Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Virendra Kumar Yadav and Version 3 by Jessie Wu.
Persistent organic pollutants (POPs) are a group of hazardous chemical compounds that originate from anthropogenic activities during production, utilization, and disposal. They can impact living beings and the environment adversely because of their ease of transportation by wind and water. The level of hazardous persistent organic pollutants is increasing every day in the environment.
- nanomaterials
- persistent organic pollutants
- remediation
1. Persistent Organic Pollutants (POPs)Ps, Source, and Fate
Persistent organic pollutants (POPs) have grabbed significant attention worldwide. POPs are defined as xenobiotic chemical compounds of different origins, but all have similar characteristics, i.e., high toxicity, bioaccumulation, hydrophobicity, environmental persistence, and ability to transfer via the FC [1][7]. POPs are carbon-containing chemicals, and due to their higher solubility in the lipids, they tend to become accumulated among the fatty tissues and can disrupt the endocrine system of organisms, therefore often referred to as endocrine disruptors (EDs) [2][8]. The physicochemical properties of POPs are responsible for their dispersion and distribution in the environment; POPs have low water solubility (log Kow 3–7); therefore, they have high adsorption, low degradation, and hydrophobic nature [3][9].
Various Categories of POPs
The utilization of POPs was restricted since 1970 in various parts of the United States of America (USA) and Europe. Moreover, there was a strict prohibition on the release of such POPs in both the above-mentioned continents [4][10]. The use and consumption of pesticides increased abruptly after the green revolution; at that time, the hazardous effects of POPs were unknown. Lately, the toxic effects of pesticides have emerged globally. The general public started to understand the toxicity of pesticides and other organic contaminants. After the Stockholm convention, POP was placed into three categories, i.e., pesticides, by-products, and industrial chemicals [5][11]. Figure 1 depicts the type and different categories of POPs.

Figure 1.
Type and categories of POPs.
POPs are the range of synthetic hazardous chemicals, produced either intentionally or unintentionally [6][12]. Pesticides fall under the category of the intentionally produced chemicals used to control pests in agriculture and houses; DDT is a known such example that was banned globally due to its extreme toxicity [7][13]. Others are industrial products or unintentionally produced chemicals, i.e., dioxins. The new class of POPs includes types of emerging contaminants such as polybrominated diphenyl ethers (PBDE), perfluorinated compounds (PF), and a list of new contaminants added day by day.
POPs can be classified as Organochlorine Pesticides (OCP); hexachlorobenzene (HCB) and other polychlorinated benzenes (PCBzs); PAHs; polychlorinated naphthalenes; PCBs; polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD and PCDF); and other contaminants of emerging concerns [8][14]. At first, during the Stockholm convention (2001), the participating countries decided to minimize or strike out the production, usage, and release of 12 key POPs popularly referred to as “the dirty dozen”. Later, ten more chemical substances were added to the group of POPs after two amendments (2009 and 2011) [9][15]. Table 1 summarizes the list of twenty-two POPs after the Stockholm Convention.
Table 1.
List of POPs as per Stockholm Convention.
| S. No | Chemical | Category |
|---|---|---|
| As per the 2001 Amendment (The Dirty Dozen) | ||
| 1 | PCB | Industrial waste/byproduct |
| 2 | PCD | Byproduct |
| 3 | PCDF | Byproduct |
| 4 | Chlordane | Pesticide |
| 5 | Mirex | Pesticide |
| 6 | Endrin | Pesticide |
| 7 | Aldrin | Pesticide |
| 8 | Dieldrin | Pesticide |
| 9 | HCB | Pesticide |
| 10 | Heptachlor | Pesticide |
| 11 | Toxaphene | Pesticide |
| 12 | DDT | Pesticide |
| As per the 2009 Amendment | ||
| 13 | Lindane | Pesticide |
| 14 | Chlordecone | Pesticide |
| 15 | Pentachloro benzene | Pesticide and Byproduct |
| 16 | Alpha-HCH | Pesticide and Byproduct |
| 17 | Beta-HCH | Pesticide and Byproduct |
| 18 | PFO and constituents PFOSF | Industrial |
| 19 | Hexabromobiphenyl | Industrial |
| 20 | Hexa-BDE and Hepta-BDE | Industrial |
| 21 | Tetra-BDE and Penta-BDE | Industrial |
| As per the 2011 Amendment | ||
| 22 | Endosulfan | Pesticide |
POPs can sustain in the environment for prolonged periods, taking decades or several centuries to be completely degraded. Due to their physicochemical properties, POPs have the tendency to travel long distances and resist degradation (biological and chemical degradation), which allows them to bioaccumulate to a deeper level via biomagnification, and their exposure can lead to severe damage to health and the environment [10][16]. Several studies suggest a range of adverse effects induced by POPs, as most of them are semivolatile compounds and can easily absorb onto the atmospheric particles and migrate into the water, air, and soil media [11][12][13][17,18,19]. POPs are rarely found in one environmental medium but are present in all media, and if tested will be found to be present in all media across the world [14][20]. POPs are found in agricultural wastes, chemical, and electronic industry waste, as well as pharmaceutical waste.
POPs are severely toxic that even the smallest concentration of them is found to be highly fatal to the organisms. POPs are generally resistant to chemical, biological, and photodegradation as they have low solubility and it is quite difficult to degrade POPs using traditional wastewater-treatment technology [15][16][21,22]. In the recent past, remediation of POPs was achieved by advanced wastewater-treatment technologies or by the combination of one or two methods. However, the most important question arises: Despite all technologies, why are POPs resistant to most degradation processes? POPs generally exhibit lipid solubility, and because of this reason, they tend to accumulate in fatty tissues of organisms. Moreover, halogenated compounds show great stability toward hydrolysis and photolytic degradation due to the nonreactivity of c-cl bonds [17][23]. The stability towards degradation and lipophilicity of POPS makes them compounds of particular concern. POPs are also divided into four levels based on their toxicity:
- (i)
-
most hazardous chemical [restricted for production and utilization];
- (ii)
-
medium level chemicals [confined to use during the production];
- (ii)
-
unintentional discharge of chemical;
- (iv)
-
use of chemicals under investigation.
As we know, POPs are extremely toxic halogenated compounds that largely impact humans either through point or non-point sources. The organic pollutants generally consist of personal care products (PCP), pesticides, organic dyes, endocrine disruptors, pharmaceutical waste, and other such contaminants of emerging concern [18][24]. The release of POPs in water bodies causes disturbance to the aquatic food chain, as POPs tend to bioaccumulate, and EPA indicated that the rate of disease caused by POPs is very high in coastal and marine ecosystems [19][25]. Thus, POPs tend to impact every living organism in some or the other way due to their hazardous nature. There arises the need for the remediation of such pollutants from the environment by applying advanced techniques, but the results show that the conventional technologies were not efficient for the complete removal of POPs as they are simply transforming the pollutants from one phase to another rather than the complete elimination [20][26]. With the advancement of nanotechnology for environment application, the focus has been shifted to the removal of POPs using nanomaterials. The present resviearchw significantly highlights the utilization of nanotechnology for the removal of POPs from the environment.
2. Advanced Nanotechnological Approaches for Removal of Persistent Organic PollutantPs
2.1. Nanocatalysis
With the ineffectiveness of conventional technologies to completely degrade and mineralize the organic pollutants, there arises the need to develop a green, innovative, and sustainable method that can destroy the POPs with much less energy consumption and chemical utilization [21][22][23][101,102,103]. Therefore, the scientific community has started looking for advanced oxidation processes as a low-cost and effective method that is proficient in oxidizing and mineralizing a range of pollutants, including POPs, due to their strong oxidizing radicals [24][104]. The use of semiconducting wide-bandgap nanomaterials for the treatment of contaminants into eco-friendly compounds comes under nanocatalysis. The semiconductor metal and metal-oxide nanomaterials have gained significant attention in POPs treatment sustainably. Several types of nanocatalyst are used for the effective degradation of POPs from wastewaters such as Fenton-based catalyst, electrocatalyst, photocatalyst, and even doped multifunctional nanocatalyst [25][26][105,106]. Photocatalysis/nanocatalysis is a well-known AOP; it is used to enhance the biodegradability of POPs by using oxidants to degrade organic pollutants by the release of highly reactive oxygen species (ROS) for the chemical reaction to occur [27][107]. Photocatalysis involves the catalytic activation in the presence of light and relies on the generation of strong radicals, i.e., H2O2, O2 •–, O3, and OH radicals, which destroy almost all organic molecules [28][108]. Photocatalysis is even effective for the remediation of volatile organic compounds (VOCs) such as PCBs, Dioxins, and PHA by producing free radicals. The process of photocatalysis starts as the nanocatalyst with a wide bandgap (such as ZnO, TiO2, WO3) becomes photoexcited in the presence of a light source (natural or artificial) and oxygen used to degrade POPs [29][30][109,110]. The photocatalytic-degradation process ideally involves the following steps, as shown in Figure 2.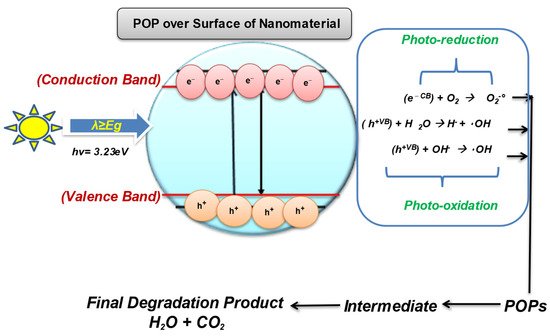
Figure 2.
Photocatalysis over the surface of the nanomaterial.
2.2. Nanoadsorption
Nanoadsorbents provide high sorption efficiency because of their extremely large surface area and sorption sites, tunable pore size, much lower intraparticle-diffusion distance, and high surface activity for effective adsorption of a vast range of organic and other pollutants [38][39][40][117,118,119]. The advantage of using nanoadsorbents is that they can be easily functionalized to make them highly selective for any pollutants [41][120]. The adsorption process has been found to be successful for the remediation of POPs such as hydrocarbon, dyes, phenols, detergents, pharmaceuticals, pesticides, and biphenyls. Figure 3 shows the types of carbon-based nanoadsorbent material with their benefits. Nanoadsorption is an easy and safe process for the remediation of POPs from water bodies. Among various technologies, nanoadsorption so far emerges as a widely efficient method for the remediation of POP. Several studies prove the efficiency of nanomaterials for the adsorption of various POPs from the wastewater, as more than 90% removal efficiency was achieved in most of the studies for up to ten cycles [42][121]. The adsorption efficiency of nanomaterial is mainly monitored by producing a complex with the surface of metal oxides and enduring a one-electron oxidation reaction under visible irradiation. Nanoadsorption is based on electrostatic interactions, hydrogen bonding, and hydrophobic interactions such as van der Waals, electron donor–acceptor, etc. [43][122]. Nanomaterials such as clay, zeolite, alumina, metal/metal oxides, activated carbon, carbon-based nanomaterials, nanocomposites, nanosheets, nanotubes, chitosan-based polymers, and graphene-based nanomaterials are extensively applied in the process of nanoadsorption [44][123]. For effective removal of POPs, the use of magnetic nanoparticles, especially iron oxide, has led away to a new class of magnetic-separation strategies. Microporous structures present in activated carbon aid the adsorption efficiency in the removal of POPs [45][46][124,125]. Carbon-based nanoadsorbents tend to interact with contaminants due to hydrophobicity, hydrogen bonding, and covalent and electrostatic interactions [47][126]. Each form has several adsorption sites that can absorb the organic pollutants due to their flexibility. Both single-walled and multiwalled carbon nanotubes have been surface-modified by increasing the porosity to generate high-energy sites to adsorb more organic pollutants over increasing the efficiency of manifolds [48][49][127,128].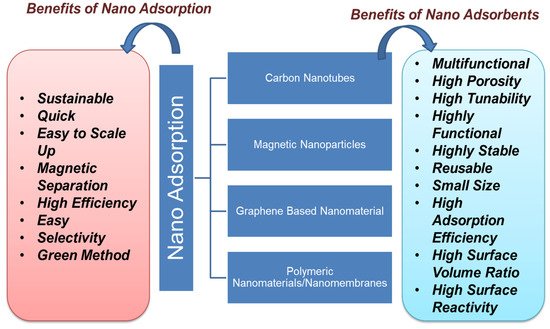
Figure 3.
Types and benefits of nanoadsorption.
