
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | François Michel Carlier | -- | 2817 | 2022-06-22 15:28:50 | | | |
| 2 | Lindsay Dong | -19 word(s) | 2798 | 2022-07-27 04:28:35 | | |
Video Upload Options
Chronic obstructive pulmonary disease (COPD), asthma and cystic fibrosis (CF) are distinct respiratory diseases that share features such as the obstruction of small airways and disease flare-ups that are called exacerbations and are often caused by infections. Along the airway epithelium, immunoglobulin (Ig) A contributes to first line mucosal protection against inhaled particles and pathogens. Dimeric IgA produced by mucosal plasma cells is transported towards the apical pole of airway epithelial cells by the polymeric Ig receptor (pIgR), where it is released as secretory IgA. Secretory IgA mediates immune exclusion and promotes the clearance of pathogens from the airway surface by inhibiting their adherence to the epithelium.
1. Introduction
2. The Mucosal S-IgA System in Homeostasis
2.1. Production and Structure of S-IgA and pIgR
2.2. Transcytosis of d-IgA and Functions of S-IgA
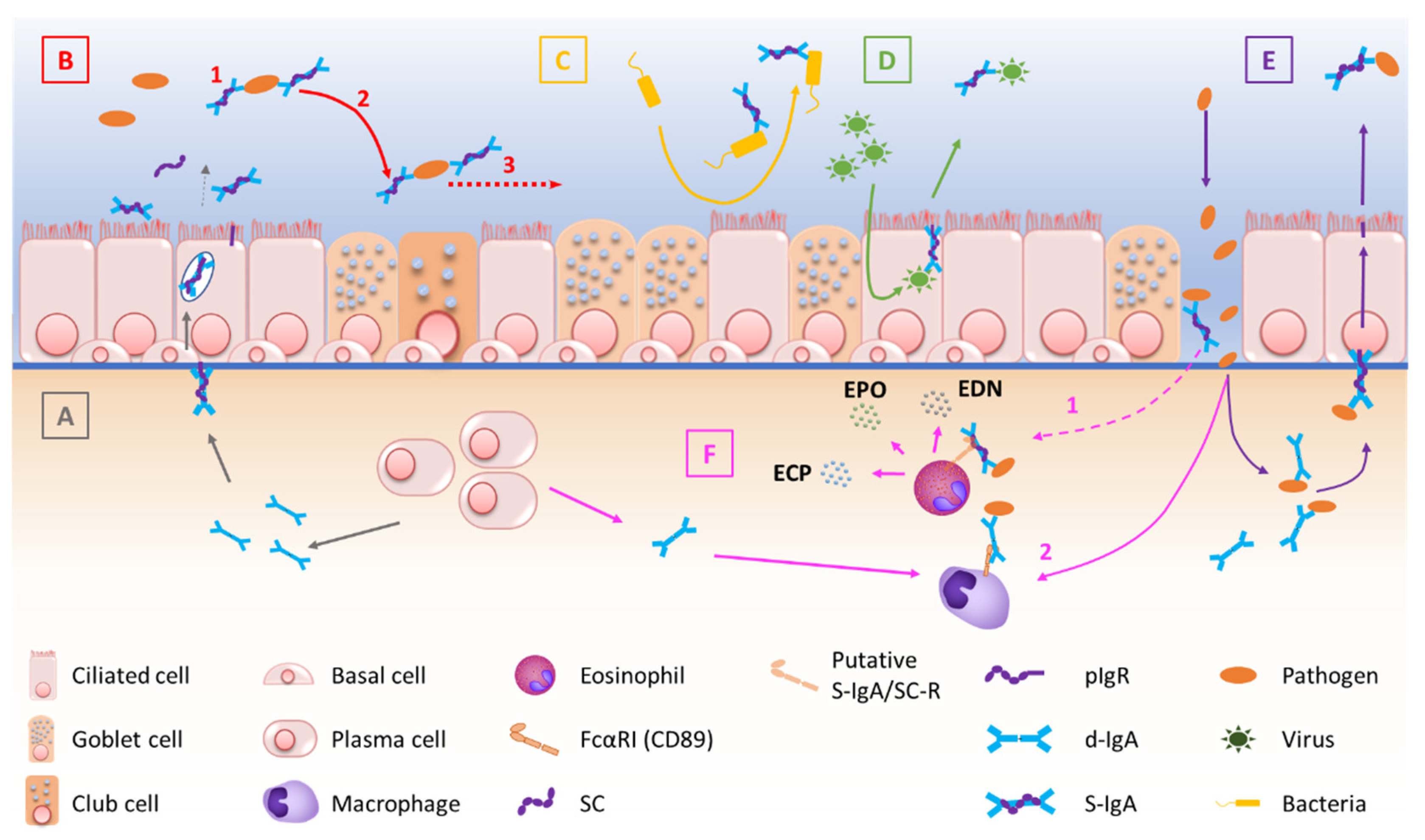
2.3. Regulation of S-IgA Production
3. The Mucosal S-IgA System in Airway Disease
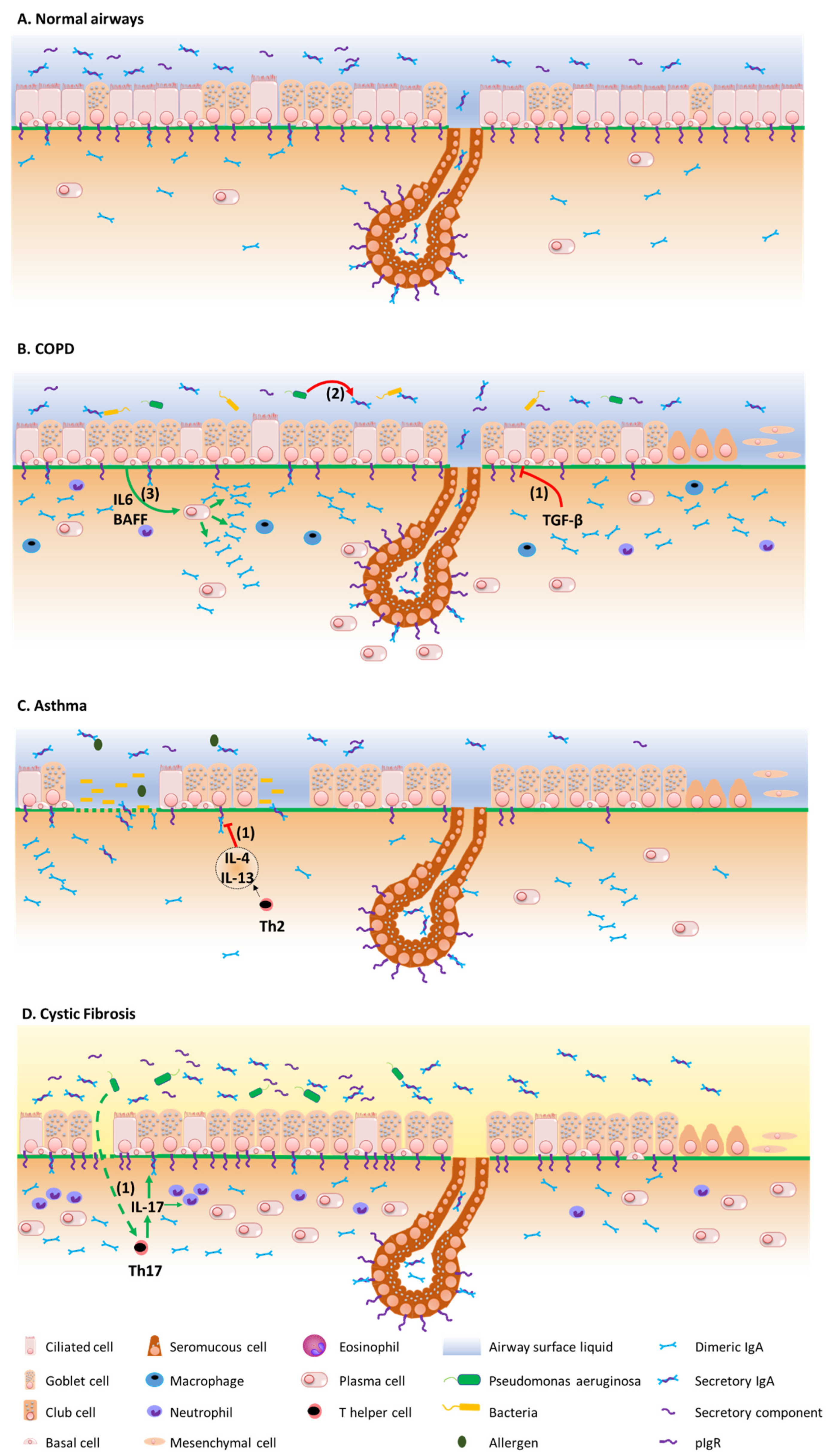
3.1. COPD
3.2. Asthma
3.3. Cystic Fibrosis
3.4. Bronchiolitis Obliterans Syndrome
References
- Corthésy, B. Multi-Faceted Functions of Secretory IgA at Mucosal Surfaces. Front. Immunol. 2013, 4, 185.
- Carlier, F.M.; Sibille, Y.; Pilette, C. The epithelial barrier and immunoglobulin A system in allergy. Clin. Exp. Allergy 2016, 46, 1372–1388.
- Yang, S.; Yu, M. Role of Goblet Cells in Intestinal Barrier and Mucosal Immunity. J. Inflamm. Res. 2021, 14, 3171–3183.
- Boyaka, P.N.; Fujihashi, K. Host Defenses at Mucosal Surfaces. In Clinical Immunology, 5th ed.; Rich, R.R., Fleisher, T.A., Shearer, W.T., Schroeder, H.W., Frew, A.J., Weyand, C.M., Eds.; Elsevier: London, UK, 2019; pp. 285–298.e1.
- McNabb, P.C.; Tomasi, T.B. Host Defense Mechanisms at Mucosal Surfaces. Annu. Rev. Microbiol. 1981, 35, 477–496.
- Davis, J.D.; Wypych, T.P. Cellular and functional heterogeneity of the airway epithelium. Mucosal Immunol. 2021, 14, 978–990.
- Gohy, S.; Hupin, C.; Ladjemi, M.Z.; Hox, V.; Pilette, C. Key role of the epithelium in chronic upper airways diseases. Clin. Exp. Allergy 2019, 50, 135–146.
- Travaglini, K.J.; Nabhan, A.N.; Penland, L.; Sinha, R.; Gillich, A.; Sit, R.V.; Chang, S.; Conley, S.D.; Mori, Y.; Seita, J.; et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 2020, 587, 619–625.
- Knight, D.A.; Holgate, S.T. The airway epithelium: Structural and functional properties in health and disease. Respirology 2003, 8, 432–446.
- Rock, J.R.; Randell, S.H.; Hogan, B.L.M. Airway basal stem cells: A perspective on their roles in epithelial homeostasis and remodeling. Dis. Model. Mech. 2010, 3, 545–556.
- Hogan, B.L.; Barkauskas, C.E.; Chapman, H.A.; Epstein, J.A.; Jain, R.; Hsia, C.C.; Niklason, L.; Calle, E.; Le, A.; Randell, S.H. Repair and regeneration of the respiratory system: Complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 2014, 15, 123–138.
- Boers, J.E.; Ambergen, A.W.; Thunnissen, F.B.J.M. Number and Proliferation of Basal and Parabasal Cells in Normal Human Airway Epithelium. Am. J. Respir. Crit. Care Med. 1998, 157, 2000–2006.
- Zuo, W.-L.; Shenoy, S.A.; Li, S.; O’Beirne, S.L.; Strulovici-Barel, Y.; Leopold, P.L.; Wang, G.; Staudt, M.R.; Walters, M.S.; Mason, C.; et al. Ontogeny and Biology of Human Small Airway Epithelial Club Cells. Am. J. Respir. Crit. Care Med. 2018, 198, 1375–1388.
- Hiemstra, P.S.; Bourdin, A. Club cells, CC10 and self-control at the epithelial surface. Eur. Respir. J. 2014, 44, 831–832.
- Boers, J.E.; Brok, J.L.D.; Koudstaal, J.; Arends, J.W.; Thunnissen, F.B. Number and proliferation of neuroendocrine cells in normal human airway epithelium. Am. J. Respir. Crit. Care Med. 1996, 154, 758–763.
- Linnoila, R.I. Functional facets of the pulmonary neuroendocrine system. Lab. Investig. 2006, 86, 425–444.
- Branchfield, K.; Nantie, L.; Verheyden, J.M.; Sui, P.; Wienhold, M.D.; Sun, X. Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science 2016, 351, 707–710.
- Montoro, D.T.; Haber, A.L.; Biton, M.; Vinarsky, V.; Lin, B.; Birket, S.E.; Yuan, F.; Chen, S.; Leung, H.M.; Villoria, J.; et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 2018, 560, 319–324.
- He, W.-H.; Zhang, W.-D.; Cheng, C.-C.; Lu, J.; Liu, L.; Chen, Z.-H.; Wang, W.-H. Expression characteristics of polymeric immunoglobulin receptor in Bactrian camel (Camelus bactrianus) lungs. PLoS ONE 2022, 17, e0264815.
- Blackburn, J.B.; Schaff, J.A.; Gutor, S.; Du, R.-H.; Nichols, D.; Sherrill, T.; Gutierrez, A.J.; Xin, M.K.; Wickersham, N.; Zhang, Y. Secretory cells are the primary source of pIgR in small airways. bioRxiv 2021.
- Schiller, H.B.; Montoro, D.T.; Simon, L.M.; Rawlins, E.L.; Meyer, K.B.; Strunz, M.; Braga, F.A.V.; Timens, W.; Koppelman, G.H.; Budinger, G.R.S.; et al. The Human Lung Cell Atlas: A High-Resolution Reference Map of the Human Lung in Health and Disease. Am. J. Respir. Cell Mol. Biol. 2019, 61, 31–41.
- Woof, J.M.; Russell, M.W. Structure and function relationships in IgA. Mucosal Immunol. 2011, 4, 590–597.
- Bakema, J.E.; van Egmond, M. Immunoglobulin A: A next generation of therapeutic antibodies? mAbs 2011, 3, 352–361.
- Monteiro, R.C. The Role of IgA and IgA Fc Receptors as Anti-Inflammatory Agents. J. Clin. Immunol. 2010, 30, 61–64.
- Underdown, B.J.; Schiff, J.M. Immunoglobulin A: Strategic Defense Initiative at the Mucosal Surface. Annu. Rev. Immunol. 1986, 4, 389–417.
- Li, Y.; Jin, L.; Chen, T. The Effects of Secretory IgA in the Mucosal Immune System. BioMed Res. Int. 2020, 2020, 2032057.
- Pilette, C.; Ouadrhiri, Y.; Godding, V.; Vaerman, J.-P.; Sibille, Y. Lung mucosal immunity: Immunoglobulin-A revisited. Eur. Respir. J. 2001, 18, 571–588.
- Conley, M.E.; Delacroix, D.L. Intravascular and Mucosal Immunoglobulin A: Two Separate but Related Systems of Immune Defense? Ann. Intern. Med. 1987, 106, 892.
- Burnett, D. Immunoglobulins in the lung. Thorax 1986, 41, 337–344.
- Reboldi, A.; Arnon, T.I.; Rodda, L.B.; Atakilit, A.; Sheppard, D.; Cyster, J.G. IgA production requires B cell interaction with subepithelial dendritic cells in Peyer’s patches. Science 2016, 352, aaf4822.
- Stumbles, P.A.; Upham, J.W.; Holt, P.G. Airway dendritic cells: Co-ordinators of immunological homeostasis and immunity in the respiratory tract. APMIS 2003, 111, 741–755.
- Turula, H.; Wobus, C.E. The Role of the Polymeric Immunoglobulin Receptor and Secretory Immunoglobulins during Mucosal Infection and Immunity. Viruses 2018, 10, 237.
- Asano, M.; Komiyama, K. Polymeric immunoglobulin receptor. J. Oral Sci. 2011, 53, 147–156.
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611.
- Johansen, F.-E.; Kaetzel, C. Regulation of the polymeric immunoglobulin receptor and IgA transport: New advances in environmental factors that stimulate pIgR expression and its role in mucosal immunity. Mucosal Immunol. 2011, 4, 598–602.
- Gohy, S.T. Polymeric immunoglobulin receptor down-regulation in chronic obstructive pulmonary disease. Persistence in the cultured epithelium and role of transforming growth factor-beta. Am. J. Respir Crit. Care Med. 2014, 190, 509–521.
- Loman, S.; Jansen, H.M.; Out, T.A.; Lutter, R. Interleukin-4 and interferon-gamma synergistically increase secretory component gene expression, but are additive in stimulating secretory immunoglobulin A release by Calu-3 airway epithelial cells. Immunology 1999, 96, 537.
- Ladjemi, M.Z.; Gras, D.; Dupasquier, S.; Detry, B.; Lecocq, M.; Garulli, C.; Fregimilicka, C.; Bouzin, C.; Gohy, S.; Chanez, P. Bronchial Epithelial IgA Secretion Is Impaired in Asthma. Role of IL-4/IL-13. Am. J. Res-Piratory Crit. Care Med. 2018, 197, 1396–1409.
- Ratajczak, C.; Guisset, A.; Detry, B.; Sibille, Y.; Pilette, C. Dual effect of neutrophils on pIgR/secretory component in human bronchial epithelial cells: Role of TGF-beta. J. Biomed. Biotechnol. 2010, 2010, 428618.
- Collin, A.M.; Lecocq, M.; Noel, S.; Detry, B.; Carlier, F.M.; Nana, F.A.; Bouzin, C.; Leal, T.; Vermeersch, M.; De Rose, V.; et al. Lung immunoglobulin A immunity dysregulation in cystic fibrosis. eBioMedicine 2020, 60, 102947.
- Gohy, S.; Moeremans, A.; Pilette, C.; Collin, A. Immunoglobulin A Mucosal Immunity and Altered Respiratory Epithelium in Cystic Fibrosis. Cells 2021, 10, 3603.
- Carlier, F.M.; Detry, B.; Lecocq, M.; Collin, A.M.; Verleden, S.E.; Stanciu-Pop, C.M.; Janssens, W.; Ambroise, J.; Vanaudenaerde, B.M.; Gohy, S.T. The memory of airway epithelium damage in smokers and COPD patients. bioRxiv 2021.
- Guo, M.-Y.; Chen, H.-K.; Ying, H.-Z.; Qiu, F.-S.; Wu, J.-Q. The Role of Respiratory Flora in the Pathogenesis of Chronic Respiratory Diseases. BioMed Res. Int. 2021, 2021, 6431862.
- Sato, S.; Kiyono, H. The mucosal immune system of the respiratory tract. Curr. Opin. Virol. 2012, 2, 225–232.
- Carlier, F.M.; de Fays, C.; Pilette, C. Epithelial Barrier Dysfunction in Chronic Respiratory Diseases. Front. Physiol. 2021, 12, 691227.
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222.
- Salvi, S.S.; Barnes, P.J. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009, 374, 733–743.
- Pilette, C.; Godding, V.; Kiss, R.; Delos, M.; Verbeken, E.; Decaestecker, C.; De Paepe, K.; Vaerman, J.-P.; Decramer, M.; Sibille, Y. Reduced Epithelial Expression of Secretory Component in Small Airways Correlates with Airflow Obstruction in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2001, 163, 185–194.
- Zuo, W.-L.; Rostami, M.R.; Shenoy, S.A.; LeBlanc, M.G.; Salit, J.; Strulovici-Barel, Y.; O’Beirne, S.L.; Kaner, R.J.; Leopold, P.L.; Mezey, J.G.; et al. Cell-specific expression of lung disease risk-related genes in the human small airway epithelium. Respir. Res. 2020, 21, 200.
- Polosukhin, V.V.; Cates, J.M.; Lawson, W.E.; Zaynagetdinov, R.; Milstone, A.P.; Massion, P.P.; Ocak, S.; Ware, L.B.; Lee, J.W.; Bowler, R.P. Bronchial secretory immunoglobulin a deficiency correlates with airway inflammation and progression of chronic obstructive pulmonary disease. Am. J. Respir Crit. Care Med. 2011, 184, 317–327.
- Polosukhin, V.V.; Richmond, B.W.; Du, R.-H.; Cates, J.; Wu, P.; Nian, H.; Massion, P.P.; Ware, L.B.; Lee, J.W.; Kononov, A.; et al. Secretory IgA Deficiency in Individual Small Airways Is Associated with Persistent Inflammation and Remodeling. Am. J. Respir. Crit. Care Med. 2017, 195, 1010–1021.
- Kuruvilla, M.E.; Lee, F.E.-H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233.
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725.
- Alhamwe, B.A.; Miethe, S.; Von Strandmann, E.P.; Potaczek, D.P.; Garn, H. Epigenetic Regulation of Airway Epithelium Immune Functions in Asthma. Front. Immunol. 2020, 11, 1747.
- Peters, U.; Dixon, A.E.; Forno, E. Obesity and asthma. J. Allergy Clin. Immunol. 2018, 141, 1169–1179.
- Potaczek, D.P.; Miethe, S.; Schindler, V.; Alhamdan, F.; Garn, H. Role of airway epithelial cells in the development of different asthma phenotypes. Cell. Signal. 2020, 69, 109523.
- Frey, A.; Lunding, L.P.; Ehlers, J.C.; Weckmann, M.; Zissler, U.M.; Wegmann, M. More than Just a Barrier: The Immune Functions of the Airway Epithelium in Asthma Pathogenesis. Front. Immunol. 2020, 11, 761.
- Nahm, D.-H.; Kim, H.-Y.; Park, H.-S. Elevation of specific immunoglobulin A antibodies to both allergen and bacterial antigen in induced sputum from asthmatics. Eur. Respir. J. 1998, 12, 540–545.
- Peebles, R.S.; Hamilton, R.G.; Lichtenstein, L.M.; Schlosberg, M.; Liu, M.C.; Proud, D.; Togias, A. Antigen-specific IgE and IgA antibodies in bronchoalveolar lavage fluid are associated with stronger antigen-induced late phase reactions. Clin. Exp. Allergy 2001, 31, 239–248.
- Orivuori, L.; Loss, G.; Roduit, C.; Dalphin, J.C.; Depner, M.; Genuneit, J.; Lauener, R.; Pekkanen, J.; Pfefferle, P.; Riedler, J. Soluble immunoglobulin A in breast milk is inversely associated with atopic dermatitis at early age: The PASTURE cohort study. Clin. Exp. Allergy 2014, 44, 102–112.
- Schaffer, F.M.; Monteiro, R.C.; Volanakis, J.E.; Cooper, M.D. IgA deficiency. Immunodefic. Rev. 1991, 3, 15–44.
- Kim, W.-J.; Choi, I.S.; Kim, C.S.; Lee, J.-H.; Kang, H.-W. Relationship between serum IgA level and allergy/asthma. Korean J. Intern. Med. 2017, 32, 137–145.
- Orivuori, L.; Mustonen, K.; Roduit, C.; Braun-Fahrländer, C.; Dalphin, J.-C.; Genuneit, J.; Lauener, R.; Pfefferle, P.; Riedler, J.; Weber, J.; et al. Immunoglobulin A and immunoglobulin G antibodies against β-lactoglobulin and gliadin at age 1 associate with immunoglobulin E sensitization at age 6. Pediatric Allergy Immunol. 2014, 25, 329–337.
- Hupin, C.; Rombaux, P.; Bowen, H.; Gould, H.; Lecocq, M.; Pilette, C. Downregulation of polymeric immunoglobulin receptor and secretory IgA antibodies in eosinophilic upper airway diseases. Allergy 2013, 68, 1589–1597.
- Ratjen, F.; Bell, S.C.; Rowe, S.M.; Goss, C.H.; Quittner, A.L.; Bush, A. Cystic fibrosis. Nat. Rev. Dis. Prim. 2015, 1, 15010.
- Collin, A.M.; Lecocq, M.; Detry, B.; Carlier, F.M.; Bouzin, C.; de Sany, P.; Hoton, D.; Verleden, S.; Froidure, A.; Pilette, C.; et al. Loss of ciliated cells and altered airway epithelial integrity in cystic fibrosis. J. Cyst. Fibros. 2021, 20, e129–e139.
- Marshall, L.J.; Perks, B.; Bodey, K.; Suri, R.; Bush, A.; Shute, J.K. Free Secretory Component from Cystic Fibrosis Sputa Displays the Cystic Fibrosis Glycosylation Phenotype. Am. J. Respir. Crit. Care Med. 2004, 169, 399–406.
- Hodson, M.E.; Morris, L.; Batten, J.C. Serum immunoglobulins and immunoglobulin G subclasses in cystic fibrosis related to the clinical state of the patient. Eur. Respir. J. 1988, 1, 701–705.
- Hassan, J.; Feighery, C.; Bresnihan, B.; Keogan, M.; Fitzgerald, M.X.; Whelan, A. Serum IgA and IGg Subclasses during Treatment for Acute Respiratory Exacerbation in Cystic Fibrosis: Analysis of Patients Colonised with Mucoid or Non-Mucoid Strains of Pseudomonas Aeruginosa. Immunol. Investig. 1994, 23, 1–13.
- Van Bever, H.P.; Gigase, P.L.; De Clerck, L.S.; Bridts, C.H.; Franckx, H.; Stevens, W.J. Immune complexes and Pseudomonas aeruginosa antibodies in cystic fibrosis. Arch. Dis. Child. 1988, 63, 1222–1228.
- Konstan, M.W.; Hilliard, K.A.; Norvell, T.M.; Berger, M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am. J. Respir Crit. Care Med. 1994, 150, 448–454.
- Verleden, G.M.; Raghu, G.; Meyer, K.C.; Glanville, A.R.; Corris, P. A new classification system for chronic lung allograft dysfunction. J. Hear. Lung Transplant 2014, 33, 127–133.
- Verleden, G.M.; Glanville, A.R.; Lease, E.D.; Fisher, A.J.; Calabrese, F.; Corris, P.A.; Ensor, C.R.; Gottlieb, J.; Hachem, R.R.; Lama, V. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J. Heart Lung Transplant 2019, 38, 493–503.
- Martinu, T.; Howell, D.N.; Davis, R.D.; Steele, M.P.; Palmer, S.M. Pathologic correlates of bronchiolitis obliterans syn-drome in pulmonary retransplant recipients. Chest 2006, 129, 1016–1023.
- Le Pavec, J.; Pradère, P.; Gigandon, A.; Dauriat, G.; Dureault, A.; Aguilar, C.; Henry, B.; Lanternier, F.; Savale, L.; Dolidon, S.; et al. Risk of Lung Allograft Dysfunction Associated With Aspergillus Infection. Transplant. Direct 2021, 7, e675.
- Fisher, C.E.; Preiksaitis, C.M.; Lease, E.D.; Edelman, J.; Kirby, K.A.; Leisenring, W.M.; Raghu, G.; Boeckh, M.; Limaye, A.P. Symptomatic Respiratory Virus Infection and Chronic Lung Allograft Dysfunction. Clin. Infect. Dis. 2015, 62, 313–319.
- Gregson, A.L. Infectious Triggers of Chronic Lung Allograft Dysfunction. Curr. Infect. Dis. Rep. 2016, 18, 21.
- Bastian, A.; Tunkel, C.; Lins, M.; Bottcher, H.; Hirt, S.W.; Cremer, J.; Bewig, B. Immunoglobulin A and secretory immunoglobulin A in the bronchoalveolar lavage from patients after lung transplantation. Clin. Transplant. 2000, 14, 580–585.
- Vandermeulen, E.; Verleden, S.E.; Bellon, H.; Ruttens, D.; Lammertyn, E.; Claes, S.; Vandooren, J.; Ugarte-Berzal, E.; Schols, D.; Emonds, M.-P.; et al. Humoral immunity in phenotypes of chronic lung allograft dysfunction: A broncho-alveolar lavage fluid analysis. Transpl. Immunol. 2016, 38, 27–32.
- Murthy, S.C.; Avery, R.K.; Budev, M.; Gupta, S.; Pettersson, G.B.; Nowicki, E.R.; Mehta, A.; Chapman, J.T.; Rajeswaran, J.; Blackstone, E.H. Low pretransplant IgA level is associated with early post–lung transplant seromucous infection. J. Thorac. Cardiovasc. Surg. 2018, 156, 882–891.e8.
- Chambers, D.C.; Davies, B.; Mathews, A.; Yerkovich, S.T.; Hopkins, P.M. Bronchiolitis obliterans syndrome, hypogammaglobulinemia, and infectious complications of lung transplantation. J. Hear. Lung Transplant. 2013, 32, 36–43.




