Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mario Allegra | -- | 2362 | 2022-07-29 11:48:40 | | | |
| 2 | Vivi Li | Meta information modification | 2362 | 2022-08-01 04:00:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Restivo, I.; Attanzio, A.; Tesoriere, L.; Allegra, M. Suicidal Erythrocyte Death in Metabolic Syndrome. Encyclopedia. Available online: https://encyclopedia.pub/entry/25655 (accessed on 07 February 2026).
Restivo I, Attanzio A, Tesoriere L, Allegra M. Suicidal Erythrocyte Death in Metabolic Syndrome. Encyclopedia. Available at: https://encyclopedia.pub/entry/25655. Accessed February 07, 2026.
Restivo, Ignazio, Alessandro Attanzio, Luisa Tesoriere, Mario Allegra. "Suicidal Erythrocyte Death in Metabolic Syndrome" Encyclopedia, https://encyclopedia.pub/entry/25655 (accessed February 07, 2026).
Restivo, I., Attanzio, A., Tesoriere, L., & Allegra, M. (2022, July 29). Suicidal Erythrocyte Death in Metabolic Syndrome. In Encyclopedia. https://encyclopedia.pub/entry/25655
Restivo, Ignazio, et al. "Suicidal Erythrocyte Death in Metabolic Syndrome." Encyclopedia. Web. 29 July, 2022.
Copy Citation
Eryptosis is a coordinated, programmed cell death culminating with the disposal of cells without disruption of the cell membrane and the release of endocellular oxidative and pro-inflammatory milieu. While providing a convenient form of death for erythrocytes, dysregulated eryptosis may result in a series of detrimental and harmful pathological consequences highly related to the endothelial dysfunction (ED). Metabolic syndrome (MetS) is described as a cluster of cardiometabolic factors (hyperglycemia, dyslipidemia, hypertension and obesity) that increases the risk of cardiovascular complications such as those related to diabetes and atherosclerosis.
eryptosis
metabolic syndrome
diabetes
dyslipidemia
hypertension
obesity
atherosclerosis
vascular damage
oxidative stress
endothelial dysfunction
1. Eryptosis
1.1. Mechanisms
The lifespan of erythrocytes (RBC) is limited by senescence [1][2]. Within 100–120 days, aged RBC are cleared from circulation through a mechanism involving the clustering and/or breakdown of the anion exchanger protein band 3 (AE1). This is regarded as the central step of an immune-mediated pathway, eventually leading to the disruption of the AE1-dependent cytoskeletal connections to the lipid bilayer. The process, then, culminates with vesicle generation, volume, density and shape changes and senescent antigens uncovering [2].
During the course of their natural ageing and prior to senescence, RBC may experience injury that weakens their integrity, function and survival. Under these circumstances, RBC undergo hemolysis that implies the rupture of the cell membrane and the release of hemoglobin (Hb). Should this happened, Hb is filtered by renal glomeruli and subsequently precipitates in the acidic lumen of renal tubules, eventually leading to renal failure [3][4].
Alternatively, RBC may enter a suicidal death program named eryptosis [5]. Similar to the apoptotic death of nucleated cells, eryptosis is a coordinated, programmed cell death eventually culminating with the disposal of cells without disruption of the cell membrane and release of intracellular oxidative and pro-inflammatory milieu [6]. Along these lines, eryptosis can be regarded as a “soft” mechanism removing, prior to hemolysis, defective, infected or otherwise potentially deleterious RBC [7].
The hallmarks of eryptosis are similar to those of apoptosis i.e., cell shrinkage, membrane blebbing and exposure of phosphatidylserine (PS) on the cell membrane. The process can be triggered by several physio-pathological cell stressors, such as hypertonic shock, energy deprivation, increased temperature and oxidative stress [4][8]. Moreover, a number of xenobiotics, heavy metals, drugs and physiological mediators can also be involved in the activation of the process [8][9].
From a mechanistic perspective, eryptosis is orchestrated by an amazingly complex cellular machinery involving Ca++, reactive oxygen and nitrogen species (RONS), ceramide, caspases, nitric oxide (NO) and a variety of kinases (Figure 1).
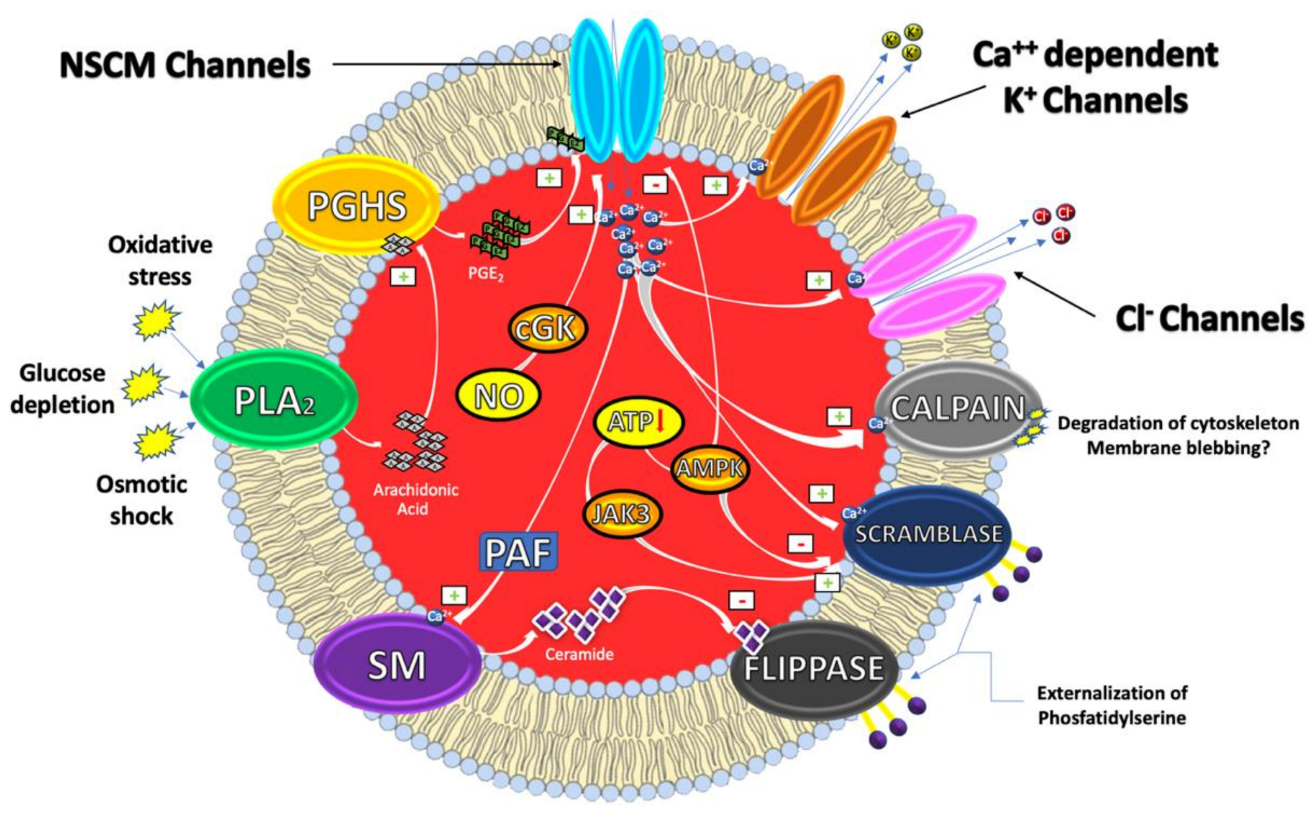
Figure 1. Eryptosis signaling.
It has, indeed, been demonstrated that cytosolic Ca2+ concentrations can play a pivotal role in the eryptotic process triggered by oxidative stress, glucose depletion and osmotic shock. Under these conditions, the pro-eryptotic stimuli activates phospholipase A2 (PLA2) that releases arachidonic acid (AA) in turn metabolized by prostaglandin endoperoxide synthase (PGHS). The resulting increase of prostaglandin E2 (PGE2) synthesis then activates and opens nonselective cationic membrane (NSCM) channels, leading to an increase of endocellular levels of Ca++ that activates several molecular targets including Ca++-dependent K+ channels. The subsequent membrane hyperpolarization, increases the electrical driving force for Cl− exit. The resulting cellular loss of KCl, with osmotically obliged water efflux, determines RBC shrinkage [9][10]. On the other hand, Ca++ influx, together with platelet activating factor (PAF), activates membrane sphingomyelinase (SM) responsible for the increase of ceramide levels that plays a crucial role in eryptosis. Indeed, Ca++ and ceramide, respectively, activate scramblase and inhibit flippases involved in PS externalization. Finally, an increase of Ca++ levels also stimulates cysteine-calpain endopeptidases, responsible for the degradation of RBC cytoskeleton and for membrane blebbing that increases the adhesiveness of RBC. Once the exposure of PS takes place, RBC is recognized by circulating macrophages with specific PS receptors and engulfed to ensure its effective removal from circulation [4][11].
Beside Ca++ and/or ceramide increase, other signaling pathways, involving kinases, NO and caspases further contribute to modulate the eryptotic process. Indeed, in RBC subjected to energy depletion or oxidative stress, Janus-activated kinase 3 (JAK3) can be phosphorylated, activated and stimulates cell membrane scrambling. On the other hand, hyperthermia or energy deprivation can activate the energy sensing AMP-activated kinase 1 α (AMPK1 α) that inhibits eryptosis. Furthermore, during oxidative stress conditions, eryptosis is also stimulated through the activation of casein kinase 1 α (CK1 α) while it is further inhibited by cGMP-dependent protein kinase (cGKI). This latter can be stimulated by NO, usually stored in RBC, and released upon Hb deoxygenation [9][12].
Interestingly, NO together with NO-donors such as nitroprusside, have been demonstrated to inhibit Ca++-induced eryptosis. The mechanism through which NO interferes with the Ca++ signaling seems to involve downstream mediators without affecting Ca++ levels and involving caspases [13].
As in nucleated cells, caspases are expressed in RBC, where they cleave the AE1 and stimulate PS exposure. However, in contrast to apoptosis of nucleated cells, they do not always play a dominant role in the eryptotic process. Caspase activation is, indeed, involved in the eryptosis induced by leukotrienes and α-lipoic acid and fostered by oxidative stress. Conversely, Ca++ entry and Ca++-dependent cell membrane scrambling do not require the activation of caspases [9][12].
While providing a convenient form of death for RBC (by counteracting hemolysis and its complications), dysregulated eryptosis may result in a series of detrimental and harmful pathological consequences. In this regard, it has been shown that eryptosis underlies and fosters several different clinical conditions or diseases such as anemias, cytostatic-induced malignancies, sepsis, psychosis and malaria [8][12][13].
2. Eryptosis, Hyperglycemia and Diabetes
DM is a group of metabolic disorders hallmarked by a long-standing hyperglycemia, oxidative stress and reduced secretion and/or efficacy of insulin. All these metabolic dysregulations eventually lead to vascular complications that may enhance the risk of stroke, heart and kidney diseases [14]. Retinopathy, nephropathy, neuropathy and atherosclerosis are, indeed, among the most serious DM long-term complications [14][15] and make DM an alarming clinical and public health problem [16][17]. The International Diabetes Federation estimates that just under half a billion people are living with DM worldwide and the number is projected to increase by 25% in 2030 and by 51% in 2045 [18].
RBC lifespan has been shown to be reduced in DM patients [19]. In agreement, anemia is prevalent in 14–45% of DM-affected subjects and cannot be attributed to a decreased RBC formation, as reticulocyte number is increased [20][21]. Instead, increasing evidence has revealed that DM-related anemia may result, at least in part, from an enhanced eryptosis [22][23].
From a mechanistic perspective, DM-induced eryptosis has been related to glycoxidation, an oxidative stress-dependent glycation of biomolecules regarded as a DM hallmark [24][25][26]. This process, via hyperglycemia-mediated and oxidative stress-dependent mechanisms, leads to a dysfunctional alteration of RBC morphology and culminates with the increase of PS levels on cell surface [8].
According to the glycoxidation hypothesis, a long-lasting increase of blood glucose levels accelerates the glycation of free amino groups in RBC membrane proteins. Such non-enzymatic modification of proteins leads to the formation and accumulation of both early and advanced glycation end-products, EGEs and AGEs, respectively [27][28][29]. The latter mediate cross-linking of cellular biomolecules, eventually leading to the accumulation of misfolded, aggregated and nonfunctional proteins. When hyperglycemia is associated with oxidative stress, less reactive EGEs are increasingly been converted into higher reactive AGEs. Among the AGEs, carboxymethyl-lysine (CML), carboxyethyl-lysine (CEL) and methylglyoxal (MG) play the major role in DM.
CML and CEL induce insulin resistance, β-cell dysfunction, vascular toxicity and are involved in the development of diabetic complications [29][30][31]. Relevantly, they have been recovered in DM patients at a plasma concentration of 4.9 and 1.7 μM, respectively [32][33].
In addition to their effects on the liver, pancreas, muscle and EC, a novel physio-pathological role for both CML and CEL has recently emerged. Indeed, these AGEs have been shown to induce eryptosis-related morphological changes in RBC that completely lose their normal discoid shape and become acanthocytes [27]. The mechanisms through which CML and CEL promote these dysfunctional and morphological RBC modifications seems to be related to the pro-oxidant nature of these compounds and mediated by cytoskeleton modifications.
MG derives from the metabolism of sugars, amino acids and lipids and is able to react with free arginine residues of proteins forming Arg-pyrimidine adducts [34]. Interestingly, this dicarbonylic compound has gained significance in the etio-pathogenesis of DM, being able to impair insulin signaling and mediate DM-related vascular complications [35][36]. Relevantly, in vivo studies clearly show an increase of MG concentrations in the plasma of DM patients, in a range between 0.3 and 0.5 µM [37][38][39][40].
Along with CML and CEL, MG can also activate the eryptotic program at concentrations of physio-pathological relevance [41]. Interestingly enough, not only has MG been recovered in the plasma of DM patients, but also it can be generated inside RBC. In vitro evidence clearly showed that MG levels are increased in RBC exposed to high-glucose levels in response to the increase in the triosephosphate pool of glycolytic intermediates [42][43].
From a mechanistic perspective the pro-eryptotic role of MG may be connected with its ability to induce energy depletion and oxidative stress without involving alterations of Ca++ homeostasis [41]. Indeed, MG can interfere with glyceraldehyde-3-phosphate dehydrogenase, inhibiting glycolysis and thus decreasing ATP intracellular concentrations. The resulting energy depletion may, in turn, compromise GSH synthesis, thus interfering with anti-oxidative defense. Relevantly, DM patients exhibit a significant reduction of GSH plasma concentration and, interestingly, this condition positively correlates with diabetic complications. Moreover, and besides its effect on GSH levels, MG can also impair RBC antioxidative defenses, inactivating glutathione peroxidase (GPx). Other mechanisms could support MG-induced eryptosis, such as crosslinking of matrix proteins or glycosylation of plasma proteins [41].
Beside specific, AGEs-related pro-eryptotic mechanisms, other crucial molecular details have been unveiled in the activation of the DM-induced, RBC suicidal death program.
In contrast with other pro-eryptotic stimuli, DM induces eryptosis through a mechanism that appears to be independent of intracellular Ca++ variations. In this regard, an inhibition of the cation channel, possibly related to the employment of Ca++ channel blocking drugs by DM patients, has been suggested. Moreover, the lack of increased intracellular Ca++ concentration could explain the absence of cell shrinkage, in contrast to the majority of eryptotic stimulators that decreases RBC volume through the activation of Ca++-sensitive K+ channels [44].
The activation of eryptosis in DM has also been related to intracellular ceramide levels [44]. The amount of this lipid signaling molecule is significantly upregulated in RBC from DM patients. What is noteworthy, is that it correlates with the percentage of PS exposure, suggesting it could be an important candidate in the pathogenesis of DM-related eryptosis [44].
As previously stated, caspase-3 activation is a key element in the eryptotic process triggered by a number of conditions. In line with this, in vivo studies showed that RBC from DM patients undergo eryptosis through the activation of caspase-3, which is strongly correlated to the extent of hyperglycemia and extracellular oxidative stress. Caspase-3 activation can be considered as an effector mechanism contributing, together with other molecular machineries, to the reduction of diabetic RBC lifespan [23].
Mechanistic evaluations of DM-related eryptosis should also consider the therapeutic approach of DM patient. Indeed, a wide variety of xenobiotics and drugs can influence RBC morphology and PS-exposure [4]. Eryptosis, thus, can also be modulated by treatment of the patients, e.g., with the use of Ca++ channel blockers or antioxidants. Moreover, diabetic complications, such as nephropathy, dehydration, iron deficiency and inflammation, may also contribute to the activation of eryptosis [4].
Beyond mechanistic investigations and in the light of the ability of eryptotic RBC to induce ED, another key issue is to establish whether eryptosis can exert a pathophysiological role in DM-related vascular complications.
These life-threatening events associated with DM are deeply dependent on glycoxidation-induced microcirculation impairment and ED [14][26]. Relevantly, ex vivo evidence shows that enhanced PS exposure on eryptotic RBC from DM patients fosters RBC adhesion to the EC [45]. It has been suggested that the loss of lipid asymmetry could be responsible for the increased adhesiveness of RBC to vascular endothelium and for their ability to impair microcirculation [45]. Moreover, clinical data suggest that DM-induced eryptosis, through the impairment of RBC deformability induced by caspase-3 activation, could contribute to the pathogenesis of the hypertensive complications of DM patients [23]. Overall, ex vivo and in vivo evidence could support a role for eryptosis in the development of ED associated to DM-related vascular complications. Additional experimental effort is required to gain more mechanistic insights and to further characterize its clinical relevance.
Besides its pathological consequences, the activation of the eryptotic process in DM also deserves particular attention from a diagnostic perspective.
Glycoxidation-mediated reduction of the RBC lifetime may, indeed, affect the interpretation of glycated Hb (HbA1c) concentration, which is widely used to monitor metabolic control in diabetic patients. In fact, the degree of Hb glycation strongly depends on both blood glucose concentration and time of exposure. RBC life span negatively correlates with glycemia and averages ~80 days in patients with poor metabolic control as compared to the normal range of 123 days [46]. Accordingly, the resulting HbA1c value maybe lower in diabetic patients with high plasma levels of AGEs and could not fully mirror an eventual poor metabolic control.
3. Eryptosis, Dyslipidemia and Atherosclerosis
Dyslipidemia of MetS patients results from the concerted action of insulin resistance and obesity and has recently been described as “metabolic dyslipidemia” [47]. Clinically, it is shown as hypertriglyceridemia with low HDL plasma levels and increased small dense LDL/LDL ratio. According to data from 2009 to 2012, such a condition affects more than 100 million U.S. adults, aged 20 years or older [48].
Over the last years, a novel modulatory role for RBC in the development of dyslipidemia-induced cardiovascular complications, such as those related to atherosclerosis, has emerged. RBC, indeed, can become entrapped within atherosclerotic lesions at sites of intraplaque hemorrhage. Here they are actively taken up by macrophages, with the extent of RBC extravasation and foam cell formation, proportional to plaque development [49][50][51]. Along these lines, the involvement of eryptosis in dyslipidemia-induced vascular complications has started to gain researchers’ attention.
Clinical evidence shows that RBC from dyslipidemic patients are characterized by a grade of eryptosis significantly higher than healthy subjects [52]. The mechanisms, underlying the activation of the eryptotic program in such patients, do not involve any alteration of Ca++ homeostasis. Rather, the increased levels of PS externalization on cell membrane appear to be dependent on endocellular oxidative stress. Coherently, RBC form dyslipidemic patients show reduced amount of plasma GSH and increased levels of lipid peroxidation markers, with respect to healthy subjects [52].
References
- Bosman, G.J.C.G.M.; Willekens, F.L.A.; Werre, J.M. Erythrocyte aging: A more than superficial resemblance to apoptosis? Cell. Physiol. Biochem. 2005, 16, 1–8.
- Arese, P.; Turrini, F.; Schwarzer, E. Band 3/complement-mediated recognition and removal of normally senescent and pathological human erythrocytes. Cell. Physiol. Biochem. 2005, 16, 133–146.
- Lang, F.; Qadri, S.M. Mechanisms and significance of eryptosis, the suicidal death of erythrocytes. Blood Purif. 2012, 33, 125–130.
- Lang, E.; Qadri, S.M.; Lang, F. Killing me softly—Suicidal erythrocyte death. Int. J. Biochem. Cell Biol. 2012, 44, 1236–1243.
- Haus, J.M.; Kashyap, S.R.; Kasumov, T.; Zhang, R.; Kelly, K.R.; Defronzo, R.A.; Kirwan, J.P. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 2009, 58.
- Lang, F.; Gulbins, E.; Lang, P.A.; Zappulla, D.; Föller, M. Ceramide in suicidal death of erythrocytes. Cell. Physiol. Biochem. 2010, 26, 21–28.
- Adams, J.M.; Pratipanawatr, T.; Berria, R.; Wang, E.; DeFronzo, R.A.; Sullards, M.C.; Mandarino, L.J. Ceramide Content Is Increased in Skeletal Muscle from Obese Insulin-Resistant Humans. Diabetes 2004, 53.
- Lang, E.; Lang, F. Triggers, inhibitors, mechanisms, and significance of eryptosis: The suicidal erythrocyte death. Biomed. Res. Int. 2015.
- Repsold, L.; Joubert, A.M. Eryptosis: An Erythrocyte’s Suicidal Type of Cell Death. Biomed. Res. Int. 2018.
- Pretorius, E.; Du Plooy, J.N.; Bester, J. A Comprehensive Review on Eryptosis. Cell. Physiol. Biochem. 2016, 39, 1977–2000.
- Lang, F.; Abed, M.; Lang, E.; Föller, M. Oxidative stress and suicidal erythrocyte death. Antioxid. Redox Signal. 2014, 21.
- Qadri, S.M.; Bissinger, R.; Solh, Z.; Oldenborg, P.A. Eryptosis in health and disease: A paradigm shift towards understanding the (patho) physiological implications of programmed cell death of erythrocytes. Blood Rev. 2017, 31, 349–361.
- Lang, E.; Lang, F. Mechanisms and pathophysiological significance of eryptosis, the suicidal erythrocyte death. Semin. Cell Dev. Biol. 2015, 39, 35–42.
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820.
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070.
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252.
- Lam, C.S.Y.; Benzie, I.F.F.; Choi, S.W.; Chan, L.Y.L.; Yeung, V.T.F.; Woo, G.C. Relationships among diabetic retinopathy, antioxidants, and glycemic control. Optom. Vis. Sci. 2011, 88.
- Gorban de Lapertosa, S.; Fereira de Moura, A.; Decroux, C.; Duke, L.; Hammond, L.; Jacobs, E.; Kaundal, A.; Li, J.; Liu, J.; Ohlrogge, A.E.; et al. Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019.
- Deray, G.; Heurtier, A.; Grimaldi, A.; Launay Vacher, V.; Isnard Bagnis, C. Anemia and diabetes. Am. J. Nephrol. 2004, 24, 522–526.
- Gauci, R.; Hunter, M.; Bruce, D.G.; Davis, W.A.; Davis, T.M.E. Anemia complicating type 2 diabetes: Prevalence, risk factors and prognosis. J. Diabetes Complic. 2017, 31.
- Singh, D.K.; Winocour, P.; Farrington, K. Erythropoietic stress and anemia in diabetes mellitus. Nat. Rev. Endocrinol. 2009, 5, 204–210.
- Calderón-Salinas, J.V.; Muñoz-Reyes, E.G.; Guerrero-Romero, J.F.; Rodríguez-Morán, M.; Bracho-Riquelme, R.L.; Carrera-Gracia, M.A.; Quintanar-Escorza, M.A. Eryptosis and oxidative damage in type 2 diabetic mellitus patients with chronic kidney disease. Mol. Cell. Biochem. 2011, 357.
- Maellaro, E.; Leoncini, S.; Moretti, D.; Del Bello, B.; Tanganelli, I.; De Felice, C.; Ciccoli, L. Erythrocyte caspase-3 activation and oxidative imbalance in erythrocytes and in plasma of type 2 diabetic patients. Acta Diabetol. 2013, 50.
- Vlassopoulos, A.; Lean, M.E.J.; Combet, E. Role of oxidative stress in physiological albumin glycation: A neglected interaction. Free Radic. Biol. Med. 2013, 60.
- Huebschmann, A.G.; Regensteiner, J.G.; Vlassara, H.; Reusch, J.E.B. Diabetes and advanced glycoxidation end products. Diabetes Care 2006, 29, 1420–1432.
- Vlassara, H.; Striker, G.E. Advanced Glycation Endproducts in Diabetes and Diabetic Complications. Endocrinol. Metab. Clin. North. Am. 2013, 42, 697–719.
- Awasthi, S.; Gayathiri, S.K.; Ramya, R.; Duraichelvan, R.; Dhason, A.; Saraswathi, N.T. Advanced Glycation-Modified Human Serum Albumin Evokes Alterations in Membrane and Eryptosis in Erythrocytes. Appl. Biochem. Biotechnol. 2015, 177.
- Ahmed, N. Advanced glycation endproducts—Role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21.
- Vlassara, H.; Uribarri, J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. Diabetes Rep. 2014, 14.
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222.
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146.
- Ikeda, K.; Higashi, T.; Sano, H.; Jinnouchi, Y.; Yoshida, M.; Araki, T.; Ueda, S.; Horiuchi, S. Ne-(carboxymethyl)lysine protein adduct is a major immunological epitope in proteins modified with advanced glycation end products of the maillard reaction. Biochemistry 1996, 35.
- Reddy, S.; Bichler, J.; Wells-Knecht, K.J.; Thorpe, S.R.; Baynes, J.W. Nε-(Carboxymethyl) lysine Is a Dominant Advanced Glycation End Product (AGE) Antigen in Tissue Proteins. Biochemistry 1995, 34.
- Rabbani, N.; Thornalley, P.J. The critical role of methylglyoxal and glyoxalase 1 in diabetic nephropathy. Diabetes 2014, 63, 50–52.
- Riboulet-Chavey, A.; Pierron, A.; Durand, I.; Murdaca, J.; Giudicelli, J.; Van Obberghen, E. Methylglyoxal impairs the insulin signaling pathways independently of the formation of intracellular reactive oxygen species. Diabetes 2006, 55.
- Matafome, P.; Sena, C.; Seiça, R. Methylglyoxal, obesity, and diabetes. Endocrine 2013, 43, 472–484.
- Beisswenger, P.J.; Howell, S.K.; Touchette, A.D.; Lal, S.; Szwergold, B.S. Metformin reduces systemic methylglyoxal levels in type 2 diabetes. Diabetes 1999, 48.
- Kilhovd, B.K.; Giardino, I.; Torjesen, P.A.; Birkeland, K.I.; Berg, T.J.; Thornalley, P.J.; Brownlee, M.; Hanssen, K.F. Increased serum levels of the specific AGE-compound methylglyoxal-derived hydroimidazolone in patients with type 2 diabetes. Metabolism 2003, 33.
- Lapolla, A.; Flamini, R.; Dalla Vedova, A.; Senesi, A.; Reitano, R.; Fedele, D.; Basso, E.; Seraglia, R.; Traldi, P. Glyoxal and methylglyoxal levels in diabetic patients: Quantitative determination by a new GC/MS method. Clin. Chem. Lab. Med. 2003, 41.
- Nagai, R.; Deemer, E.K.; Brock, J.W.; Thorpe, S.R.; Baynes, J.W. Effect of glucose concentration on formation of AGEs in erythrocytes In Vitro. Ann. N. Y. Acad. Sci. 2005, 1043, 146–150.
- Nicolay, J.; Schneider, J.; Niemoeller, O.; Artunc, F.; Portero-Otin, M.; Haik, G.; Thornalley, P.; Schleicher, E.; Wieder, T.; Lang, F. Stimulation of suicidal erythrocyte death by methylglyoxal. Cell. Physiol. Biochem. 2006, 40.
- Thornalley, P.J.; Jahan, I.; Ng, R. Suppression of the accumulation of triosephosphates and increased formation of methylglyoxal in human red blood cells during hyperglycaemia by thiamine In Vitro. J. Biochem. 2001, 129.
- Thornalley, P.J. Modification of the glyoxalase system in human red blood cells by glucose In Vitro. Biochem. J. 1988, 254.
- Kempe-Teufel, D.S.; Bissinger, R.; Qadri, S.M.; Wagner, R.; Peter, A.; Lang, F. Cellular markers of eryptosis are altered in type 2 diabetes. Clin. Chem. Lab. Med. 2018, 56, e177–e180.
- Wali, R.K.; Jaffe, S.; Kumar, D.; Kalra, V.K. Alterations in organization of phospholipids in erythrocytes as factor in adherence to endothelial cells in diabetes mellitus. Diabetes 1988, 62.
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V. Obesity and dyslipidemia. Metabolism 2019, 92, 71–81.
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in Obesity: Mechanisms and Potential Targets. Nutrients 2013, 5, 1218–1240.
- Kopin, L.; Lowenstein, C. In the Clinic® dyslipidemia. Ann. Intern. Med. 2017, 167.
- Schnabel, R.B.; Baumert, J.; Barbalic, M.; Dupuis, J.; Ellinor, P.T.; Durda, P.; Dehghan, A.; Bis, J.C.; Illig, T.; Morrison, A.C.; et al. Duffy antigen receptor for chemokines (Darc) polymorphism regulates circulating concentrations of monocyte chemoattractant protein-1 and other inflammatory mediators. Blood 2010, 115.
- Kolodgie, F.D.; Gold, H.K.; Burke, A.P.; Fowler, D.R.; Kruth, H.S.; Weber, D.K.; Farb, A.; Guerrero, L.J.; Hayase, M.; Kutys, R.; et al. Intraplaque Hemorrhage and Progression of Coronary Atheroma. N. Engl. J. Med. 2003, 32.
- Virmani, R.; Roberts, W.C. Extravasated erythrocytes, iron, and fibrin in atherosclerotic plaques of coronary arteries in fatal coronary heart disease and their relation to luminal thrombus: Frequency and significance in 57 necropsy patients and in 2958 five mm segments of 224 majo. Am. Heart J. 1983, 105.
- Pinzón-Díaz, C.E.; Calderón-Salinas, J.V.; Rosas-Flores, M.M.; Hernández, G.; López-Betancourt, A.; Quintanar-Escorza, M.A. Eryptosis and oxidative damage in hypertensive and dyslipidemic patients. Mol. Cell. Biochem. 2018, 11.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Entry Collection:
Hypertension and Cardiovascular Diseases
Revisions:
2 times
(View History)
Update Date:
01 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




