Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Virendra Kumar Yadav | -- | 3447 | 2022-08-01 09:17:43 | | | |
| 2 | Jessie Wu | + 5 word(s) | 3452 | 2022-08-02 05:31:58 | | | | |
| 3 | Jessie Wu | Meta information modification | 3452 | 2022-08-02 05:33:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Boulkhessaim, S.; Gacem, A.; Khan, S.H.; Amari, A.; Yadav, V.K.; Harharah, H.N.; Elkhaleefa, A.M.; Yadav, K.K.; Rather, S.; Ahn, H.; et al. Nanotechnological Approaches for Removal of Persistent Organic Pollutants. Encyclopedia. Available online: https://encyclopedia.pub/entry/25719 (accessed on 03 March 2026).
Boulkhessaim S, Gacem A, Khan SH, Amari A, Yadav VK, Harharah HN, et al. Nanotechnological Approaches for Removal of Persistent Organic Pollutants. Encyclopedia. Available at: https://encyclopedia.pub/entry/25719. Accessed March 03, 2026.
Boulkhessaim, Salim, Amel Gacem, Samreen Heena Khan, Abdelfattah Amari, Virendra Kumar Yadav, Hamed N. Harharah, Abubakr M. Elkhaleefa, Krishna Kumar Yadav, Sami-Ullah Rather, Hyun-Jo Ahn, et al. "Nanotechnological Approaches for Removal of Persistent Organic Pollutants" Encyclopedia, https://encyclopedia.pub/entry/25719 (accessed March 03, 2026).
Boulkhessaim, S., Gacem, A., Khan, S.H., Amari, A., Yadav, V.K., Harharah, H.N., Elkhaleefa, A.M., Yadav, K.K., Rather, S., Ahn, H., & Jeon, B. (2022, August 01). Nanotechnological Approaches for Removal of Persistent Organic Pollutants. In Encyclopedia. https://encyclopedia.pub/entry/25719
Boulkhessaim, Salim, et al. "Nanotechnological Approaches for Removal of Persistent Organic Pollutants." Encyclopedia. Web. 01 August, 2022.
Copy Citation
Persistent organic pollutants (POPs) are a group of hazardous chemical compounds that originate from anthropogenic activities during production, utilization, and disposal. They can impact living beings and the environment adversely because of their ease of transportation by wind and water. The level of hazardous persistent organic pollutants is increasing every day in the environment.
nanomaterials
persistent organic pollutants
remediation
1. Persistent Organic Pollutants (POPs), Source, and Fate
Persistent organic pollutants (POPs) have grabbed significant attention worldwide. POPs are defined as xenobiotic chemical compounds of different origins, but all have similar characteristics, i.e., high toxicity, bioaccumulation, hydrophobicity, environmental persistence, and ability to transfer via the FC [1]. POPs are carbon-containing chemicals, and due to their higher solubility in the lipids, they tend to become accumulated among the fatty tissues and can disrupt the endocrine system of organisms, therefore often referred to as endocrine disruptors (EDs) [2]. The physicochemical properties of POPs are responsible for their dispersion and distribution in the environment; POPs have low water solubility (log Kow 3–7); therefore, they have high adsorption, low degradation, and hydrophobic nature [3].
Various Categories of POPs
The utilization of POPs was restricted since 1970 in various parts of the United States of America (USA) and Europe. Moreover, there was a strict prohibition on the release of such POPs in both the above-mentioned continents [4]. The use and consumption of pesticides increased abruptly after the green revolution; at that time, the hazardous effects of POPs were unknown. Lately, the toxic effects of pesticides have emerged globally. The general public started to understand the toxicity of pesticides and other organic contaminants. After the Stockholm convention, POP was placed into three categories, i.e., pesticides, by-products, and industrial chemicals [5]. Figure 1 depicts the type and different categories of POPs.
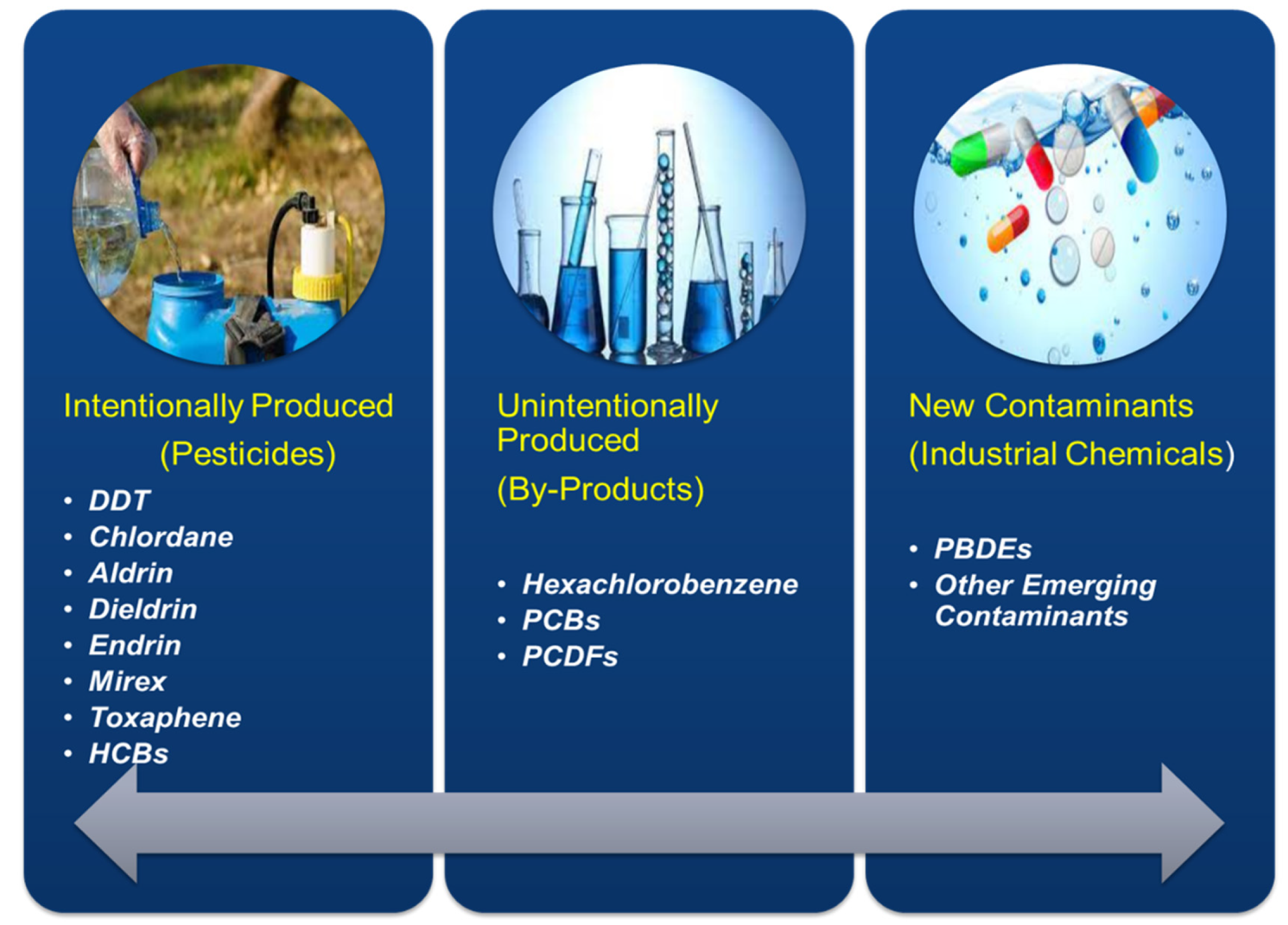
Figure 1. Type and categories of POPs.
POPs are the range of synthetic hazardous chemicals, produced either intentionally or unintentionally [6]. Pesticides fall under the category of the intentionally produced chemicals used to control pests in agriculture and houses; DDT is a known such example that was banned globally due to its extreme toxicity [7]. Others are industrial products or unintentionally produced chemicals, i.e., dioxins. The new class of POPs includes types of emerging contaminants such as polybrominated diphenyl ethers (PBDE), perfluorinated compounds (PF), and a list of new contaminants added day by day.
POPs can be classified as Organochlorine Pesticides (OCP); hexachlorobenzene (HCB) and other polychlorinated benzenes (PCBzs); PAHs; polychlorinated naphthalenes; PCBs; polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD and PCDF); and other contaminants of emerging concerns [8]. At first, during the Stockholm convention (2001), the participating countries decided to minimize or strike out the production, usage, and release of 12 key POPs popularly referred to as “the dirty dozen”. Later, ten more chemical substances were added to the group of POPs after two amendments (2009 and 2011) [9]. Table 1 summarizes the list of twenty-two POPs after the Stockholm Convention.
Table 1. List of POPs as per Stockholm Convention.
| S. No | Chemical | Category |
|---|---|---|
| As per the 2001 Amendment (The Dirty Dozen) | ||
| 1 | PCB | Industrial waste/byproduct |
| 2 | PCD | Byproduct |
| 3 | PCDF | Byproduct |
| 4 | Chlordane | Pesticide |
| 5 | Mirex | Pesticide |
| 6 | Endrin | Pesticide |
| 7 | Aldrin | Pesticide |
| 8 | Dieldrin | Pesticide |
| 9 | HCB | Pesticide |
| 10 | Heptachlor | Pesticide |
| 11 | Toxaphene | Pesticide |
| 12 | DDT | Pesticide |
| As per the 2009 Amendment | ||
| 13 | Lindane | Pesticide |
| 14 | Chlordecone | Pesticide |
| 15 | Pentachloro benzene | Pesticide and Byproduct |
| 16 | Alpha-HCH | Pesticide and Byproduct |
| 17 | Beta-HCH | Pesticide and Byproduct |
| 18 | PFO and constituents PFOSF | Industrial |
| 19 | Hexabromobiphenyl | Industrial |
| 20 | Hexa-BDE and Hepta-BDE | Industrial |
| 21 | Tetra-BDE and Penta-BDE | Industrial |
| As per the 2011 Amendment | ||
| 22 | Endosulfan | Pesticide |
POPs can sustain in the environment for prolonged periods, taking decades or several centuries to be completely degraded. Due to their physicochemical properties, POPs have the tendency to travel long distances and resist degradation (biological and chemical degradation), which allows them to bioaccumulate to a deeper level via biomagnification, and their exposure can lead to severe damage to health and the environment [10]. Several studies suggest a range of adverse effects induced by POPs, as most of them are semivolatile compounds and can easily absorb onto the atmospheric particles and migrate into the water, air, and soil media [11][12][13]. POPs are rarely found in one environmental medium but are present in all media, and if tested will be found to be present in all media across the world [14]. POPs are found in agricultural wastes, chemical, and electronic industry waste, as well as pharmaceutical waste.
POPs are severely toxic that even the smallest concentration of them is found to be highly fatal to the organisms. POPs are generally resistant to chemical, biological, and photodegradation as they have low solubility and it is quite difficult to degrade POPs using traditional wastewater-treatment technology [15][16]. In the recent past, remediation of POPs was achieved by advanced wastewater-treatment technologies or by the combination of one or two methods. However, the most important question arises: Despite all technologies, why are POPs resistant to most degradation processes? POPs generally exhibit lipid solubility, and because of this reason, they tend to accumulate in fatty tissues of organisms. Moreover, halogenated compounds show great stability toward hydrolysis and photolytic degradation due to the nonreactivity of c-cl bonds [17]. The stability towards degradation and lipophilicity of POPS makes them compounds of particular concern. POPs are also divided into four levels based on their toxicity:
- (i)
-
most hazardous chemical [restricted for production and utilization];
- (ii)
-
medium level chemicals [confined to use during the production];
- (ii)
-
unintentional discharge of chemical;
- (iv)
-
use of chemicals under investigation.
POPs are extremely toxic halogenated compounds that largely impact humans either through point or non-point sources. The organic pollutants generally consist of personal care products (PCP), pesticides, organic dyes, endocrine disruptors, pharmaceutical waste, and other such contaminants of emerging concern [18]. The release of POPs in water bodies causes disturbance to the aquatic food chain, as POPs tend to bioaccumulate, and EPA indicated that the rate of disease caused by POPs is very high in coastal and marine ecosystems [19]. Thus, POPs tend to impact every living organism in some or the other way due to their hazardous nature. There arises the need for the remediation of such pollutants from the environment by applying advanced techniques, but the results show that the conventional technologies were not efficient for the complete removal of POPs as they are simply transforming the pollutants from one phase to another rather than the complete elimination [20]. With the advancement of nanotechnology for environment application, the focus has been shifted to the removal of POPs using nanomaterials. The present research significantly highlights the utilization of nanotechnology for the removal of POPs from the environment.
2. Advanced Nanotechnological Approaches for Removal of Persistent Organic Pollutants
2.1. Nanocatalysis
With the ineffectiveness of conventional technologies to completely degrade and mineralize the organic pollutants, there arises the need to develop a green, innovative, and sustainable method that can destroy the POPs with much less energy consumption and chemical utilization [21][22][23]. Therefore, the scientific community has started looking for advanced oxidation processes as a low-cost and effective method that is proficient in oxidizing and mineralizing a range of pollutants, including POPs, due to their strong oxidizing radicals [24]. The use of semiconducting wide-bandgap nanomaterials for the treatment of contaminants into eco-friendly compounds comes under nanocatalysis. The semiconductor metal and metal-oxide nanomaterials have gained significant attention in POPs treatment sustainably. Several types of nanocatalyst are used for the effective degradation of POPs from wastewaters such as Fenton-based catalyst, electrocatalyst, photocatalyst, and even doped multifunctional nanocatalyst [25][26]. Photocatalysis/nanocatalysis is a well-known AOP; it is used to enhance the biodegradability of POPs by using oxidants to degrade organic pollutants by the release of highly reactive oxygen species (ROS) for the chemical reaction to occur [27]. Photocatalysis involves the catalytic activation in the presence of light and relies on the generation of strong radicals, i.e., H2O2, O2 •–, O3, and OH radicals, which destroy almost all organic molecules [28]. Photocatalysis is even effective for the remediation of volatile organic compounds (VOCs) such as PCBs, Dioxins, and PHA by producing free radicals. The process of photocatalysis starts as the nanocatalyst with a wide bandgap (such as ZnO, TiO2, WO3) becomes photoexcited in the presence of a light source (natural or artificial) and oxygen used to degrade POPs [29][30]. The photocatalytic-degradation process ideally involves the following steps, as shown in Figure 2.
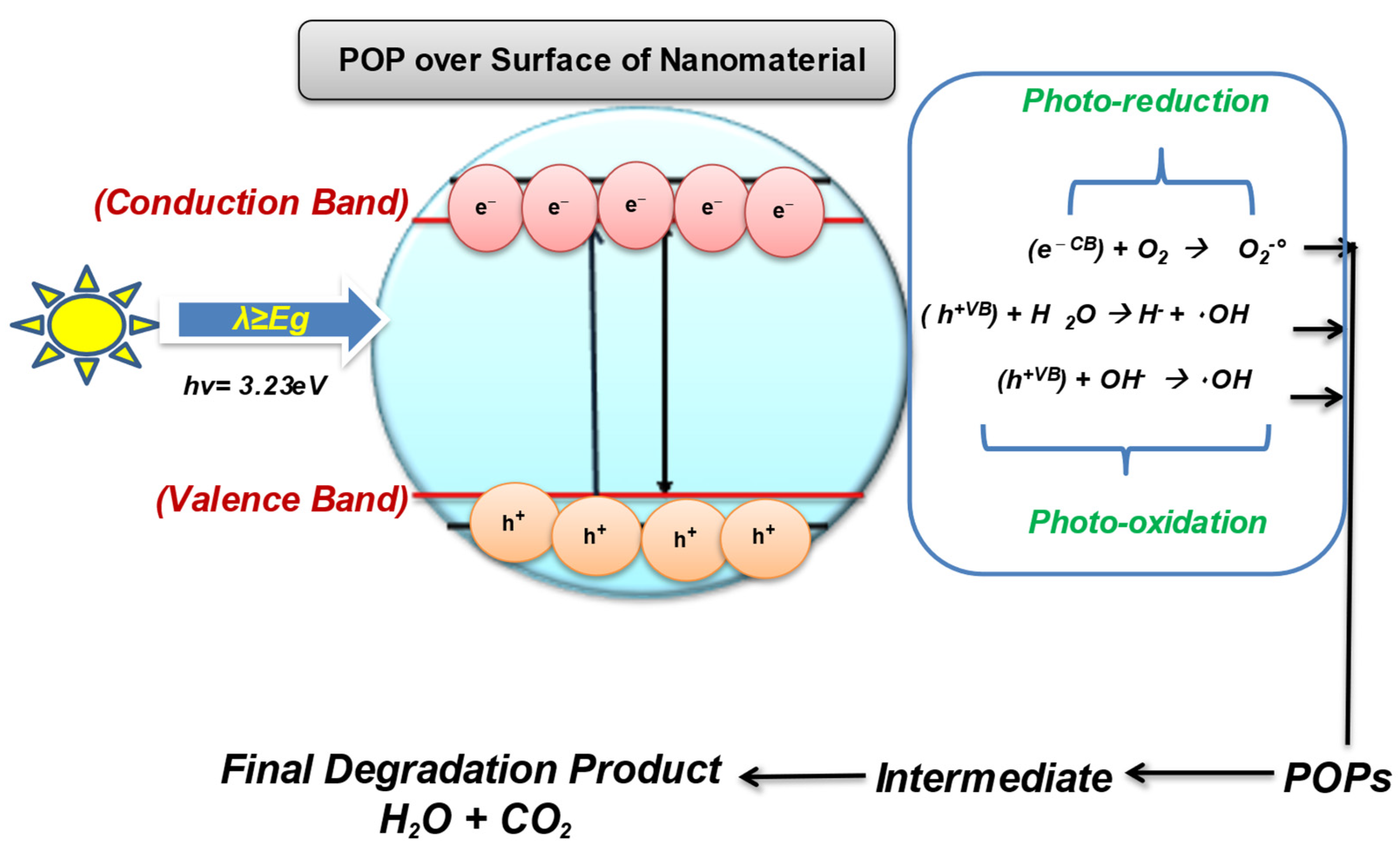
Figure 2. Photocatalysis over the surface of the nanomaterial.
As of now, TIO2 and ZnO are the most widely utilized semiconductors for the degradation of POPs. The heterogeneous photocatalyst is efficient for removing highly hydrophobic POPs from the environment. The surface, pore-volume, and structure of the semiconducting material are deciding parameters to consider while selecting the ideal catalyst [31][32]. The surface properties and crystal structure of the material can be tuned to boost the degradation efficiency of the photocatalyst [33]. However, the main demerits related to photocatalysis is the removal of nanomaterial from the reaction media once the process is over.
Lwin et al. (2019) synthesized a cubic ZnO-SnO2 nanocomposite via the solvothermal method and used it for the degradation of tetracycline hydrochloride by photocatalysis. The as-synthesized nanocomposite material was analyzed by advanced instrumentation. The degradation result shows that ZnO-SnO2 nanocomposite shows remarkable photostability even after four consecutive cycles, providing a successful method for the remediation of POPs that can also be used for the remediation of other organic contaminants [34].
Amir et al. (2016) reported a MnFe2O4@PANI@Ag nanocatalyst to degrade azo dyes from industrial waste. The degradation result proves that as-synthesized nanocatalyst has a high potential to degrade the azo dyes, and the best advantage is that the nanocomposites can be easily detached by applying the external magnet and can be used for the next cycle with the same efficiency [35].
Khan et al. (2018) synthesized magnetic Fe-ZnO nanocomposite material via the sonochemical process to remediate Chlorpyrifos pesticide from the aqueous solution. The result shows that Fe-ZnO nanocomposite was quick to degrade the pesticide with good stability and reusability. The results show up to 90% degradation and the nanocomposite could be removed by applying the external magnet [36].
Chen et al. (2022) explored Mn-based nanocomposites to degrade bisphenol A, as the potential of pristine manganese oxides (Mn3O4) for the remediation of organic substances has not been explored yet. The study involves the activation of Mn3O4-based peroxymonsulfate to degrade bisphenol A (BPA) in different water systems. The results show remarkable mineralization (75.9%) with efficient removal of BPA (96.7%) at optimum parameters in 60 min. The nanocomposite shows the stability of Mn3O4, long-term performance, and eight cycles of reusability with only an 11% reduction in BPA removal. The activated-sludge inhibition method used to check the toxicity of BPA after degradation was shown to be significantly repressed [37].
Photocatalysis is undoubtedly the most widely employed process for wastewater treatment. High efficiency, sustainability, and good results make photocatalysis a method of choice for the degradation of a wide range of organic and inorganic pollutants.
2.2. Nanoadsorption
Nanoadsorbents provide high sorption efficiency because of their extremely large surface area and sorption sites, tunable pore size, much lower intraparticle-diffusion distance, and high surface activity for effective adsorption of a vast range of organic and other pollutants [38][39][40]. The advantage of using nanoadsorbents is that they can be easily functionalized to make them highly selective for any pollutants [41]. The adsorption process has been found to be successful for the remediation of POPs such as hydrocarbon, dyes, phenols, detergents, pharmaceuticals, pesticides, and biphenyls.
Figure 3 shows the types of carbon-based nanoadsorbent material with their benefits. Nanoadsorption is an easy and safe process for the remediation of POPs from water bodies. Among various technologies, nanoadsorption so far emerges as a widely efficient method for the remediation of POP. Several studies prove the efficiency of nanomaterials for the adsorption of various POPs from the wastewater, as more than 90% removal efficiency was achieved in most of the studies for up to ten cycles [42]. The adsorption efficiency of nanomaterial is mainly monitored by producing a complex with the surface of metal oxides and enduring a one-electron oxidation reaction under visible irradiation. Nanoadsorption is based on electrostatic interactions, hydrogen bonding, and hydrophobic interactions such as van der Waals, electron donor–acceptor, etc. [43]. Nanomaterials such as clay, zeolite, alumina, metal/metal oxides, activated carbon, carbon-based nanomaterials, nanocomposites, nanosheets, nanotubes, chitosan-based polymers, and graphene-based nanomaterials are extensively applied in the process of nanoadsorption [44]. For effective removal of POPs, the use of magnetic nanoparticles, especially iron oxide, has led away to a new class of magnetic-separation strategies. Microporous structures present in activated carbon aid the adsorption efficiency in the removal of POPs [45][46]. Carbon-based nanoadsorbents tend to interact with contaminants due to hydrophobicity, hydrogen bonding, and covalent and electrostatic interactions [47]. Each form has several adsorption sites that can absorb the organic pollutants due to their flexibility. Both single-walled and multiwalled carbon nanotubes have been surface-modified by increasing the porosity to generate high-energy sites to adsorb more organic pollutants over increasing the efficiency of manifolds [48][49].
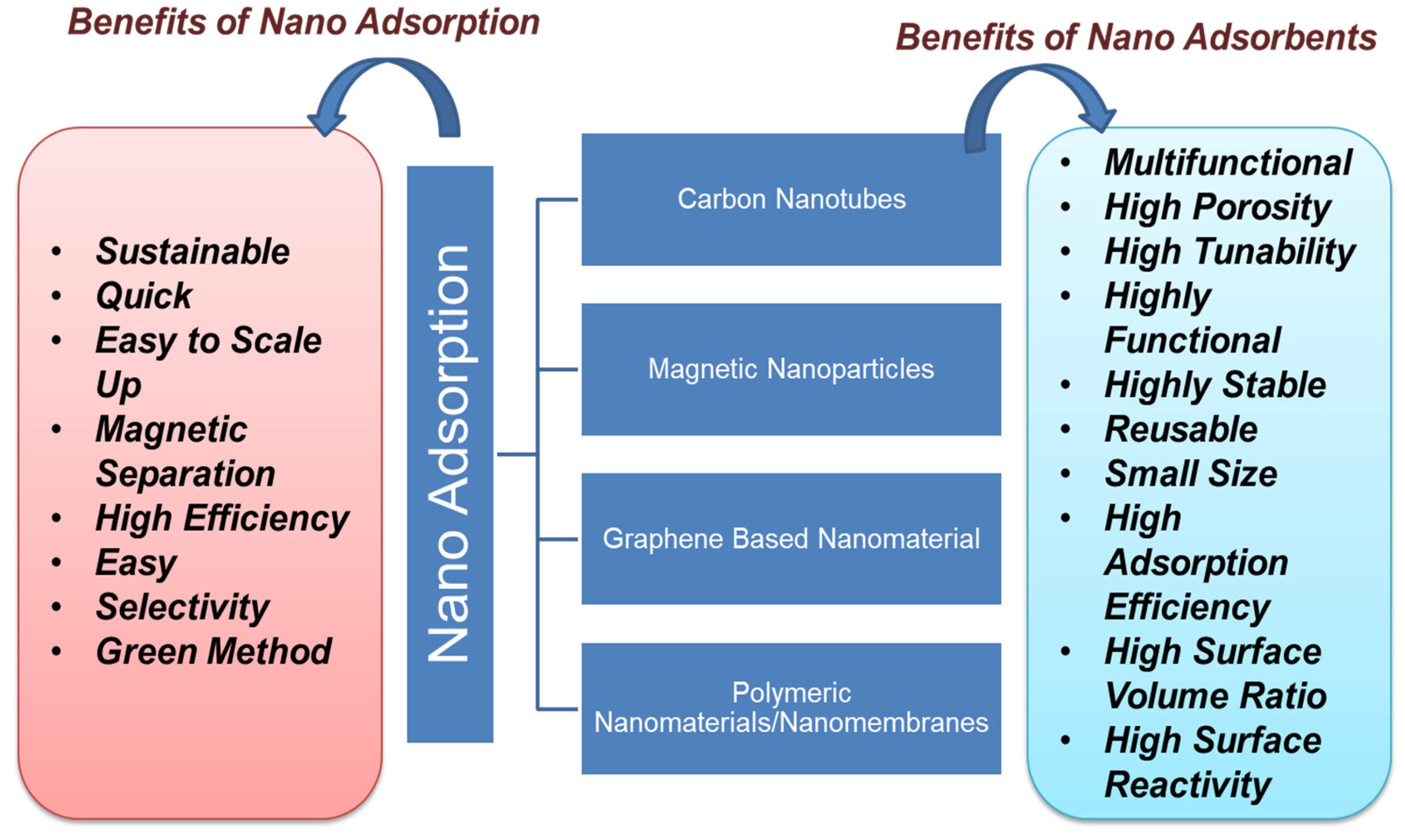
Figure 3. Types and benefits of nanoadsorption.
A study was conducted by Ali et al. (2018) for the adsorption of cyanazine using green-synthesized iron nanocomposites. The result shows the quick removal of cyanazine from water due to low contact time [50]. In another study by Mahdavi et al. (2021), aminoguanidine-modified magnetic graphene oxide was used for the efficient remediation of chlorpyrifos from water. The desorption of chlorpyrifos was analyzed by HPLC-MS, and the results show remarkable desorption by using a synthesized nanoadsorbent [51].
Izanloo et al. (2019) successfully synthesized bifunctional nanoadsorbent (Fe3O4@SiO2@NH2@SH) for the remediation of 2,4-dichlorophenoxyacetic acid (2,4-D) and lead from synthetic wastewater. The adsorption process follows the Langmuir isotherm with second-order kinetics. Based on the study, it was noticed that pH plays a key role in the adsorption of organic contaminants. The result also shows that the synthesized nanoadsorbent was so efficient that it can be used for several cycles without losing its desorption efficiency [52].
Mohammadi et al. (2018) modified magnetic Fe3O4@SiO2@NH2 nanoadsorbent for the remediation of 2,4-Dichlorophenoxyacetic acid (2,4-D) and 2-methyl-4-chlorophenoxyacetic acid (MCPA) from aqueous solution. The effects of pH, time, dosage, and initial concentration of the pollutants were studied to obtain a better insight into the synthesized material. The result shows that amino-functionalized Fe3O4@SiO2@NH2 is an effective adsorbent for the remediation of phenoxy-acid herbicides from water due to its advantages such as easy and rapid separation of the target pollutant from the solution [53].
Dehghani et al. (2019) investigated the adsorption of diazinon on multiwalled CNTs in a batch reactor. The results show that 100% remediation of diazinon was achieved at pH 6 in just 15 min. The result shows that the highly efficient MWCNTs can be used for the remediation of different pesticides from an aqueous solution [54].
Kalhor et al. (2018) synthesized amino-functionalized nanosilica (NH2-SHNS) nanoadsorbent for removal of imidacloprid pesticide from wastewater. The as-synthesized nanoadsorbent had a spherical shape in the size range of 70–250 nm. Parameters such as pH, temperature, dosage, and concentration of pesticide were investigated and observed that the adsorption equilibrium was matched with the Redlich–Peterson isotherm and follows pseudo-first-order reaction kinetics [55].
Sahoo et al. (2020) synthesized magnetically separable GO/g-C3N4-Fe3O4 nanocomposite using the hydrothermal process for the remediation of methylene blue and tetracycline from wastewater. The result exhibited that nanoadsorbents can be used for up to 5 cycles without losing their efficiency. It was observed that the adsorption of pollutants is pH-dependent, and maximum adsorption capacity was achieved at pH 3 for tetracycline and pH 9 for methylene blue. The higher adsorption efficiency is due to hydrogen bonding and π-π interaction. The adsorption data follow pseudo-second-order kinetics and are best-fitted to the Langmuir isotherm [56].
Nikzad et al. (2019) studied the adsorption of diazinon by magnetic guar-gum MMT (montmorillonite) from aqueous solutions. The magnetic MMT was synthesized via the chemical coprecipitation method and was found in a size range of 50–130 nm. The adsorption kinetics follows the pseudo-second-order model, also best following the Langmuir isotherm. The magnetic MMT shows excellent adsorption efficiency for the removal of diazinon [57].
In a similar study, Peralta et al. (2020) synthesized silica-based nanoadsorbents for the remediation of several POPs. The hybrid magnetic iron-oxide nanoparticles were covered with silica and 3-(trimethoxysilyl) propyl-octadecyl dimethyl-ammonium chloride; it is further modified to obtain the final nanoadsorbent [58].
The process of nanoadsorbents is widely used as a low-cost, effective, and sustainable treatment technology. It is most successful for the removal of heavy metals from wastewater due to its reusable efficiency and it does not require high operation and maintenance costs. Magnetic nanoadsorbents are easy to separate from the reaction medium by applying the external magnet, which is one of the greatest advantages of using nanoadsorbents.
2.3. Nanofiltration
Nanotechnology has paved the way to advance water treatment systems by using nanofiltration membranes [59]. Membrane processes such as microfiltration (MF), reverse osmosis (RO), ultrafiltration (UF), and nanofiltration (NF) are pressure-driven filtration techniques and are considered highly effective processes for the treatment of wastewaters [60]. They are considered alternative methods for the remediation of organic micropollutants for the water bodies. Though the treatment of wastewaters using membrane processes is costly, they are the best alternative to conventional techniques as their removal efficiency is very high [61]. The nanoparticle can be incorporated into the membranes either by surface immobilization, blending, or surface grafting for developing the membranes with desirable functionality and characteristics [62]. By using the electrospinning method, polymeric or composite nanofibrous membranes can be developed to compose ultrafine nanofibers by using materials such as ceramics, biomass wastes, polymers, or metals in the range of 10–1000 nm [63][64].
Out of all membrane techniques, NF and RO proved their efficiency for the effective filtration for the remediation of micro/trace organic pollutants. NF is comparatively more efficient for the remediation of pollutants than RO (a drawback of high energy consumption and maintenance cost), where filtration is caused by different mechanisms, i.e., convection, diffusion, and charge effects [65][66][67]. NF is effective for the remediation of micropollutants due to its small pore sizes, high efficiency, and user-friendliness [68]. Several polymers (natural and synthetic) have been used for the preparation of nanofiltration membranes such as polypropylene, polyvinyl fluoride, polyacrylonitrile, and most commonly cellulose acetate, as they are effective in the removal of POPs [69][70][71][72]. Nanofibers have stable adsorption structures due to their loose bundles as compared to nanotubes and nanoparticles. Nanofibers have been found to be efficient for the removal of pesticides from wastewaters through their molecular propagation mechanism; furthermore, when semiconducting materials are used for the synthesis of nanofibers, they can add the photocatalytic property [73]. Several nanocomposite nanofiber (ZnO-cellulose acetate, TiO2-graphene, etc.) membranes exhibit strong photocatalytic efficiency for the remediation of dye compounds [74]. In addition, the immobilization of magnetic nanoparticles with the membrane was found efficient for the remediation of organic pollutants, and doping with TiO2 for photocatalytic degradation shows good results [75].
For the quality and efficient removal or range of organic/inorganic pollutants, single or combinations of filtration techniques (i.e., ultrafiltration; microfiltration; nanofiltration, and a combination of two or more) have been utilized. Moreover, a combination of filtration techniques with biological or chemical methods is known for the efficient remediation of persistent organic pollutants from wastewaters [76]. However, for the successful implementation of membrane processes, the following factors need to be considered: type of membrane, membrane modules, membrane composition, and most importantly membrane interaction with the pollutant [77].
Nanofiltration is a pressure-driven technique based on hydrodynamics between the membrane surface and membrane nanopores and is efficient in the remediation of low-molecular-weight compounds with a size range between 1–10 nm [78]. By reducing the hardness of organic pollutants, nanofilters help to reduce the ionic strength of the solution. The effectiveness of filtration is vastly dependent on the surface concentration of the membrane, its porosity, and charge [79]. The electrospinning technique is used for the preparation of high-quality nanofibrous membranes [80]. Nanofiltration effectively removes almost all dissolved salts and rejects multi, di- and univalent ions, so it is highly efficient for the treatment of arsenic in drinking water [81].
The study was conducted by Karimi et al. (2016) for the effective removal of atrazine and diazinon from wastewater by using a thin-film composite polyamide nanofiltration membrane synthesized via interfacial polymerization. The results show that diazinon was better rejected than atrazine. The water permeability and diazinon rejection increased from 22 L/m2/h and 95.2% for the unmodified membrane to about 41.56 L/m2/h and 98.8% for the 2% (w/v) TEA modified membrane showing a significant improvement in the performance of poly (piperazine amide) TFC NF membranes for pesticides removal [82].
Wang et al. (2020) synthesized a novel nanocomposite with catalytic property (Al-MOF/Fe3O4/PDA@Ag) by loading silver nanoparticles (Ag) onto the magnetic Al-MOF/Fe3O4/PDA. The as-synthesized composite shows higher removal efficiency for various organic pollutants (CIP, NOR, and MO) in a short period. The catalyst could be easily separated by the application of an external magnet and also shows good reusability and stability [83].
Membrane filtration is found to be the safest technology, and NF is excellent for the removal of low-molecular-weight compounds. NF is the only filtration technology known for the removal of pesticides and other organic contaminants successfully. However, membrane blockage and fouling are the drawback of filtration technology, which can be overcome by the use of hybrid technologies.
References
- Lefebvre, T.; Fréour, T.; Ploteau, S.; Le Bizec, B.; Antignac, J.P.; Cano-Sancho, G. Associations between human internal chemical exposure to Persistent Organic Pollutants (POPs) and In Vitro Fertilization (IVF) outcomes: Systematic review and evidence map of human epidemiological evidence. Reprod. Toxicol. 2021, 105, 184–197.
- Adithya, S.; Jayaraman, R.S.; Krishnan, A.; Malolan, R.; Gopinath, K.P.; Arun, J.; Govarthanan, M. A critical review on the formation, fate and degradation of the persistent organic pollutant hexachlorocyclohexane in water systems and waste streams. Chemosphere 2021, 271, 129866.
- Jacob, J. A review of the accumulation and distribution of persistent organic pollutants in the environment. Int. J. Biosci. Biochem. Bioinform. 2013, 3, 657.
- Lallas, P.L. The Stockholm Convention on persistent organic pollutants. Am. J. Int. Law 2001, 95, 692–708.
- Xie, L.; Du, T.; Wang, J.; Ma, Y.; Ni, Y.; Liu, Z.; Wang, J. Recent advances on heterojunction-based photocatalysts for the degradation of persistent organic pollutants. Chem. Eng. J. 2021, 426, 130617.
- Kanzari, F.; Syakti, A.D.; Asia, L.; Malleret, L.; Piram, A.; Mille, G.; Doumenq, P. Distributions and sources of persistent organic pollutants (aliphatic hydrocarbons, PAHs, PCBs and pesticides) in surface sediments of an industrialized urban river (Huveaune), France. Sci. Total Environ. 2014, 478, 141–151.
- Venier, M.; Salamova, A.; Hites, R.A. How to distinguish urban vs. agricultural sources of persistent organic pollutants? Curr. Opin. Environ. Sci. Health 2019, 8, 23–28.
- Guo, W.; Pan, B.; Sakkiah, S.; Yavas, G.; Ge, W.; Hong, H.Z. Persistent organic pollutants in food: Contamination sources, health effects and detection methods. Int. J. Environ. Res. Public Health 2019, 16, 4361.
- Eljarrat, E.; Barcelo, D. Priority lists for persistent organic pollutants and emerging contaminants based on their relative toxic potency in environmental samples. TrAC Trends Anal. Chem. 2003, 1, 655–665.
- Göktaş, R.K.; MacLeod, M. Remoteness from sources of persistent organic pollutants in the multi-media global environment. Environ. Pollut. 2016, 217, 33–41.
- Adeola, F.O. Boon or bane? The environmental and health impacts of persistent organic pollutants (POPs). Hum. Ecol. Rev. 2004, 11, 27–35.
- Abelsohn, A.; Gibson, B.L.; Sanborn, M.D.; Weir, E. Identifying and managing adverse environmental health effects: Persistent organic pollutants. CMAJ 2002, 166, 1549–1554.
- Qing Li, Q.; Loganath, A.; Seng Chong, Y.; Tan, J.; Philip Obbard, J. Persistent organic pollutants and adverse health effects in humans. J. Toxicol. Environ. Health Part A 2006, 69, 1987–2005.
- Gaur, N.; Narasimhulu, K.; PydiSetty, Y. Recent advances in the bio-remediation of persistent organic pollutants and its effect on environment. J. Clean. Prod. 2018, 198, 1602–1631.
- Sun, B.; Li, Q.; Zheng, M.; Su, G.; Lin, S.; Wu, M.; Meng, B. Recent advances in the removal of persistent organic pollutants (POPs) using multifunctional materials: A review. Environ. Pollut. 2020, 265, 114908.
- Nguyen, V.H.; Smith, S.M.; Wantala, K.; Kajitvichyanukul, P. Photocatalytic remediation of persistent organic pollutants (POPs): A review. Arab. J. Chem. 2020, 13, 8309–8337.
- Karthigadevi, G.; Manikandan, S.; Karmegam, N.; Subbaiya, R.; Chozhavendhan, S.; Ravindran, B.; Awasthi, M.K. Chemico-nanotreatment methods for the removal of persistent organic pollutants and xenobiotics in water—A review. Bioresour. Technol. 2021, 324, 124678.
- Ebrahiem, E.E.; Al-Maghrabi, M.N.; Mobarki, A.R. Removal of organic pollutants from industrial wastewater by applying photo-Fenton oxidation technology. Arab. J. Chem. 2017, 10, S1674–S1679.
- Thirunavukkarasu, A.; Nithya, R.; Sivashankar, R. A review on the role of nanomaterials in the removal of organic pollutants from wastewater. Rev. Environ. Sci. Bio/Technol. 2020, 19, 751–778.
- Kang, J.W. Removing environmental organic pollutants with bioremediation and phytoremediation. Biotechnol. Lett. 2014, 36, 1129–1139.
- Modi, S.; Yadav, V.K.; Gacem, A.; Ali, I.H.; Dave, D.; Khan, S.H.; Jeon, B.H. Recent and Emerging Trends in Remediation of Methylene Blue Dye from Wastewater by Using Zinc Oxide Nanoparticles. Water 2022, 14, 1749.
- Gnanamoorthy, G.; Ali, D.; Yadav, V.K.; Dhinagaran, G.; Venkatachalam, K.; Narayanan, V. New construction of Fe3O4/rGO/ZnSnO3 nanocomposites enhanced photoelectro chemical properties. Opt. Mater. 2020, 109, 110353.
- Waghmode, T.R.; Kurade, M.B.; Sapkal, R.T.; Bhosale, C.H.; Jeon, B.H.; Govindwar, S.P. Sequential photocatalysis and biological treatment for the enhanced degradation of the persistent azo dye methyl red. J. Hazard. Mater. 2019, 371, 115–122.
- Khan, S.H.; Yadav, V.K. Advanced oxidation processes for wastewater remediation: An overview. In Removal of Emerging Contaminants Through Microbial Processes, Springer: Singapore, 2021; pp. 71–93.
- Doll, T.E.; Frimmel, F.H. Removal of selected persistent organic pollutants by heterogeneous photocatalysis in water. Catal. Today 2005, 101, 195–202.
- Li, K.; Yan, L.; Zeng, Z.; Luo, S.; Luo, X.; Liu, X.; Guo, Y. Fabrication of H3PW12O40-doped carbon nitride nanotubes by one-step hydrothermal treatment strategy and their efficient visible-light photocatalytic activity toward representative aqueous persistent organic pollutants degradation. Appl. Catal. B Environ. 2014, 156, 141–152.
- Vaya, D.; Surolia, P.K. Semiconductor based photocatalytic degradation of pesticides: An overview. Environ. Technol. Innov. 2020, 20, 101128.
- Devipriya, S.; Yesodharan, S. Photocatalytic degradation of pesticide contaminants in water. Sol. Energy Mater. Sol. Cells 2005, 86, 309–348.
- Hadei, M.; Mesdaghinia, A.; Nabizadeh, R.; Mahvi, A.H.; Rabbani, S.; Naddafi, K. A comprehensive systematic review of photocatalytic degradation of pesticides using nano TiO2. Environ. Sci. Pollut. Res. 2021, 28, 13055–13071.
- Kurade, M.B.; Ha, Y.H.; Xiong, J.Q.; Govindwar, S.P.; Jang, M.; Jeon, B.H. Phytoremediation as a green biotechnology tool for emerging environmental pollution: A step forward towards sustainable rehabilitation of the environment. Chem. Eng. J. 2021, 415, 129040.
- Valizadeh, S.; Lee, S.S.; Baek, K.; Choi, Y.J.; Jeon, B.H.; Rhee, G.H.; Park, Y.K. Bioremediation strategies with biochar for polychlorinated biphenyls (PCBs)-contaminated soils: A review. Environ. Res. 2021, 200, 111757.
- Choi, E.; Cho, I.H.; Park, J. The effect of operational parameters on the photocatalytic degradation of pesticide. J. Environ. Sci. Health Part B 2004, 39, 53–64.
- Bano, K.; Kaushal, S.; Singh, P.P. A review on photocatalytic degradation of hazardous pesticides using heterojunctions. Polyhedron 2021, 209, 115465.
- Lwin, H.M.; Zhan, W.; Song, S.; Jia, F.; Zhou, J. Visible-light photocatalytic degradation pathway of tetracycline hydrochloride with cubic structured ZnO/SnO2 heterojunction nanocatalyst. Chem. Phys. Lett. 2019, 736, 136806.
- Amir, M.; Kurtan, U.; Baykal, A.; Sözeri, H. MnFe2O4@ heterogeneous nanocatalyst for degradation of industrial aqueous organic pollutants. J. Mater. Sci. Technol. 2016, 32, 134–141.
- Khan, S.H.; Pathak, B.; Fulekar, M.H. Synthesis, characterization and photocatalytic degradation of chlorpyrifos by novel Fe: ZnO nanocomposite material. Nanotechnol. Environ. Eng. 2018, 3, 13.
- Chen, L.; Maqbool, T.; Hou, C.; Fu, W.; Zhang, X. Mechanistic study of oxidative removal of bisphenol A by pristine nanocatalyst Mn3O4/peroxymonosulfate. Sep. Purif. Technol. 2022, 281, 119882.
- Tara, N.; Siddiqui, S.I.; Rathi, G.; Chaudhry, S.A.; Asiri, A.M. Nano-engineered adsorbent for the removal of dyes from water: A review. Curr. Anal. Chem. 2020, 16, 14–40.
- Yadav, V.K.; Ali, D.; Khan, S.H.; Gnanamoorthy, G.; Choudhary, N.; Yadav, K.K.; Manhrdas, S. Synthesis and characterization of amorphous iron oxide nanoparticles by the sonochemical method and their application for the remediation of heavy metals from wastewater. Nanomaterials 2020, 10, 1551.
- Yadav, V.K.; Choudhary, N.; Khan, S.H.; Malik, P.; Inwati, G.K.; Suriyaprabha, R.; Ravi, R.K. Synthesis and Characterisation of Nano-Biosorbents and Their Applications for Waste Water Treatment. In Handbook of Research on Emerging Developments and Environmental Impacts of Ecological Chemistry; IGI Global: Hershey, PA, USA, 2020; pp. 252–290.
- Harja, M.; Ciobanu, G. Eco-friendly nano-adsorbents for pollutant removal from wastewaters. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O., Martínez, L., Kharisov, B., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–22.
- Khan, S.H.; Alaie, S.A. Green nanomaterials for environmental applications. In Green Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 365–396.
- Ghosh, S.; Malloum, A.; Bornman, C.; Othmani, A.; Osagie, C.; Esfahani, Z.; Dehghani, M.H. Novel green adsorbents for removal of aniline from industrial effluents: A review. J. Mol. Liq. 2022, 345, 118167.
- Sadegh, H.; Ali, G.A. Potential applications of nanomaterials in wastewater treatment: Nanoadsorbents performance. In Research Anthology on Synthesis, Characterization, and Applications of Nanomaterials; IGI Global: Hershey, PA, USA, 2021; pp. 1230–1240.
- Kurniawan, T.A.; Sillanpää, M.E.; Sillanpää, M. Nanoadsorbents for remediation of aquatic environment: Local and practical solutions for global water pollution problems. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1233–1295.
- Subbaiah, M.P.; Kalimuthu, P.; Jung, J.; Jeon, B.H. Recent advances in effective capture of inorganic mercury from aqueous solutions through sulfurized 2D-material-based adsorbents. J. Mater. Chem. A 2021, 9, 18086–18101.
- Gul, A.; Khaligh, N.G.; Julkapli, N.M. Surface modification of carbon-based nanoadsorbents for the advanced wastewater treatment. J. Mol. Struct. 2021, 1235, 130148.
- Hussain, N.; Bilal, M.; Iqbal, H.M. Carbon-based nanomaterials with multipurpose attributes for water treatment: Greening the 21st-century nanostructure materials deployment. Biomater. Polym. Horiz. 2022, 1, 48–58.
- Hu, X.; You, S.; Li, F.; Liu, Y. Recent advances in antimony removal using carbon-based nanomaterials: A review. Front. Environ. Sci. Eng. 2022, 16, 48.
- Ali, I.; Alharbi, O.M.; Alothman, Z.A.; Alwarthan, A. Facile and eco-friendly synthesis of functionalized iron nanoparticles for cyanazine removal in water. Colloids Surf. B Biointerfaces 2018, 171, 606–613.
- Mahdavi, V.; Taghadosi, F.; Dashtestani, F.; Bahadorikhalili, S.; Farimani, M.M.; Ma’mani, L.; Khaneghah, A.M. Aminoguanidine modified magnetic graphene oxide as a robust nanoadsorbent for efficient removal and extraction of chlorpyrifos residue from water. J. Environ. Chem. Eng. 2021, 9, 106117.
- Izanloo, M.; Esrafili, A.; Jafari, A.J.; Farzadkia, M.; Behbahani, M.; Sobhi, H.R. Application of a novel bi-functional nanoadsorbent for the simultaneous removal of inorganic and organic compounds: Equilibrium, kinetic and thermodynamic studies. J. Mol. Liq. 2019, 273, 543–550.
- Mohammadi, F.; Esrafili, A.; Kermani, M.; Behbahani, M. Application of modified magnetic nanoparticles with amine groups as an efficient solid sorbent for simultaneous removal of 2, 4-Dichlorophenoxyacetic acid and 2-methyl-4-chlorophenoxyacetic acid from aqueous solution: Optimization and modeling. J. Iran. Chem. Soc. 2018, 15, 421–429.
- Dehghani, M.H.; Kamalian, S.; Shayeghi, M.; Yousefi, M.; Heidarinejad, Z.; Agarwal, S.; Gupta, V.K. High-performance removal of diazinon pesticide from water using multi-walled carbon nanotubes. Microchem. J. 2019, 145, 486–491.
- Kalhor, M.M.; Rafati, A.A.; Rafati, L.; Rafati, A.A. Synthesis, characterization and adsorption studies of amino-functionalized silica nano hollow sphere as an efficient adsorbent for removal of imidacloprid pesticide. J. Mol. Liq. 2018, 266, 453–459.
- Sahoo, S.K.; Padhiari, S.; Biswal, S.K.; Panda, B.B.; Hota, G. Fe3O4 nanoparticles functionalized GO/g-C3N4 nanocomposite: An efficient magnetic nano adsorbent for adsorptive removal of organic pollutants. Mater. Chem. Phys. 2020, 244, 122710.
- Nikzad, S.; Amooey, A.A.; Alinejad-Mir, A. Adsorption of diazinon from aqueous solutions by magnetic guar gum-montmorillonite. Chem. Data Collect. 2019, 20, 100187.
- Peralta, M.E.; Mártire, D.O.; Moreno, M.S.; Parolo, M.E.; Carlos, L. Versatile nano adsorbents based on magnetic mesostructured silica nanoparticles with tailored surface properties for organic pollutants removal. J. Environ. Chem. Eng. 2021, 9, 104841.
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254.
- Mustereţ, C.P.; Teodosiu, C. Removal of Persistent Organic Pollutants from Textile Wastewater By Membrane Processes. Environ. Eng. Manag. J. 2007, 6, 175–187.
- Luis, P.; Saquib, M.; Vinckier, C.; Van der Bruggen, B. Effect of membrane filtration on ozonation efficiency for removal of atrazine from surface water. Ind. Eng. Chem. Res. 2011, 50, 8686–8692.
- Fujii, S.; Polprasert, C.; Tanaka, S.; Hong Lien, N.P.; Qiu, Y. New POPs in the water environment: Distribution, bioaccumulation and treatment of perfluorinated compounds–a review paper. J. Water Supply Res. Technol. AQUA 2007, 56, 313–326.
- Tibi, F.; Charfi, A.; Cho, J.; Kim, J. Fabrication of polymeric membranes for membrane distillation process and application for wastewater treatment: Critical review. Process Saf. Environ. Prot. 2020, 141, 190–201.
- Sarkar, S.; Chakraborty, S. Nanocomposite polymeric membrane a new trend of water and wastewater treatment: A short review. Groundw. Sustain. Dev. 2021, 12, 100533.
- Vázquez-Núñez, E.; Molina-Guerrero, C.E.; Peña-Castro, J.M.; Fernández-Luqueño, F.; de la Rosa-Álvarez, M. Use of nanotechnology for the bioremediation of contaminants: A review. Processes 2020, 8, 826.
- Ng, L.Y.; Chua, H.S.; Ng, C.Y. Incorporation of graphene oxide-based nanocomposite in the polymeric membrane for water and wastewater treatment: A review on recent development. J. Environ. Chem. Eng. 2021, 9, 105994.
- Lau, W.J.; Ismail, A.F. Polymeric nanofiltration membranes for textile dye wastewater treatment: Preparation, performance evaluation, transport modelling, and fouling control—A review. Desalination 2009, 245, 321–348.
- Oatley-Radcliffe, D.L.; Walters, M.; Ainscough, T.J.; Williams, P.M.; Mohammad, A.W.; Hilal, N. Nanofiltration membranes and processes: A review of research trends over the past decade. J. Water Process Eng. 2017, 19, 164–171.
- Mulyanti, R.; Susanto, H. Wastewater treatment by nanofiltration membranes. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 142, p. 012017.
- Yoon, Y.; Lueptow, R.M. Removal of organic contaminants by RO and NF membranes. J. Membr. Sci. 2005, 261, 76–86.
- Agenson, K.O.; Oh, J.I.; Urase, T. Retention of a wide variety of organic pollutants by different nanofiltration/reverse osmosis membranes: Controlling parameters of process. J. Membr. Sci. 2003, 225, 91–103.
- Paul, M.; Jons, S.D. Chemistry and fabrication of polymeric nanofiltration membranes: A review. Polymer 2016, 103, 417–456.
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565.
- Molinari, R.; Mungari, M.; Drioli, E.; Di Paola, A.; Loddo, V.; Palmisano, L.; Schiavello, M. Study on a photocatalytic membrane reactor for water purification. Catal. Today 2000, 55, 71–78.
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184.
- Argurio, P.; Fontananova, E.; Molinari, R.; Drioli, E. Photocatalytic membranes in photocatalytic membrane reactors. Processes 2018, 6, 162.
- Zhang, W.; Ding, L.; Luo, J.; Jaffrin, M.Y.; Tang, B. Membrane fouling in photocatalytic membrane reactors (PMRs) for water and wastewater treatment: A critical review. Chem. Eng. J. 2016, 302, 446–458.
- Marchetti, P.; Jimenez Solomon, M.F.; Szekely, G.; Livingston, A.G. Molecular separation with organic solvent nanofiltration: A critical review. Chem. Rev. 2014, 114, 10735–10806.
- Hilal, N.; Al-Zoubi, H.; Darwish, N.A.; Mohamma, A.W.; Arabi, M.A. A comprehensive review of nanofiltration membranes: Treatment, pretreatment, modelling, and atomic force microscopy. Desalination 2004, 170, 281–308.
- Tul Muntha, S.; Kausar, A.; Siddiq, M. Advances in polymeric nanofiltration membrane: A review. Polym. Plast. Technol. Eng. 2017, 56, 841–856.
- Shon, H.K.; Phuntsho, S.; Chaudhary, D.S.; Vigneswaran, S.; Cho, J. Nanofiltration for water and wastewater treatment–a mini review. Drink. Water Eng. Sci. 2013, 6, 47–53.
- Karimi, H.; Rahimpour, A.; Kebria, M.R.Z. Pesticides removal from water using modified piperazine-based nanofiltration (NF) membranes. Desalination Water Treat. 2016, 57, 24844–24854.
- Wang, Y.; He, L.; Li, Y.; Jing, L.; Wang, J.; Li, X. Ag NPs supported on the magnetic Al-MOF/PDA as nanocatalyst for the removal of organic pollutants in water. J. Alloys Compd. 2020, 828, 154340.
More
Information
Subjects:
Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.5K
Entry Collection:
Wastewater Treatment
Revisions:
3 times
(View History)
Update Date:
02 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





