
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | 书卫 书卫 肖 | -- | 1039 | 2022-07-21 04:04:05 | | | |
| 2 | 书卫 书卫 肖 | -38 word(s) | 1001 | 2022-07-21 04:11:40 | | | | |
| 3 | 书卫 书卫 肖 | + 494 word(s) | 1495 | 2022-07-21 05:14:07 | | | | |
| 4 | Catherine Yang | Meta information modification | 1495 | 2022-07-21 07:36:03 | | |
Video Upload Options
The stability of copper-based catalysts is an important property that affects the catalytic efficiency, which determines the service life of the catalytic base in the methanol steam reforming (MSR) reaction, and plays an important role in the sustainable production of hydrogen.
1. Introduction
With the booming economy, energy consumption and harmful gas emissions have increased sharply[1][2][3], and the decline of fossil fuels has become a major obstacle to sustainable development. With the needs of global sustainable development, we urgently need some new fuels. Hydrogen is a well-known clean energy carrier, and fuel cells can convert the chemical energy in fuel hydrogen and oxidant oxygen into electricity (sustainable energy).Hydrogen can come from many sources[4][5], for example, photolysis of water for hydrogen production[6][7][8], traditional fossil fuel hydrogen production[9][10], biomass hydrogen production[11][12][13], and hydrogen production from water electrolysis[14][15]. In recent years, more and more studies have been conducted on methanol steam reforming. Methanol reforming produces hydrogen with low CO selectivity and high hydrogen selectivity, and has little effect on the electrode toxicity of proton exchange membrane fuel cells[16]. Moreover, methanol steam reforming does not require the vaporization step in hydrogen production, which can bring good economic benefits[17][18]. Liquid methanol (CH3OH) is a perfect hydrogen carrier that is more facile to transport than hydrogen gas[19][20][21]. There are many ways to produce methanol, such as the synthesis gas to methanol and the direct oxidation of methane to methanol[22]. However, many scientists have called for “green methanol” from renewable hydrogen and CO2 hydrogenation[23]. There are also many ways to synthesize methanol from renewable energy such as biomass, wind power, and solar energy. For example, many works have reported methanol synthesis directly from photo/electronic catalytic CO2 reduction in water[24]. It is very useful for the industry and our society to produce methanol from renewable energy using CO2 as a raw material. In addition, when the captured CO2 source is biomass, it is called bio-methanol[25]. This means that methanol could also be obtained through thermochemical and biochemical conversion of biomass gasification and electrolysis[26]. Gautam et al. have provided an excellent review on the current trends and future perspective of bio-methanol as a renewable fuel from waste biomass[26]. Bio-fuels (e.g., bio-methanol, bio-ethanol, biodiesel) would be a significant alternative fuel for the future. Compared with other fossil fuels, methanol with a low carbon atom and high hydrogen-to-carbon ratio can significantly reduce the occurrence of side reactions[27][28][29].
There are four typical ways to produce hydrogen from methanol: methanol decomposition (MD)[30][31][32], partial oxidation of methanol (POM)[33][34][35], steam reforming of methanol (SRM)[9][36], and oxidative steam reforming of methanol (OSRM)[37][38][39]. Methanol reforming can produce a large amount of hydrogen, which is one of the important reasons why it is widely studied by researchers[40][41][42]. SRM also contains two side reactions, which are methanol decomposition and water gas shift reactions[43].
The catalyst is the key factor that affects the hydrogen production efficiency of methanol reforming. The deactivation of the catalyst can easily reduce the yield of hydrogen and the lifetime of the catalyst. Noble metals have high catalytic activity and stability, but the cost is too high, limiting their large-scale application[44][45][46]. Copper-based catalysts have low cost and excellent catalytic activity, and they are good candidates for methanol reforming for hydrogen production process[19][20][27]. For example, CuO-ZnO-Al2O3 catalysts are often used in methanol reforming to produce hydrogen, and their performance is also very good[47][48]. Bagherzadeh et al. investigated the effect of adding ZrO2-CeO2 to CuO-ZnO-Al2O3 catalysts, and found that the selectivity for H2 was high and the selectivity for CO was low[49]. Mohtashami et al. introduced ZrO2 to a Cu/ZnO catalyst and studied its MSR (Methanol Steam Reforming) performance, and the methanol conversion reached up to 97.8% with the selectivity for H2 of 99%[48]. However, Cu-based catalysts suffer thermal instabilities[50], such as spontaneous combustion, sintering, and deactivation[22][51][52]. The reports have shown that when the temperature is higher than 300 °C, the copper particles in the copper-based catalyst are easy to sinter[53]. There is also a by-product methyl formate produced in methanol reforming that promotes catalyst deactivation through pyrolysis[22]. Thus, how to improve their stability is an important and meaningful topic.
In addition to the factors of the copper-based catalyst itself, the methanol reforming hydrogen production reactor also has a great influence on the stability of the catalyst, for example, methanol steam reforming is a strong endothermic reaction, which requires the reactor temperature not to be too high[54]. Moreover, the production of the reactor is relatively complicated, and requires relatively complex technology and high cost. With the development of technology, the design of the reactor can become simpler and simpler, and the more likely it is that a reactor that makes the catalyst more stable can be created. It has been reported in the literature that the reactors used for hydrogen production from methanol reforming are mainly packed bed reactors[55]. However, this kind of reactor requires high temperature, which is its disadvantage, so other reactors have been studied in recent years, such as membrane reactors[56][57] and microporous reactors[58][59]. Moreover, in recent years, many researchers have made great efforts in the design of methanol reforming reactors and have achieved good results; for example, Mironova et al. designed a flow reactor with a Pd-Cu membrane in which methanol steam reforming can achieve a high hydrogen yield compared to conventional reactors[60], while Wang et al. designed a rib-type microreactor for methanol steam reforming and found that the conversion rate of methanol reached 99.4% [59]. With the development of science and technology, 3D printing technology is also used to design catalysts[61]; this technology can design a reactor suitable for catalysts. Moreover, other technologies, such as plasma-assisted reactors and solar-powered MSR reactors[55][62] or the novel solar triple-line photothermal chemical energy and heat storage medium reactor proposed by Du et al., can effectively prevent the deactivation of the catalyst and achieve the stability of the reaction[62].
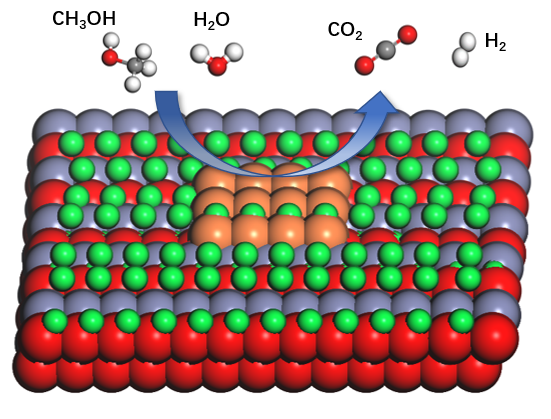
Figure 1. The reaction process of methanol reforming for hydrogen production.
2. Research Progress on Avoiding Deactivation of Copper-Based Catalysts
Improving the stability of copper-based catalysts is of great significance for improving the efficiency of methanol reforming to hydrogen production. However, there are also many difficulties. In the future, researchers should make great efforts in this regard.
The preparation method affects the copper dispersion, microstructural properties, and surface areas of copper-based catalysts, which determine the catalytic performance, especially the catalytic stability. Herein, we selected the preparation of Cu-ZnO series catalysts as examples. According to the previous literature, there is an interaction between Cu and Zn[20], and this interaction is helpful to enhance the catalyst activity. In addition, the preparation method has a great influence on the metal–support interaction. Therefore, optimizing the preparation method is of great significance for the improvement of catalyst activity. There are several traditional preparation methods for the synthesis of methanol reforming catalysts, such as the hydrothermal method, dipping method, co-precipitation method, and sol-gel method[48]. Sanches et al. prepared a Cu/ZnO catalyst by the co-precipitation method[63], and Liao et al. synthesized a CuO/ZnO/CeO2/ZrO2 catalyst by the one-step hydrothermal method[64]. A series of CuZn/MCM-41 catalysts were prepared by the co-impregnation method[65] and achieved good results. After the catalyst was operated for 5 h, the methanol conversion rate was stable at 88%, and the H2 selectivity was 91%. The effects of synthesis methods on the catalyst were also compared. By comparing the performance of catalysts synthesized by one-pot hydrothermal synthesis, co-impregnation, continuous impregnation, and copper impregnation in MSR, it was found that the catalyst synthesized by the co-impregnation method had the best activity.
In order to improve catalyst stability and activity, researchers have added various promoters. For instance, Pu et al. added Sc2O3 to Cu/ZnO and found that it has good stability and activity in methanol reforming for hydrogen production[66], in which Sc3+ increases the copper dispersion and enhances the intermetallic interaction. A similar effect makes it suitable to add Mg to Cu/ZnO/Al2O3, which enhances the catalyst activity by enhancing the Cu-ZnO interaction and increasing the Cu dispersion[67]. The addition of promoters changes the structural properties of the catalyst. For example, Sanches et al.[63] added ZrO2 to Cu/ZnO, and found that ZrO2 clusters in the catalyst could reduce the formation of CO. The addition of ZrO2 induces microstrains in the Cu and ZnO lattices and promotes the formation of CuO, and CuO is easily reduced. Mohtashami et al. found that ZrO2 can reduce CuO size and increase CuO dispersion[48]. Some researchers have also worked to prevent catalyst sintering. The addition of ZrO2 to Cu/ZnO by Huang et al. improved catalyst durability[68]. Different promoters have different effects on the same catalyst. To reduce carbon deposition, Lorenzut et al. introduced Ni and Co into Cu/ZnO/Al2O3, and the carbon deposition was also improved due to the alloying of Ni with Cu[69]. For the traditional Cu/ZnO catalyst, the biggest problem is its durability. ZrO2 is a good promoter and we need to find more useful promoters.
References
- Yang, Z.; Lei, Z.; Ge, B.; Xiong, X.; Jin, Y.; Jiao, K.; Chen, F.; Peng, S. Development of catalytic combustion and CO2 capture and conversion technology. Int. J. Coal Sci. Technol. 2021, 8, 377–382.
- Zhang, X.; Song, X.; Wang, J.; Su, W.; Zhou, B.; Bai, Y.; Yu, G. Physico-chemical structure evolution characteristics of coal char during gasification in the presence of iron-based waste catalyst. Int. J. Coal Sci. Technol. 2020, 7, 456–463.
- Liu, X.; Yang, Q. Recovery of vanadium and tungsten from waste selective catalytic reduction catalysts by K2CO3 roasting and water leaching followed by CaCl2 precipitation. Int. J. Coal Sci. Technol. 2021, 8, 727–736.
- Hwang, B.-Y.; Sakthinathan, S.; Chiu, T.-W. Production of hydrogen from steam reforming of methanol carried out by self-combusted CuCr1-xFexO2 (x = 0–1) nanopowders catalyst. Int. J. Hydrog. Energy 2019, 44, 2848–2856.
- Xiang, Y.; He, J.; Sun, N.; Fan, Y.; Yang, L.; Fang, C.; Kuai, L. Hollow mesoporous CeO2 microspheres for efficient loading of Au single-atoms to catalyze the water-gas shift reaction. Micropor. Mesopor. Mater. 2020, 308, 110507.
- Xiang, Q.; Li, F.; Zhang, D.; Liao, Y.; Zhou, H. Plasma-based surface modification of g-C3N4 nanosheets for highly efficient photocatalytic hydrogen evolution. Appl. Surf. Sci. 2019, 495, 143520.
- Guo, Y.; Mao, L.; Tang, Y.; Shang, Q.; Cai, X.; Zhang, J.; Hu, H.; Tan, X.; Liu, L.; Wang, H.; et al. Concentrating electron and activating H-OH bond of absorbed water on metallic NiCo2S4 boosting photocatalytic hydrogen evolution. Nano Energy. 2022, 95, 107028.
- Lin, Q.; Liang, S.; Wang, J.; Zhang, R.; Wang, X. Cadmium sulfide 3D photonic crystal with hierarchically ordered macropores for highly efficient photocatalytic hydrogen generation. Inorg. Chem. 2022, 61, 2920–2928.
- Yaakob, Z.; Kamarudin, S.K.; Daud, W.R.W.; Yosfiah, M.R.; Lim, K.L.; Kazemian, H. Hydrogen production by methanol-steam reforming using Ni-Mo-Cu/γ-alumina trimetallic catalysts. Asia-Pac. J. Chem. Eng. 2010, 5, 862–868.
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260.
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230.
- Levin, D.B.; Pitt, L.; Love, M. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrogen Energy. 2004, 29, 173–185.
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzym. Microb. Technol. 2006, 38, 569–582.
- Janssen, H.; Bringmann, J.C.; Emonts, B.; Schroeder, V. Safety-related studies on hydrogen production in high-pressure electrolysers. Int. J. Hydrog. Energy 2004, 29, 759–770.
- Turner, J.; Sverdrup, G.; Mann, M.K.; Maness, P.-C.; Kroposki, B.; Ghirardi, M.; Evans, R.J.; Blake, D. Renewable hydrogen production. Int. J. Energ. Res. 2008, 32, 379–407.
- Zhao, J.; Shi, R.; Li, Z.; Zhou, C.; Zhang, T. How to make use of methanol in green catalytic hydrogen production? Nano Select 2020, 1, 12–29.
- Lin, L.; Yu, Q.; Peng, M.; Li, A.; Yao, S.; Tian, S.; Liu, X.; Li, A.; Jiang, Z.; Gao, R.; et al. Atomically dispersed Ni/α-MoC catalyst for hydrogen production from methanol/water. J. Am. Chem. Soc. 2021, 143, 309–317.
- Huber, G.W.; Shabaker, J.W.;Dumesic, J.A. Raney Ni-Sn catalyst for H2 production from biomass-derived hydrocarbons. Science. 2003, 300, 2075–2077.
- Ribeirinha, P.; Mateos-Pedrero, C.; Boaventura, M.; Sousa, J.; Mendes, A. CuO/ZnO/Ga2O3 catalyst for low temperature MSR reaction: Synthesis, characterization and kinetic model. Appl. Catal. B Environ. 2018, 221, 371–379.
- Wang, S.-S.; Su, H.-Y.; Gu, X.-K.; Li, W.-X. Differentiating intrinsic reactivity of copper, copper–zinc alloy, and copper/zinc oxide interface for methanol steam reforming by first-principles theory. J. Phys. Chem. C 2017, 121, 21553–21559.
- Kim, W.; Mohaideen, K.K.; Seo, D.J.; Yoon, W.L. Methanol-steam reforming reaction over Cu-Al-based catalysts derived from layered double hydroxides. Int. J. Hydrogen Energy 2017, 42, 2081–2087.
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol steam reforming for hydrogen production. Chem. Rev. 2007, 107, 3992–4021.
- Li, M.M.-J.; Tsang, S.C.E. Bimetallic catalysts for green methanol production via CO2 and renewable hydrogen: A mini-review and prospects. Catal. Sci. Technol. 2018, 8, 3450–3464.
- Tackett, B.M.; Gomez, E.; Chen, J.G. Net reduction of CO2 via its thermocatalytic and electrocatalytic transformation reactions in standard and hybrid processes. Nat. Catal. 2019, 2, 381–386.
- Ali, S.; Sørensen, K.; Nielsen, M.P. Modeling a novel combined solid oxide electrolysis cell (SOEC)—Biomass gasification renewable methanol production system. Renew. Energy 2020, 154, 1025–1034.
- Gautam, P.; Neha; Upadhyay, S.N.; Dubey, S.K. Bio-methanol as a renewable fuel from waste biomass: Current trends and future perspective. Fuel 2020, 273, 117783.
- Lei, Y.; Luo, Y.; Li, X.; Lu, J.; Mei, Z.; Peng, W.; Chen, R.; Chen, K.; Chen, D.; He, D. The role of samarium on Cu/Al2O3 catalyst in the methanol steam reforming for hydrogen production. Catal. Today 2018, 307, 162–168.
- Wang, F.; Li, L.; Liu, Y. Effects of flow and operation parameters on methanol steam reforming in tube reactor heated by simulated waste heat. Int. J. Hydrogen Energy 2017, 42, 26270–26276.
- Lytkina, A.A.; Orekhova, N.V.; Ermilova, M.M.; Yaroslavtsev, A.B. The influence of the support composition and structure (MХZr1-XO2-δ) of bimetallic catalysts on the activity in methanol steam reforming. Int. J. Hydrogen Energy 2018, 43, 198–207.
- Spassova, I.; Tsontcheva, T.; Velichkova, N.; Khristova, M.; Nihtianova, D. Catalytic reduction of NO with decomposed methanol on alumina-supported Mn–Ce catalysts. J. Colloid Interface Sci. 2012, 374, 267–277.
- Tsoncheva, T.; Gallo, A.; Scotti, N.; Dimitrov, M.; Delaigle, R.; Gaigneaux, E.M.; Kovacheva, D.; Dal Santo, V.; Ravasio, N. Optimization of the preparation procedure of cobalt modified silicas as catalysts in methanol decomposition. Appl. Catal. A Gen. 2012, 417-418, 209–219.
- Mihaylov, M.; Tsoncheva, T.; Hadjiivanov, K. Structure sensitivity of methanol decomposition on Ni/SiO2 catalysts. J. Mater. Sci. 2011, 46, 7144–7151.
- Li, C.-L.; Lin, Y.-C. Catalytic partial oxidation of methanol over copper–zinc based catalysts: A comparative study of alumina, zirconia, and magnesia as promoters. Catal. Lett. 2010, 140, 69–76.
- Li, C.-L.; Jiang, B.-S.; Fanchiang, W.-L.; Lin, Y.-C. The effect of Pd content in LaMnO3 for methanol partial oxidation. Catal. Commun. 2011, 16, 165–169.
- Kapran, A.Y.; Soloviev, S.O.; Orlyk, S.N. Decomposition and partial oxidation of methanol over metal oxide Cu–Zn–Ce-based monoliths. React. Kinet. Mech. Cat. 2010, 101, 343–353.
- Tang, H.-Y.; Erickson, P.; Yoon, H.C.; Liao, C.-H. Comparison of steam and autothermal reforming of methanol using a packed-bed low-cost copper catalyst. Int. J. Hydrogen Energy 2009, 34, 7656–7665.
- Yong, S.T.; Hidajat, K.; Kawi, S. Reaction study of auto thermal steam reforming of methanol to hydrogen using a novel nano CuZnAl-catalyst. J. Power Sources 2004, 131, 91–95.
- Yoon, H.C.; Erickson, P.A.; Kim, H.-M. Lowering the O2/CH3OH ratio in autothermal reforming of methanol by using a reduced copper-based catalyst. Int. J. Hydrog. Energy 2008, 33, 6619–6626.
- Chen, W.-H.; Syu, Y.-J. Thermal behavior and hydrogen production of methanol steam reforming and autothermal reforming with spiral preheating. Int. J. Hydrog. Energy 2011, 36, 3397–3408.
- Ploner, K.; Delir Kheyrollahi Nezhad, P.; Watschinger, M.; Schlicker, L.; Bekheet, M.F.; Gurlo, A.; Gili, A.; Doran, A.; Schwarz, S.; Stöger-Pollach, M.; et al. Steering the methanol steam reforming performance of Cu/ZrO2 catalysts by modification of the Cu-ZrO2 interface dimensions resulting from Cu loading variation. Appl. Catal. A Gen. 2021, 623, 118279.
- Azenha, C.; Lagarteira, T.; Mateos-Pedrero, C.; Mendes, A. Production of hydrogen from methanol steam reforming using CuPd/ZrO2 catalysts–Influence of the catalytic surface on methanol conversion and CO selectivity. Int. J. Hydrog. Energy 2021, 46, 17490–17499.
- Ruano, D.; Pabón, B.M.; Azenha, C.; Mateos-Pedrero, C.; Mendes, A.; Perez-Dieste, V.; Concepción, P. Influence of the ZrO2 crystalline phases on the nature of ctive sites in PdCu/ZrO2 catalysts for the methanol steam reforming reaction—An in situ spectroscopic study. Catalysts 2020, 10, 1005.
- Li, G.; Gu, C.; Zhu, W.; Wang, X.; Yuan, X.; Cui, Z.; Wang, H.; Gao, Z. Hydrogen production from methanol decomposition using Cu-Al spinel catalysts. J. Clean. Prod. 2018, 183, 415–423.
- Liu, D.; Men, Y.; Wang, J.; Kolb, G.; Liu, X.; Wang, Y.; Sun, Q. Highly active and durable Pt/In2O3/Al2O3 catalysts in methanol steam reforming. Int. J. Hydrog. Energy 2016, 41, 21990–21999.
- Martinelli, M.; Jacobs, G.; Graham, U.M.; Davis, B.H. Methanol steam reforming: Na doping of Pt/YSZ provides fine tuning of selectivity. Catalysts 2017, 7, 148.
- Köpfle, N.; Mayr, L.; Schmidmair, D.; Bernardi, J.; Knop-Gericke, A.; Hävecker, M.; Klötzer, B.; Penner, S. A comparative discussion of the catalytic activity and CO2-selectivity of Cu-Zr and Pd-Zr (Intermetallic) compounds in methanol steam reforming. Catalysts 2017, 7, 53.
- Fornari, A.C.; Menechini Neto, R.; Lenzi, G.G.; dos Santos, O.A.A.; de Matos, L.M.J. Utilization of sol-gel CuO-ZnO-Al2O3 catalysts in the methanol steam reforming for hydrogen production. Can. J. Chem. Eng. 2017, 95, 2258–2271.
- Mohtashami, Y.; Taghizadeh, M. Performance of the ZrO2 promoted CuZnO catalyst supported on acetic acid-treated MCM-41 in methanol steam reforming. Int. J. Hydrog. Energy 2019, 44, 5725–5738.
- Bagherzadeh, S.B.; Haghighi, M.; Rahemi, N. Novel oxalate gel coprecipitation synthesis of ZrO2-CeO2-promoted CuO-ZnO-Al2O3 nanocatalyst for fuel cell-grade hydrogen production from methanol: Influence of ceria-zirconia loading. Energy Convers Manag. 2017, 134, 88–102.
- Dong, C.; Yu, Q.; Ye, R.P.; Su, P.; Liu, J.; Wang, G.H. Hollow carbon sphere nanoreactors loaded with PdCu nanoparticles: Void-confinement effects in liquid-phase hydrogenations. Angew. Chem. Int. Ed. Engl. 2020, 59, 18374–18379.
- Yong, S.T.; Ooi, C.W.; Chai, S.P.; Wu, X.S. Review of methanol reforming-Cu-based catalysts, surface reaction mechanisms, and reaction schemes. Int. J. Hydrog. Energy 2013, 38, 9541–9552.
- Ke, C.; Lin, Z. Density functional theory based micro- and macro-kinetic studies of Ni-catalyzed methanol steam reforming. Catalysts 2020, 10, 349.
- Twigg, M.V.; Spencer, M.S. Deactivation of copper metal catalysts for methanol decomposition, methanol steam reforming and methanol synthesis. Top. Catal. 2003, 22, 191–203.
- Khani, Y.; Bahadoran, F.; Soltanali, S.; Ahari, J.S. Hydrogen production by methanol steam reforming on a cordierite monolith reactor coated with Cu–Ni/LaZnAlO4 and Cu–Ni/γ-Al2O3 catalysts. Res. Chem. Intermed. 2018, 44, 925–942.
- Kang, J.; Song, Y.; Kim, T.; Kim, S. Recent trends in the development of reactor systems for hydrogen production via methanol steam reforming. Int. J. Hydrog. Energy 2022, 47, 3587–3610.
- Ranjekar, A.M.; Yadav, G.D. Steam reforming of methanol for hydrogen production: A critical analysis of catalysis, processes, and scope. Ind. Eng. Chem. Res. 2021, 60, 89–113.
- Kim, S.; Yun, S.-W.; Lee, B.; Heo, J.; Kim, K.; Kim, Y.-T.; Lim, H. Steam reforming of methanol for ultra-pure H2 production in a membrane reactor: Techno-economic analysis. Int. J. Hydrog. Energy 2019, 44, 2330–2339.
- Zhuang, X.; Xu, X.; Li, L.; Deng, D. Numerical investigation of a multichannel reactor for syngas production by methanol steam reforming at various operating conditions. Int. J. Hydrog. Energy 2020, 45, 14790–14805.
- Wang, G.; Wang, F.;Chen, B. Performance study on methanol steam reforming rib micro-reactor with waste heat recovery. Energies 2020, 13, 1564.
- Mironova, E.Y.; Lytkina, A.A.; Ermilova, M.M.; Orekhova, N.V.; Zhilyaeva, N.A.; Roshan, N.R.; Ievlev, V.M.; Yaroslavtsev, A.B. Methanol steam reforming in a reactor with a palladium–copper membrane in the presence of a nickel–copper catalyst. Pet. Chem. 2020, 60, 1232–1238.
- Li, X.; Rezaei, F.; Rownaghi, A.A. 3D-printed zeolite monoliths with hierarchical porosity for selective methanol to light olefin reaction. React. Chem. Eng. 2018, 3, 733–746.
- Du, W.; Lee, M.-T.; Wang, Y.; Zhao, C.; Li, G.; Li, M. Design of a solar-driven methanol steam reforming receiver/reactor with a thermal storage medium and its performance analysis. Int. J. Hydrog. Energy 2020, 45, 33076–33087.
- Sanches, S.G.; Flores, J.H.; da Silva, M.I.P. Cu/ZnO and Cu/ZnO/ZrO2 catalysts used for methanol steam reforming. Mol. Catal. 2018, 454, 55–62.
- Liao, M.; Guo, C.; Guo, W.; Hu, T.; Xie, J.; Gao, P.; Xiao, H. One-step growth of CuO/ZnO/CeO2/ZrO2 nanoflowers catalyst by hydrothermal method on Al2O3 support for methanol steam reforming in a microreactor. Int. J. Hydrog. Energy 2021, 46, 9280–9291.
- Fasanya, O.O.; Atta, A.Y.; Myint, M.T.Z.; Dutta, J.; Jibril, B.Y. Effects of synthesis methods on performance of CuZn/MCM-41 catalysts in methanol steam reforming. Int. J. Hydrog. Energy 2021, 46, 3539–3553.
- Pu, Y.-C.; Li, S.-R.; Yan, S.; Huang, X.; Wang, D.; Ye, Y.-Y.; Liu, Y.-Q. An improved Cu/ZnO catalyst promoted by Sc2O3 for hydrogen production from methanol reforming. Fuel 2019, 241, 607–615.
- Cheng, Z.; Zhou, W.; Lan, G.; Sun, X.; Wang, X.; Jiang, C.; Li, Y. High-performance Cu/ZnO/Al2O3 catalysts for methanol steam reforming with enhanced Cu-ZnO synergy effect via magnesium assisted strategy. J. Energy. Chem. 2021, 63, 550–557.
- Huang, H.-Y.; Chen, H.-I.; Huang, Y.-J. The promotional effects of ZrO2 and Au on the CuZnO catalyst regarding the durability and activity of the partial oxidation of methanol. Catalysts 2018, 8, 345.
- Lorenzut, B.; Montini, T.; De Rogatis, L.; Canton, P.; Benedetti, A.; Fornasiero, P. Hydrogen production through alcohol steam reforming on Cu/ZnO-based catalysts. Appl. Catal. B Environ. 2011, 101, 397–408.




