for patients at intermediate-to-low surgical risk [8][9][8,9]. The number of TAVR procedures is rapidly increasing, and the continuous expansion of the population deemed suitable for TAVR [10][11][10,11] has corresponded with an impressive constant evolution in TAVR devices and materials. Indeed, these advances have significantly reduced periprocedural complications, making it safe to shorten hospital stay and improve long-term outcomes [12][13][12,13]. Moreover, these progresses have resulted in a wide armamentarium at ouresearchers' disposal, including bioprostheses presenting different dimensions, designs, and deliverability, providing the opportunity to select a device based on each patient’s clinical and anatomic characteristics. In addition, as TAVR comes of age, clinical indications for TAVR are gradually expanding, from elderly and comorbid patients affected by calcific AS to younger patients, with bicuspid aortic valve, bioprosthesis degeneration, and/or aortic regurgitation (AR), all conditions that potentially require devices with specific features.
2. Types of Transcatheter Aortic Valve Replacement (TAVR) VR Devices
Since the first transcatheter implantation of the aortic Cribier–Edwards valve (Edwards Lifesciences, Irvine, CA, USA) in 2002 [6], several new transcatheter heart valves (THV) were introduced and approved for clinical use. The development of a safe and efficacious THV is technically challenging, as the valve must be crimped before implantation and then deployed over a heavily calcified aortic valve. Over time, improvements in valve design, materials, and delivery systems facilitated the implantation of the valve in the desired position, and decreased procedural and periprocedural complications [14]. For example, the sheath size for the delivery system was reduced from 24 to 12–14 Fr to enable implantation through a narrower vascular access and to reduce vascular complications. Sealing technologies, such as an outer skirt or a pericardial wrap, contributed to reduce the rates of paravalvular leak (PVL). Moreover, frame height was decreased, and the sizes of frames’ upper cells were enlarged to facilitate continued coronary access [15][16][15,16]. THV consist of a three-leaflet valve, made of bovine or porcine pericardium or polymeric material, mounted on a radiopaque metallic scaffold (frame), made of stainless steel, nitinol, or cobalt–chromium, and wrapped by an outer sheath (skirt or wrap)—in pericardial or polymeric material—to increase the surface area contact between the device and the native valve, and mitigate the risk of significant PVL (Figure 1). According to the position of the prosthetic leaflets relative to the native valve annulus, the THV is labeled as supra- or intra-annular. Supra-annular valves usually result in a larger effective orifice area (EOA) and lower transvalvular aortic mean gradients than intra-annular THV, which have a lower frame height that eases coronary access. Some THVs can be recaptured and repositioned after implantation, while other THV are non-repositionable after deployment. The delivery systems differ regarding the degree of flexion of the distal catheter and sheath diameter. The most common delivery approach is transfemoral, but other access routes (i.e., trans-subclavian, transaortic, transapical, transcarotid and transcaval) are used, as iliofemoral and aortic vessel diseases are commonly present in TAVR patients.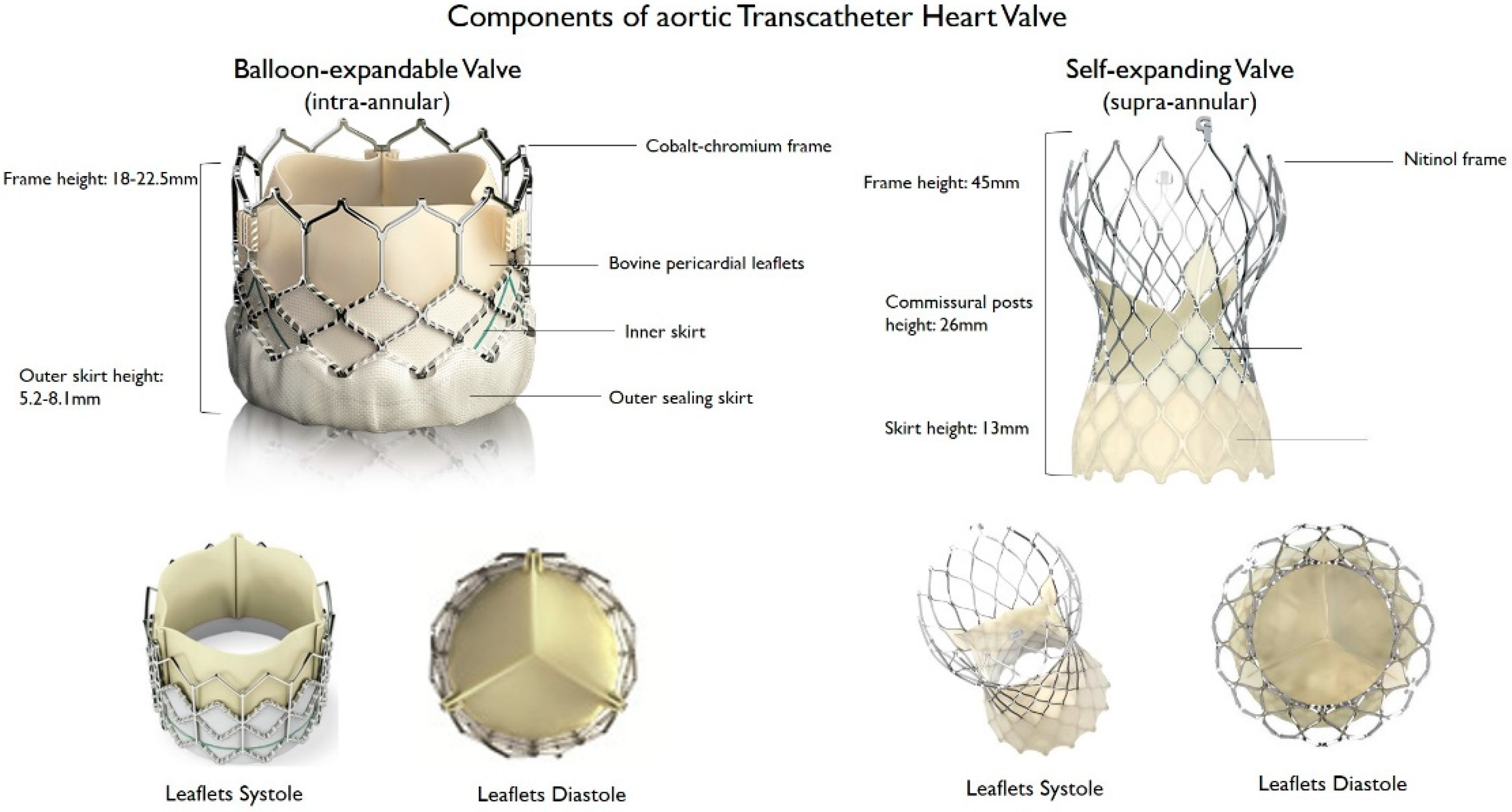
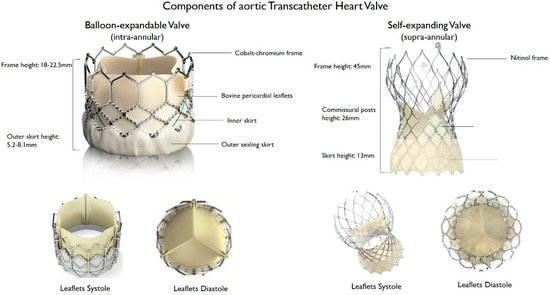
Figure 1. Main features of balloon–expandable and self–expanding aortic transcatheter heart valves.
Main features of balloon–expandable and self–expanding aortic transcatheter heart valves.
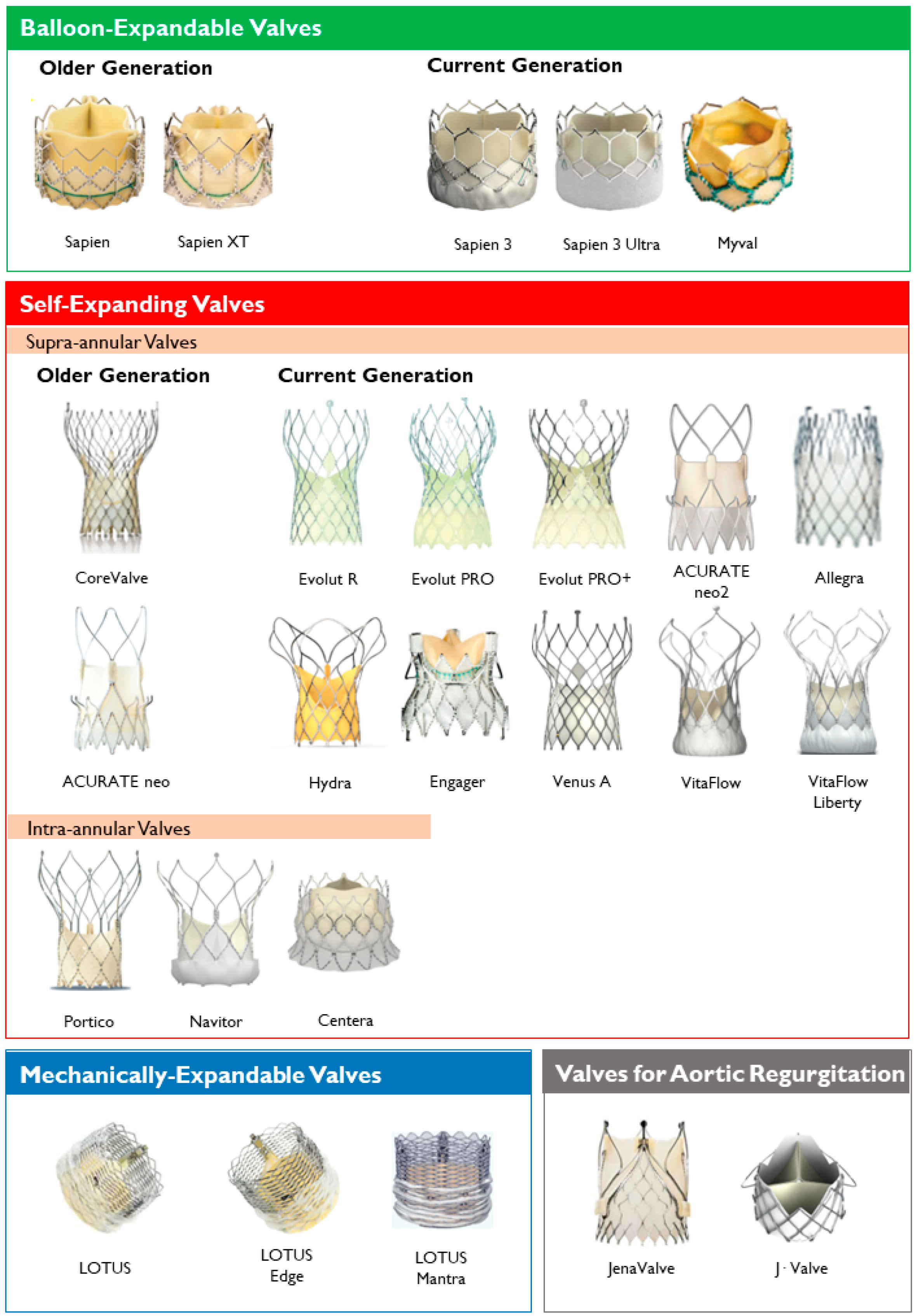
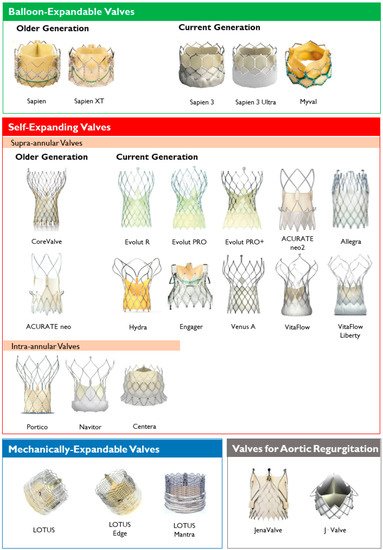
Figure 2. Transcatheter heart valves, stratified by mechanism of the valve frame expansion and leaflets position.
Transcatheter heart valves, stratified by mechanism of the valve frame expansion and leaflets position.
Table 1.
Overview of Transcatheter Aortic Valve Replacement (TAVR) Prostheses.
| Prosthesis | Frame Material | Leaflet Material | Valve Sizes (mm) | Sheath Sizes | Supra- or Intra- Annular |
Repositionable/Retrievable | Delivery Routes | FDA Approval | CE Mark Approval |
|---|---|---|---|---|---|---|---|---|---|
| Balloon-expandable | |||||||||
| Sapien | Stainless steel | Bovine pericardium | 23, 26 | 22F (23 mm), 24F (26 mm) | Intra-annular | No/No | TF, TA | ✓ | ✓ |
| Sapien XT | Cobalt-chromium | Bovine pericardium | 23, 26, 29 | 16F (23 mm), 18F (26 mm), 20F (29 mm) | Intra-annular | No/No | TF, TA, TAo | ✓ | ✓ |
| Sapien 3 | Cobalt-chromium | Bovine pericardium | 20, 23, 26, 29 | 14F (20, 23, 26 mm), 16F (29 mm) | Intra-annular | No/No | TF, TA, TAo | ✓ | ✓ |
| Sapien 3 Ultra | Cobalt-chromium | Bovine pericardium | 20, 23, 26, 29 | 14F | Intra-annular | No/No | TF | ✓ | ✓ |
| Myval THV | Nickel-cobalt | Bovine pericardium | 20, 23, 26, 29, 21.5, 24.5, 27.5, 30.5, 32 | 14F | Intra-annular | No/No | TF | ✓ | |
| Self-expanding | |||||||||
| CoreValve | Nitinol | Porcine pericardium | 23, 26, 29, 31 | 18F | Supra-annular | Yes/Yes | TF, TAo, SC | ✓ | ✓ |
| Evolut R | Nitinol | Porcine pericardium | 23, 26, 29, 34 | 14F (23, 26, 29 mm), 16F (34 mm) | Supra-annular | Yes/Yes | TF, TAo, SC | ✓ | ✓ |
| Evolut PRO | Nitinol | Porcine pericardium | 23, 26, 29, 34 | 16F | Supra-annular | Yes/Yes | TF, TAo, SC | ✓ | ✓ |
| Evolut PRO+ | Nitinol | Porcine pericardium | 23, 26, 29, 34 | 14F (23, 26, 29 mm), 16F (34 mm) | Supra-annular | Yes/Yes | TF, TAo, SC | ✓ | ✓ |
| ACURATE neo | Nitinol | Porcine pericardium | 23, 25, 27 | 18F | Supra-annular | No/No | TF, TA | ✓ | |
| ACURATE neo2 | Nitinol | Porcine pericardium | 23, 25, 27 | 14F | Supra-annular | No/No | TF, TA | ✓ | |
| Allegra | Nitinol | Bovine pericardium | 23, 27, 31 | 18F | Supra-annular | Yes/Yes | TF | ||
| Hydra | Nitinol | Bovine pericardium | 22, 26, 30 | 18F | Supra-annular | Yes/Yes | TF | ✓ | |
| Engager | Nitinol | Bovine pericardium | 23, 26 | 30F | Supra-annular | Yes/Yes | TA | ✓ | |
| Venus-A valve | Nitinol | Porcine pericardium | 23, 26, 29, 32 | Supra-annular | Yes/No | TF | |||
| VitaFlow | Nitinol | Bovine pericardium | 21, 24, 27, 30 | 16F (21, 24 mm), 18F (27, 30 mm) | Supra-annular | Yes/No | TF, TAo, CA | ||
| VitaFlow Liberty | Nitinol | Bovine pericardium | 21, 24, 27, 30 | 16F (21, 24 mm), 18F (27, 30 mm) | Supra-annular | Yes/No | TF, TAo, CA | ||
| Centera | Nitinol | Bovine pericardium | 23, 26 29 | 14F | Intra-annular | Yes/Yes | TF | ✓ | |
| Portico | Nitinol | Bovine pericardium | 23, 25, 27, 29 | 18F (23, 25 mm), 19F (27, 29 mm) | Intra-annular | Yes/Yes | TF, TAo, TAx, SC | ✓ | |
| Navitor | Nitinol | Bovine pericardium | 23, 25, 27, 29 | 14F (23, 25 mm), 15F (27, 29 mm) | Intra-annular | Yes/Yes | TF, TAo, TAx | ✓ | |
| Mechanically expandable | |||||||||
| Lotus | Nitinol | Bovine pericardium | 23, 25, 27 | 20F (23, 25 mm), 22F (27 mm) | Intra-annular | Yes/Yes | TF, TAo | ✓ | ✓ |
| Lotus Edge | Nitinol | Bovine pericardium | 23, 25, 27 | 15F | Intra-annular | Yes/Yes | TF, TAo | ✓ | ✓ |
| Lotus Mantra | Nitinol | Bovine pericardium | 23, 25, 27 | 12F | Intra-annular | Yes/Yes | TF, TAo | ✓ | ✓ |
| Aortic regurgitation | |||||||||
| JenaValve | Nitinol | Porcine pericardium | 23, 25, 27 | 19F | Intra-annular | Yes/Yes | TA | ✓ | |
| J·Valve | Nitinol | Bovine pericardium | 22, 25, 28 | 18F | Intra-annular | No/No | TA | ✓ |
TF-Transfemoral, TA-Transapical, TAo-Transaortic, TAx-Transaxillary, SC-Subclavian, CA-Carotid. FDA Approval–approved for use by the United States Food and Drug Administration. CE Mark Approval–approved for use across all EU member states, European Econamic Area, and Turkey by the European Commission. ✓ = approved.
