
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Virendra Kumar Yadav | -- | 3160 | 2022-08-01 09:19:01 | | | |
| 2 | Catherine Yang | Meta information modification | 3160 | 2022-08-01 10:00:09 | | | | |
| 3 | Catherine Yang | -76 word(s) | 3082 | 2022-09-01 04:08:52 | | |
Video Upload Options
Bacterial strains resistant to antimicrobial treatments, such as antibiotics, have emerged as serious clinical problems, necessitating the development of novel bactericidal materials. Nanostructures with particle sizes ranging from 1 to 100 nanometers have appeared recently as novel antibacterial agents, which are also known as “nanoantibiotics”. Nanomaterials have been shown to exert greater antibacterial effects on Gram-positive and Gram-negative bacteria across several studies. Antibacterial nanofilms for medical implants and restorative matters to prevent bacterial harm and antibacterial vaccinations to control bacterial infections are examples of nanoparticle applications in the biomedical sectors. The development of unique nanostructures, such as nanocrystals and nanostructured materials, is an exciting step in alternative efforts to manage microorganisms because these materials provide disrupted antibacterial effects, including better biocompatibility, as opposed to minor molecular antimicrobial systems, which have short-term functions and are poisonous.
1. Nanoparticles as Antimicrobial Agents
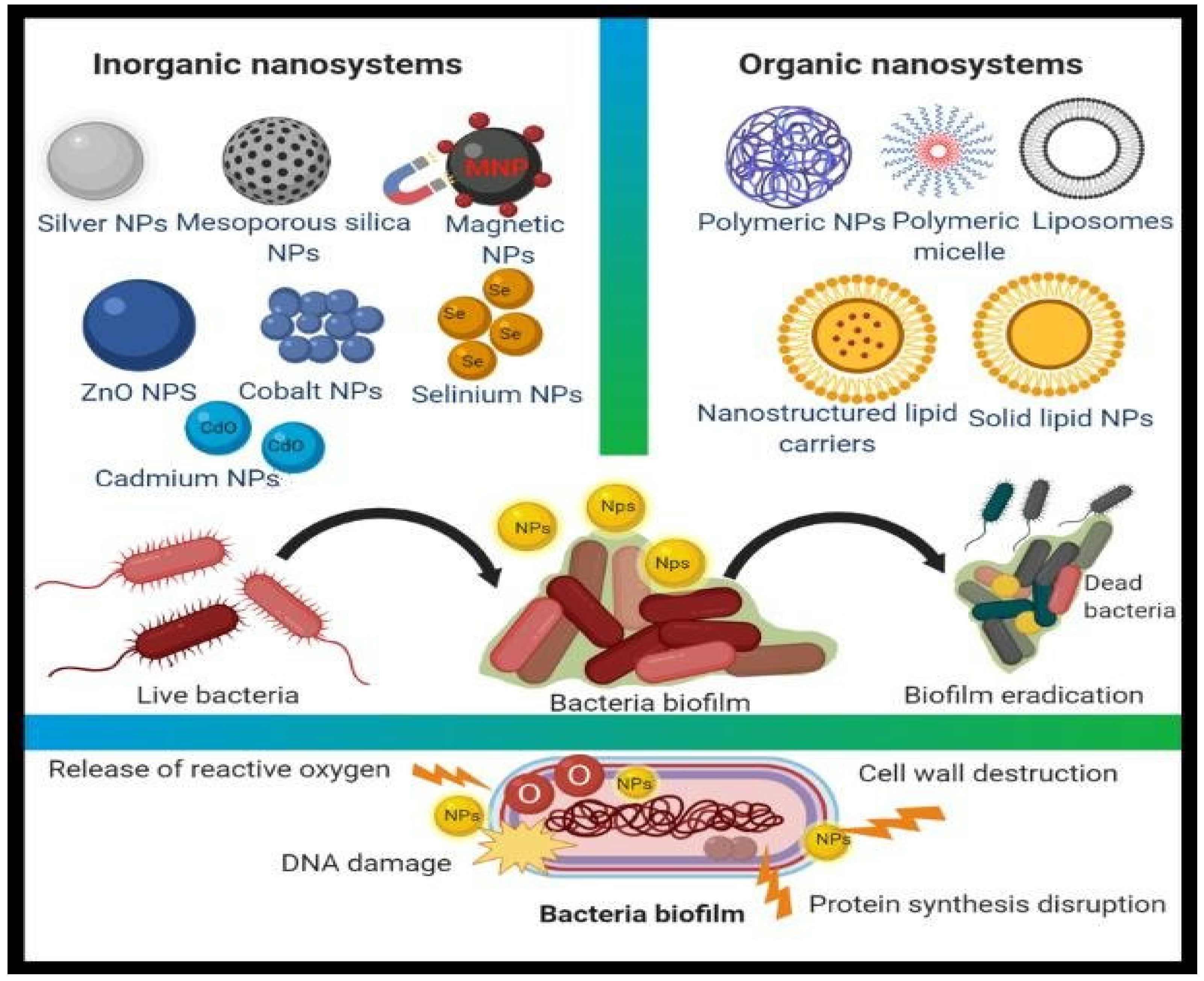
1.1. Synergistic Effect (Antibiotics within Nanoparticles)
1.2. Metal Nanoparticles (Inorganic Nanoparticles)
1.2.1. Antimicrobial Role of Silver Nanoparticles
1.2.2. Gold Nanoparticles (Au NPs)
1.3. Copper Nanoparticles (Cu NPs)
1.4. Zinc Oxide Nanoparticles (ZnO NPs)
1.5. Titanium Dioxide Nanoparticles (TiO2 NPs)
1.6. CuO Nanoparticles (CuO NPs)
2. Carbon Nanotubes as Antimicrobial Agents
| Nanomaterials | Antibiotics/Drugs | Target Bacteria/Diseases | References |
|---|---|---|---|
| Ag NPs | Ciprofloxacin, vancomycin Clotrimazole |
VRE, MRSA MRSA, S. aureus, |
[12][13] |
| Au NPs | Vancomycin, ampicillin |
MRSA, MRSA, P. aeruginosa, Enterobacter aerogenes, E. coli |
[16] |
| ZnO NPs | Ciprofloxacin, ceftazidime | MDRA. baumannii | [24] |
| Fe3O4 NPs | Ampicillin Ampicillin |
S. aureus E. coli, P. aeruginosa, MRSA |
|
| SWCNTs | Ciprofloxacin | S. aureus, P. aeruginosa, E. coli | [35][36] |
| Chitosan | Streptomycin Ciprofloxacin |
Listeria monocytogenes Uropathogenic E. coli |
[37] |
| Liposome | Pioglitazone (PIO), dexamethasone plus minocycline |
Atherosclerotic plaques Orthopedic/dental implants |
[38] |
| Exosome | Curcumin | Septic shock | [39] |
References
- Ameen, F.; Alsamhary, K.; Alabdullatif, J.A.; Alnadhari, S. A review on metal-based nanoparticles and their toxicity to beneficial soil bacteria and fungi. Ecotoxicol. Environ. Saf. 2021, 213, 112027.
- Jampilek, J.; Kralova, K. Advances in Nanostructures for Antimicrobial Therapy. Materials 2022, 15, 2388.
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292.
- Kumar, P.; Mathpal, M.C.; Inwati, G.; Ghosh, S.; Kumar, V.; Roos, W.; Swart, H. Optical and surface properties of Zn doped CdO nanorods and antimicrobial applications. Colloids Surf. A Physicochem. Eng. Asp. 2020, 605, 125369.
- Rajendran, S.; Inwati, G.K.; Yadav, V.K.; Choudhary, N.; Solanki, M.B.; Abdellattif, M.H.; Yadav, K.K.; Gupta, N.; Islam, S.; Jeon, B.-H. Enriched Catalytic Activity of TiO2 Nanoparticles Supported by Activated Carbon for Noxious Pollutant Elimination. Nanomaterials 2021, 11, 2808.
- Kumar, P.; Inwati, G.K.; Mathpal, M.C.; Ghosh, S.; Roos, W.; Swart, H. Defects induced Enhancement of Antifungal activities of Zn doped CuO nanostructures. Appl. Surf. Sci. 2021, 560, 150026.
- Varier, K.M.; Gudeppu, M.; Chinnasamy, A.; Thangarajan, S.; Balasubramanian, J.; Li, Y.; Gajendran, B. Nanoparticles: Antimicrobial Applications and Its Prospects. In Advanced Nanostructured Materials for Environmental Remediation; Naushad, M., Rajendran, S., Gracia, F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 321–355.
- Hari, N.; Thomas, T.K.; Nair, A.J. Comparative Study on the Synergistic Action of Differentially Synthesized Silver Nanoparticles with β-Cephem Antibiotics and Chloramphenicol. J. Nanosci. 2014, 2014, 201482.
- Bankier, C.; Matharu, R.; Cheong, Y.K.; Ren, G.G.; Cloutman-Green, E.; Ciric, L. Synergistic Antibacterial Effects of Metallic Nanoparticle Combinations. Sci. Rep. 2019, 9, 16074.
- Malik, P.; Inwati, G.K.; Mukherjee, T.K.; Singh, S.; Singh, M. Green silver nanoparticle and Tween-20 modulated pro-oxidant to antioxidant curcumin transformation in aqueous CTAB stabilized peanut oil emulsions. J. Mol. Liq. 2019, 291, 111252.
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Zeng, H.-Y.; Ou-Yang, Y.-S.; Chen, Y.-B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122.
- Pei, J.; Fu, B.; Jiang, L.; Sun, T. Biosynthesis, characterization, and anticancer effect of plant-mediated silver nanoparticles using Coptis chinensis. Int. J. Nanomed. 2019, 14, 1969–1978.
- Malik, P.; Katyal, V.; Malik, V.; Asatkar, A.; Inwati, G.; Mukherjee, T.K. Nanobiosensors: Concepts and variations. Int. Sch. Res. Not. 2013, 2013, 9.
- Prasher, P.; Singh, M.; Mudila, H. Silver nanoparticles as antimicrobial therapeutics: Current perspectives and future challenges. 3 Biotech 2018, 8, 411.
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248.
- Macdonald, T.J.; Wu, K.; Sehmi, S.K.; Noimark, S.; Peveler, W.J.; Du Toit, H.; Voelcker, N.H.; Allan, E.; MacRobert, A.J.; Gavriilidis, A.; et al. Thiol-Capped Gold Nanoparticles Swell-Encapsulated into Polyurethane as Powerful Antibacterial Surfaces Under Dark and Light Conditions. Sci. Rep. 2016, 6, 39272.
- Gnanamoorthy, G.; Ramar, K.; Ali, D.; Yadav, V.K.; Ahamed, A.J.; Kumar, G. Synthesis and effective performance of Photocatalytic and Antimicrobial activities of Bauhinia tomentosa Linn plants using of gold nanoparticles. Opt. Mater. 2022, 123, 111945.
- Salvadori, M.R.; Ando, R.A.; Nascimento, C.A.O.D.; Corrêa, B. Intracellular Biosynthesis and Removal of Copper Nanoparticles by Dead Biomass of Yeast Isolated from the Wastewater of a Mine in the Brazilian Amazonia. PLoS ONE 2014, 9, e87968.
- Usman, M.S.; El Zowalaty, M.E.; Shameli, K.; Zainuddin, N.; Salama, M.; Ibrahim, N.A. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int. J. Nanomed. 2013, 8, 4467–4479.
- Kruk, T.; Szczepanowicz, K.; Stefańska, J.; Socha, R.; Warszyński, P. Synthesis and antimicrobial activity of monodisperse copper nanoparticles. Colloids Surf. B Biointerfaces 2015, 128, 17–22.
- Inwati, G.K.; Kumar, P.; Roos, W.D.; Swart, H.C. Thermally induced structural metamorphosis of ZnO:Rb nanostructures for antibacterial impacts. Colloids Surf. B Biointerfaces 2020, 188, 110821.
- Armijo, L.M.; Wawrzyniec, S.J.; Kopciuch, M.; Brandt, Y.I.; Rivera, A.C.; Withers, N.J.; Cook, N.C.; Huber, D.L.; Monson, T.C.; Smyth, H.D.C.; et al. Antibacterial activity of iron oxide, iron nitride, and tobramycin conjugated nanoparticles against Pseudomonas aeruginosa biofilms. J. Nanobiotechnol. 2020, 18, 35.
- Vijayakumar, S.; Krishnakumar, C.; Arulmozhi, P.; Mahadevan, S.; Parameswari, N. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Glycosmis pentaphylla (Retz.) DC. Microb. Pathog. 2018, 116, 44–48.
- Tiwari, V.; Mishra, N.; Gadani, K.; Solanki, P.; Shah, N.A.; Tiwari, M. Mechanism of Anti-bacterial Activity of Zinc Oxide Nanoparticle Against Carbapenem-Resistant Acinetobacter baumannii. Front. Microbiol. 2018, 9, 1218.
- Gumienna, M.; Górna, B. Antimicrobial Food Packaging with Biodegradable Polymers and Bacteriocins. Molecules 2021, 26, 3735.
- Verdier, T.; Coutand, M.; Bertron, A.; Roques, C. Antibacterial Activity of TiO2 Photocatalyst Alone or in Coatings on E. coli: The Influence of Methodological Aspects. Coatings 2014, 4, 670–686.
- Arora, B.; Murar, M.; Dhumale, V. Antimicrobial potential of TiO2 nanoparticles against MDR Pseudomonas aeruginosa. J. Exp. Nanosci. 2015, 10, 819–827.
- Inwati, G.; Rao, Y.; Singh, M. In Situ Growth of Low-Dimensional Silver Nanoclusters with Their Tunable Plasmonic and Thermodynamic Behavior. ACS Omega 2017, 2, 5748–5758.
- Kumar, P.; Inwati, G.K.; Mathpal, M.C.; Maze J., W.; Swart, H. Recent Advances on Ferrites Nanomaterial’s as Photocatalyst for Environment. Adv. Nanostruct. Mater. 2022, 560, 381–409.
- Inwati, G.; Rao, Y.; Singh, M. Thermodynamically induced in Situ and Tunable Cu Plasmonic Behaviour. Sci. Rep. 2018, 8, 3006.
- Inwati, G.K.; Rao, Y.; Singh, M. In Situ Free Radical Growth Mechanism of Platinum Nanoparticles by Microwave Irradiation and Electrocatalytic Properties. Nanoscale Res. Lett. 2016, 11, 458.
- Gnanamoorthy, G.; Yadav, V.K.; Ali, D.; Narayanan, V.; Katubi KM, S.; Alarifi, S. Trigger action of copper aminophosphate (X-CuAP) nanoparticles for enhanced electrochemical, photocatalyst and biological properties. Opt. Mater. 2021, 117, 111113.
- Gnanamoorthy, G.; Yadav, V.K.; Narayanan, V. Well organized assembly of (X)-CuSnO3 nanoparticles enhanced photocatalytic and anti-bacterial properties. J. Water Process Eng. 2020, 36, 101258.
- Gnanamoorthy, G.; Yadav, V.K.; Yadav, K.K.; Ramar, K.; Alam, J.; Shukla, A.K.; Ali, F.A.A.; Alhoshan, M. Fabrication of different SnO2 nanorods for enhanced photocatalytic degradation and antibacterial activity. Environ. Sci. Pollut. Res. 2021.
- Wang, H.; Zhou, Y.; Sun, Q.; Zhou, C.; Hu, S.; Lenahan, C.; Xu, W.; Deng, Y.; Li, G.; Tao, S. Update on Nanoparticle-Based Drug Delivery System for Anti-inflammatory Treatment. Front. Bioeng. Biotechnol. 2021, 9, 630352.
- Mocan, T.; Matea, C.T.; Pop, T.; Mosteanu, O.; Buzoianu, A.D.; Suciu, S.; Puia, C.; Zdrehus, C.; Iancu, C.; Mocan, L. Carbon nanotubes as anti-bacterial agents. Cell. Mol. Life Sci. 2017, 74, 3467–3479.
- Eid, K.A.; Azzazy, H.M. Sustained broad-spectrum antibacterial effects of nanoliposomes loaded with silver nanoparticles. Nanomedicine 2013, 9, 1301–1310.
- Maja, L.; Željko, K.; Mateja, P. Sustainable technologies for liposome preparation. J. Supercrit. Fluids 2020, 165, 104984.
- Nikolić, I.; Savic, I.; Popsavin, M.; Rakic, S.J.; Mihajilov-Krstev, T.M.; Ristic, I.S.; Eric, S.P.; Savić-Gajic, I.M. Preparation, characterization and antimicrobial activity of inclusion complex of biochanin A with (2-hydroxypropyl)-β-cyclodextrin. J. Pharm. Pharmacol. 2018, 70, 1485–1493.
- Inwati, G.K.; Yadav, V.K.; Ali, I.H.; Vuggili, S.B.; Kakodiya, S.D.; Solanki, M.K.; Yadav, K.K.; Ahn, Y.; Yadav, S.; Islam, S.; et al. 2D Personality of Multifunctional Carbon Nitrides towards Enhanced Catalytic Performance in Energy Storage and Remediation. Appl. Sci. 2021, 8, 3753.
- Teixeira-Santos, R.; Gomes, M.; Gomes, L.C.; Mergulhão, F.J. Antimicrobial and anti-adhesive properties of carbon nanotube-based surfaces for medical applications: A systematic review. iScience 2021, 24, 102001.
- Malek, I.; Schaber, C.F.; Heinlein, T.; Schneider, J.J.; Gorb, S.N.; Schmitz, R.A. Vertically aligned multi walled carbon nanotubes prevent biofilm formation of medically relevant bacteria. J. Mater. Chem. B 2016, 4, 5228–5235.
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir 2008, 24, 6409–6413.
- Liu, D.; Mao, Y.; Ding, L. Carbon nanotubes as antimicrobial agents for water disinfection and pathogen control. J. Water Health 2018, 16, 171–180.
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250.
- Ana, M. Díez-Pascual, State of the Art in the Antibacterial and Antiviral Applications of Carbon-Based Polymeric Nanocomposites. Int. J. Mol. Sci. 2021, 22, 10511.
- ClinicalTrials.gov Extension Study of Liposomal Amikacin for Inhalation in Cystic Fibrosis (CF) Patients with Chronic Pseudomonas Aeruginosa (Pa) Infection. Available online: https://clinicaltrials.gov/ct2/show/NCT01316276 (accessed on 12 November 2019).
- ClinicalTrials.gov Study of Dose Escalation of Liposomal Amikacin for Inhalation (ARIKAYCE™)—Extension Phase. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03905642 (accessed on 18 November 2019).
- Ghobrial, O.G.; Derendorf, H.; Hillman, J.D. Pharmacodynamic activity of the lantibiotic MU1140. Int. J. Antimicrob. Agents 2009, 33, 70–74.
- Molchanova, N.; Hansen, P.R.; Franzyk, H. Advances in Development of Antimicrobial Peptidomimetics as Potential Drugs. Molecules 2017, 22, 1430.
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine Fight against Antibacterial Resistance: An Overview of the Recent Pharmaceutical Innovations. Pharmaceutics 2020, 12, 142.




