
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ekta Menghani | -- | 2787 | 2022-07-20 20:35:37 | | | |
| 2 | Lindsay Dong | Meta information modification | 2787 | 2022-07-21 07:46:51 | | |
Video Upload Options
Environmental factors, such as high light intensity, adverse temperature, drought, or soil salinity, are summarized as abiotic stresses and discriminated from biotic stresses that are exerted by pathogens and herbivores, for instance. It was an unexpected observation that overproduction of reactive oxygen species (ROS) is a common response to all kinds of stress investigated so far. ROS are important messengers in cell signaling, but exceeding a concentration threshold causes damage. This requires fine-tuning of ROS production and degradation rates. In general, there are two options to control cellular ROS levels, (I) ROS scavenging at the expense of antioxidant consumption and (II) enzyme-controlled degradation of ROS. As antioxidants are limited in quantity, the first strategy only allows temporarily buffering of a certain cellular ROS level. This way, it prevents spells of eventually damaging ROS concentrations.
1. Introduction
2. ROS Production
2.1. ROS Production in Metabolism
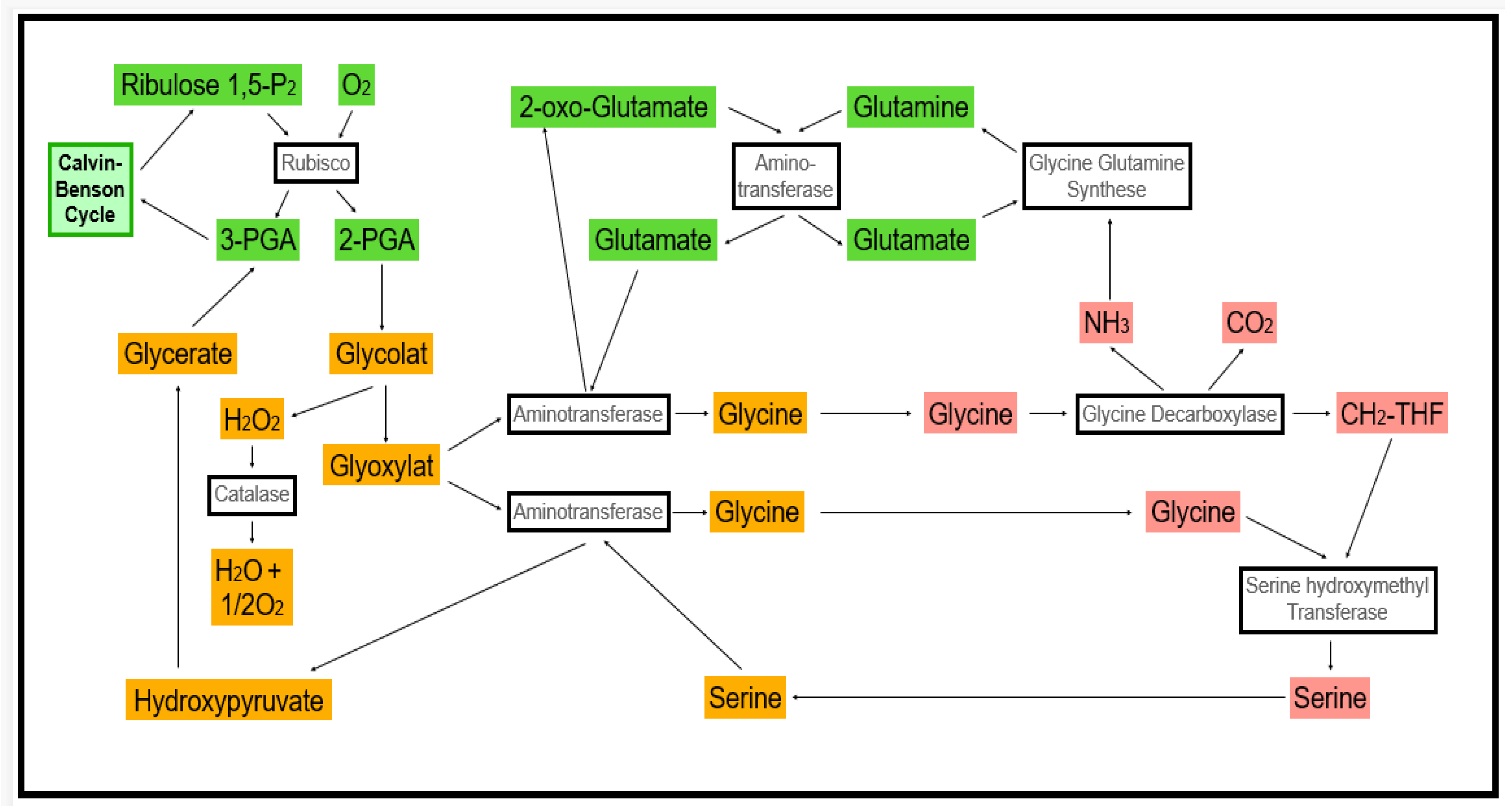
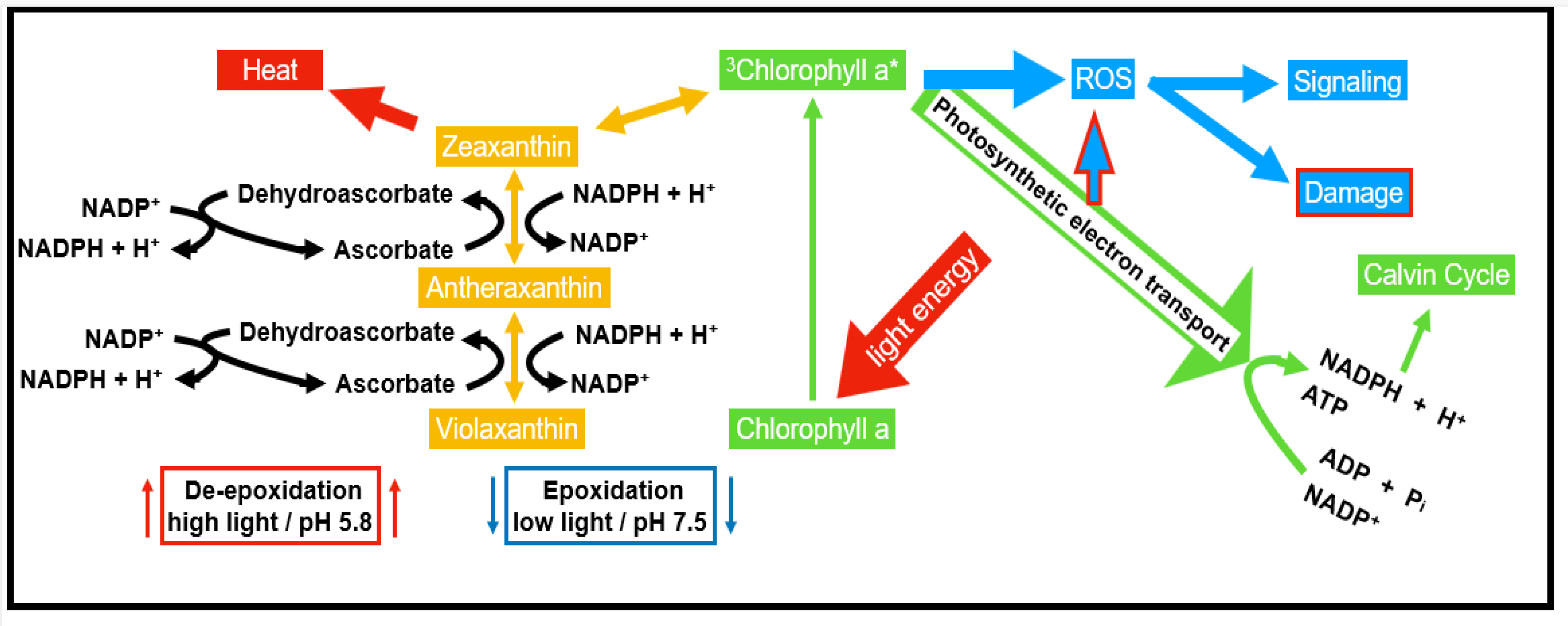
2.2. ROS Production as a Response to Pathogen Attack
2.3. Abiotic Stress-Induced ROS Production
3. Components Controlling the ROS Level in Plants
3.1. Major Antioxidants Involved
3.1.1. Ascorbic Acid (Vitamin C)
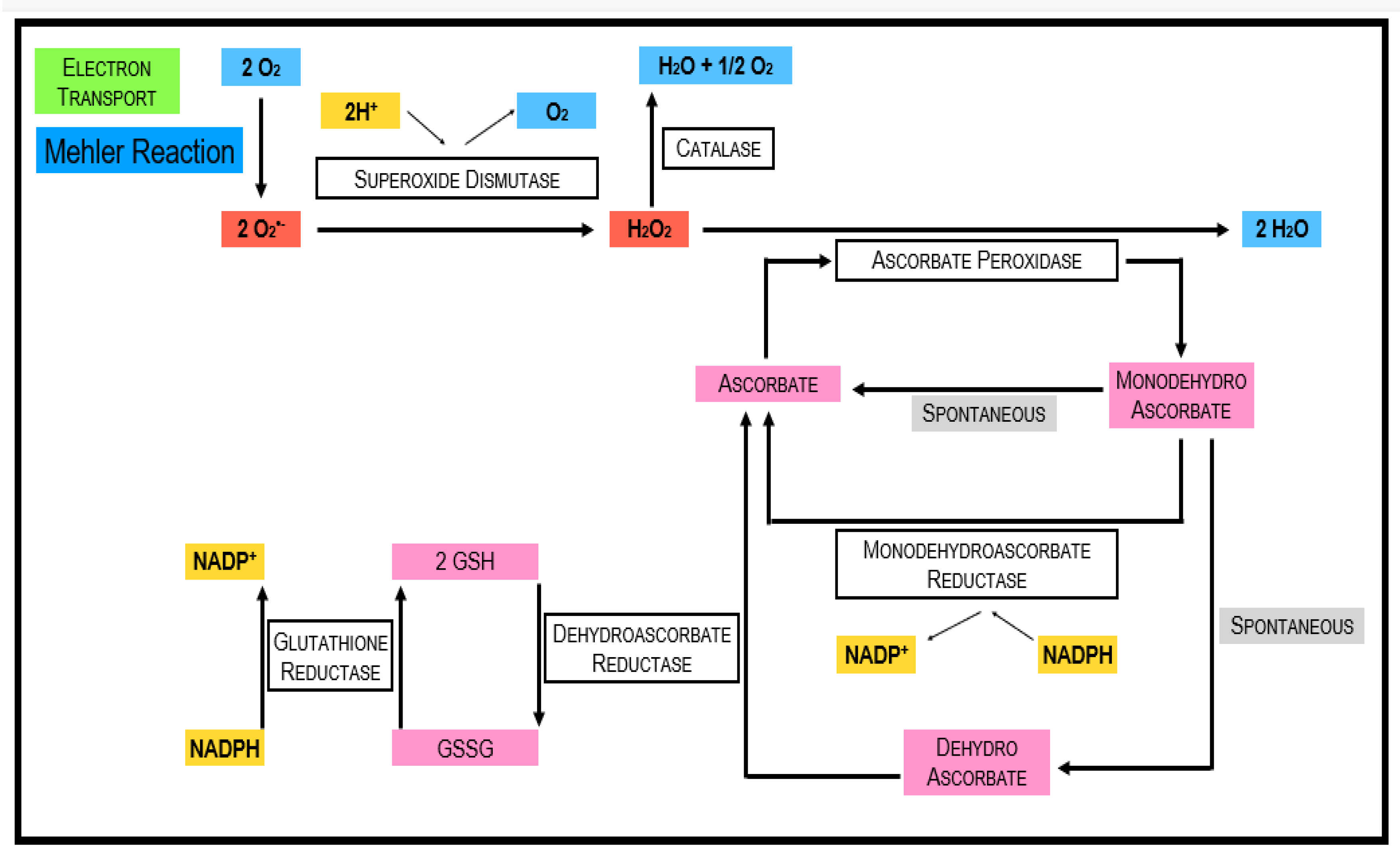
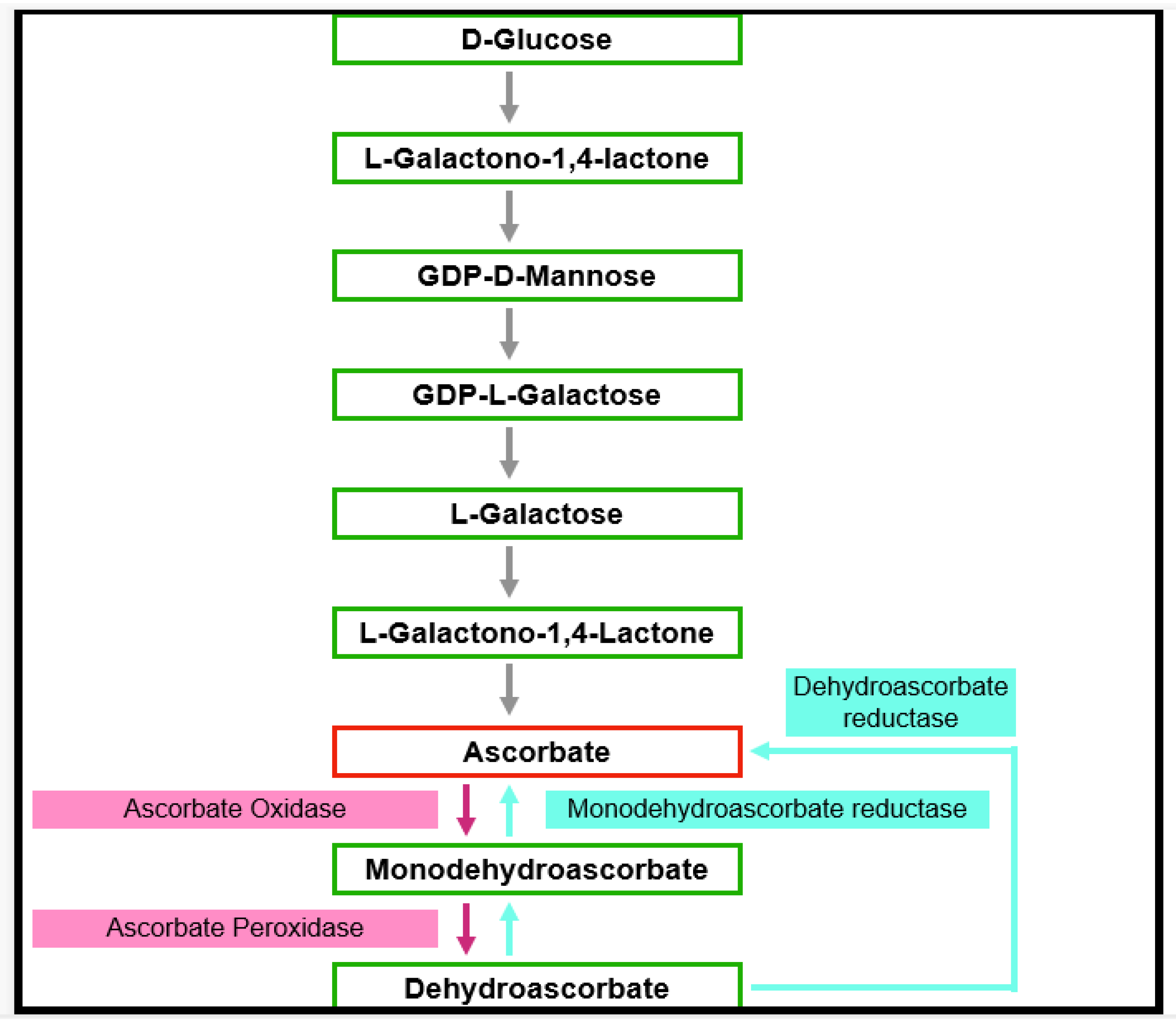
3.1.2. α-Tocopherol
3.1.3. Glutathione
3.1.4. Carotenoids
3.1.5. Flavonoids
3.2. Enzymes Catalyzing ROS Removal
3.2.1. Superoxide Dismutase (SOD; EC.1.15.1)
3.2.2. Catalases (CAT; EC 1.11.1.6)
3.2.3. Ascorbate Peroxidase (APX; E.C. 1.1.11.1)
3.2.4. Monodehydroascorbate Reductase (MDHAR; E.C. 1.6.5.4)
3.2.5. Glutathione Peroxidases (GPX, EC 1.11.1.9)
4. Regulating ROS Concentrations in Plant Cells
4.1. Stress Perception and Signaling
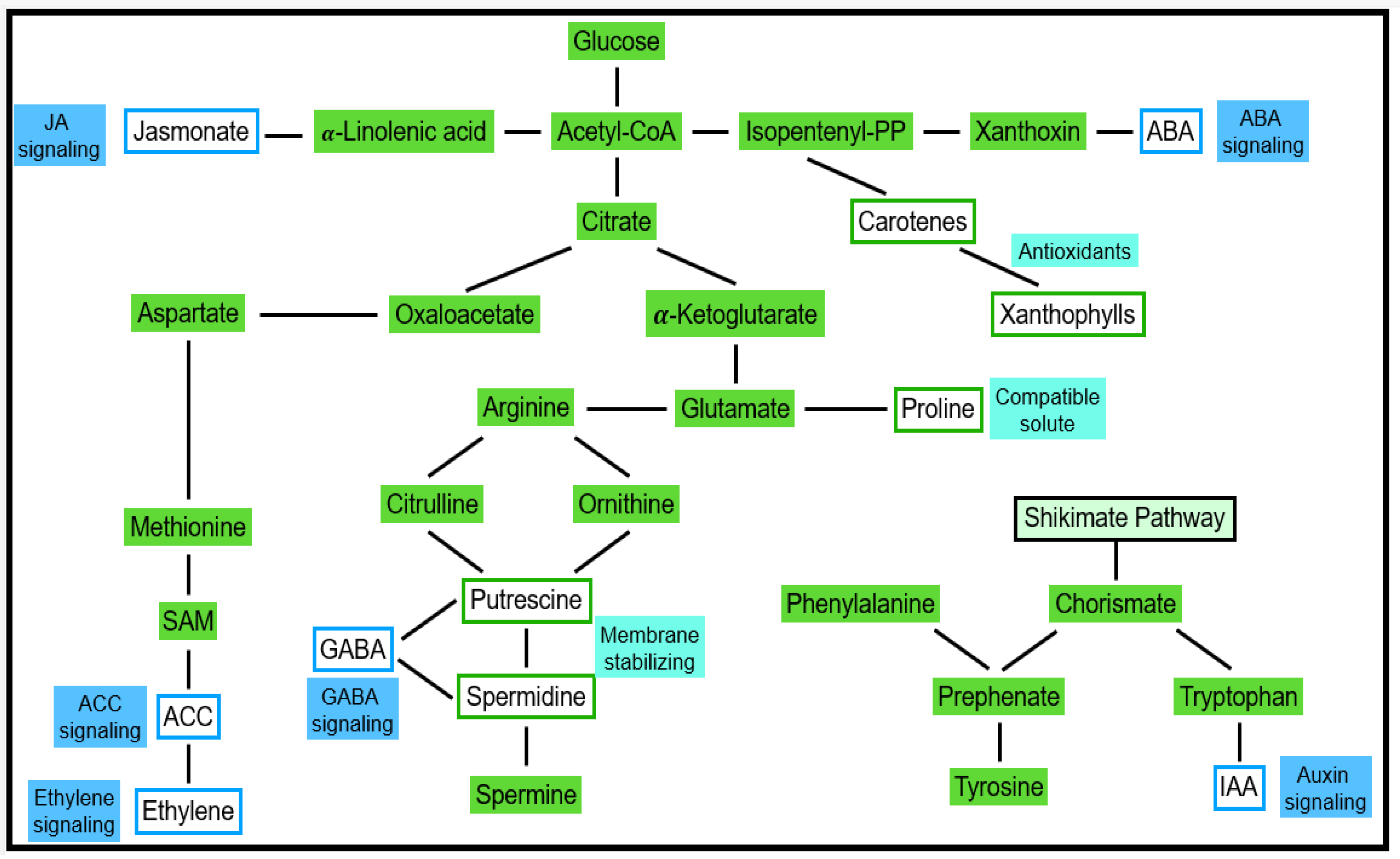
4.2. ROS in Signaling Events
4.3. Regulating the Activity of ROS Scavenging Enzymes
References
- Jaleel, C.A.; Manivannan, P.; Lakshmanan, G.M.A.; Gomathinayagam, M.; Panneerselvam, R. Alterations in morphological parameters and photosynthetic pigment responses of Catharanthus roseus under soil water deficits. Colloids Surf. B Biointerfaces 2008, 61, 298–303.
- Des Marais, D.L.; Hernandez, K.M.; Juenger, T.E. Genotype-by-environment interaction and plasticity: Exploring genomic responses of plants to the abiotic environment. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 5–29.
- Tuteja, N.; Ahmad, P.; Panda, B.B.; Tuteja, R. Genotoxic stress in plants: Shedding light on DNA damage, repair and DNA repair helicases. Mutat. Res. 2009, 681, 134–149.
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214.
- Queval, G.; Issakidis-Bourguet, E.; Hoeberichts, F.-A.; Vandorpe, M.; Gakiere, B.; Vanacker, H.; Miginiac-Maslow, M.; Van Breusegem, F.; Doctor, G. Conditional oxidative stress responses in the Arabidopsis photo respiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J. 2007, 52, 640–657.
- Mullineaux, P.M.; Exposito-Rodriguez, M.; Lassie, P.P.; Smirnoff, N. ROS-dependent signalling pathways in plants and algae exposed to high light: Comparisons with other eukaryotes. Free Radic. Biol. Med. 2018, 122, 52–64.
- Schwarzlander, M.; Fricker, M.D.; Sweetlove, L.J. Monitoring the in vivo redox state of plant mitochondria: Effect of respiratory inhibitors, abiotic stress and assessment of recovery from oxidative challenge. Biochim. Biophys. Acta-Bioenerg. 2009, 1787, 468–475.
- Huang, S.; Van Akin, O.; Schwarzlander, M.; Belt, K.; Millar, A.H. The roles of mitochondrial reactive oxygen species in cellular signaling and stress response in plants. Plant Physiol. 2016, 171, 1551–1559.
- Wojtaszek, P. Oxydation burst: An early plant response to pathogen infection. Biochem. J. 1997, 322, 681–692.
- Lichtenthaler, H.K. Vegetation stress: An introduction to the stress concept in plants. J. Plant Physiol. 1996, 148, 4–14.
- Zurbriggen, M.D.; Carrillo, N.; Tognetti, V.B.; Melzer, M.; Peskier, M.; Hause, B.; Hajirezei, M.-R. Chloroplast-generated reactive oxygen species play a major role in localized cell death during the non-host interaction between tobacco and Xanthomonas campestri pv. vesicatoria. Plant J. 2009, 60, 962–973.
- Karlusich, J.J.P.; Zurbriggen, M.D.; Shahinnia, F.; Sonnewald, S.; Sonnewald, U.; Hosseini, S.A.; Hajirezaei, M.-R.; Carrillo, N. Chloroplast redox status modulates genome-wide plant responses during the non-host interaction of Tobacco with the hemibiotrophic bacterium Xanthomonas campestris pv. vesicatoria. Front. Plant Sci. 2017, 8, 1158.
- Zurbriggen, M.D.; Carrillo, N.; Hajirezaei, M.-R. ROS signaling in the hypersensitive response. Plant Signal. Behav. 2010, 5, 393–396.
- Lichtenthaler, H. The Stress Concept in Plants: An Introduction. Ann. N. Y. Acad. Sci. 2006, 851, 187–198.
- Lebaudy, A.; Vavasseur, A.; Hosy, E.; Dreyer, I.; Leonhardt, N.; Thibaud, J.-B.; Véry, A.-A.; Simonneau, T.; Sentenac, H. Plant adaptation to fluctuating environment and biomass production are strongly dependent on guard cell potassium channels. Proc. Natl. Acad. Sci. USA 2008, 105, 5271–5276.
- Etsuo, N. Oxidative Stress and antioxidants: Distress or eustress? Arch. Biochem. Biophys. 2016, 595, 19–24.
- Obata, T.; Fernie, R. The use of metabolomics to dissect plant responses to abiotic stress. Cell. Mol. Life Sci. 2012, 69, 3225–3243.
- Pommerrenig, B.; Ludewig, F.; Cvetkovic, J.M.; Trentmann, O.; Klemens, P.A.W.; Neuhaus, H.E. In Concert: Orchestrated changes in carbohydrate homeostasis are critical for plant abiotic stress tolerance. Plant Cell Physio. 2018, 59, 1290–1299.
- Paul, M.J.; Foyer, C.H. Sink regulation of photosynthesis. J. Exp. Botany 2001, 52, 1383–1400.
- Xiong, L.; Schumaker, K.S.; Zhu, J.K. Cell signaling during cold, drought, and salt stress. Plant Cell 2002, 14, 165–183.
- Foyer, C.H.; Trebst, A.; Noctor, G. Protective and signaling functions of ascorbate, glutathione and tocopherol in chloroplasts. In Advances in Photosynthesis and Respiration: Photoprotection, Photoinhibition, Gene Regulation, and Environment; Demmig-Adams, B., Adams, W.W., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 241–268.
- Gallie, D.R. L-Ascorbic Acid: A Multifunctional Molecule Supporting Plant Growth and Development. Scientifica 2013, 1–24.
- Barata-Soares, A.D.; Gomez, M.L.P.A.; de Mesquita, C.H.; Franco, M.; Lajolo, F.M. Ascorbic acid biosynthesis: A precursor study on plants. Braz. J. Plant Physiol. 2004, 16, 147–154.
- Srivalli, B.; Chinnusamy, V.; Khanna-Chopra, R. Antioxidant defense in response to abiotic stresses in plants. J. Plant Biol. 2003, 30, 121–139.
- Fiedler, E.; Soll, J.; Schultz, G. The formation of homogentisate in the biosynthesis of tocopherol and plastoquinone in spinach chloroplasts. Planta 1982, 155, 511–515.
- Heintze, A.; Goerlach, J.; Leuschner, C.; Hoppe, P.; Hagelstein, P.; Schulze-Siebert, D.; Schultz, G. Plastidic isoprenoid synthesis during chloroplast development. Plant Physiol. 1990, 93, 1121–1127.
- Pellaud, S.; Mené-Saffrané, L. Metabolic Origins and Transport of Vitamin E Biosynthetic Precursors. Front. Plant Sci. 2017, 8, 1959.
- Hausmann, N.; Werhahn, W.; Huchzermeyer, B.; Braun, H.P.; Papenbrock, J. How to document the purity of mitochondria prepared from green tissue of pea, tobacco and Arabidopsis thaliana. Phyton 2003, 3, 215–229.
- Hare, P.D.; Cress, W.A.; Staden, J.V. Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 1998, 21, 535–553.
- Igamberdiev, A.U.; Hill, R.D. Nitrate, NO and haemoglobin in plant adaptation to hypoxia: An alternative to classic fermentation pathways. J. Exp. Bot. 2004, 55, 2473–2482.
- Ledford, H.K.; Niyogi, K.K. Singlet oxygen and photo-oxidative stress management in plants and algae. Plant Cell Environ. 2005, 28, 10.
- Noctor, G. Metabolic signalling in defence and stress: The central roles of soluble redox couples. Plant Cell Environ. 2006, 29, 409–425.
- Millar, A.H.; Mittova, V.; Kiddle, G.; Heazlewood, J.L.; Bartoli, C.G.; Theodoulou, F.L.; Foyer, C.H. Control of ascorbate synthesis by respiration and its implications for stress responses. Plant Physiol. 2003, 133, 443–447.
- Mullineaux, P.M.; Rausch, T. Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Photosyn. Res. 2005, 86, 459–474.
- May, M.; Vernoux, T.; Leaver, C.; Van Montagu, M.; Inze, D. Glutathione homeostasis in plants: Implications for environmental sensing and plant development. J. Exp. Bot. 1998, 49, 649–667.
- Horst, I.; Heimann, L.; Peterhansel, C. Signal integration on plant promoters A case study in maize. Plant Signal. Behav. 2013, 8, e25389.
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76.
- Gill, S.S.; Anjum, N.A.; Gill, R.; Yadav, S.; Hasanuzzaman, M.; Fujita, M.; Mishra, P.; Sabat, S.C.; Tuteja, N. Superoxide dismutase—Mentor of abiotic stress tolerance in crop plants. Environ. Sci. Pollut. Res. 2015, 22, 10375–10394.
- Frugoli, J.A.; Zhong, H.H.; Nuccio, M.L.; McCourt, P.; McPeek, M.A.; Thomas, T.L.; McClung, C.R. Catalase is encoded by a multigene family in Arabidopsis thaliana (L.). Heynh. Plant Physiol. 1996, 112, 327–336.
- Willekens, H.; Villarroel, R.; Van Montagu, M.; Inzé, D.; Van Camp, W. Molecular identification of catalases from Nicotiana plumbaginifolia (L.). FEBS Lett. 1994, 352, 79–83.
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279.
- Davletova, S.; Rizhsky, L.; Liang, H.; Shengqiang, Z.; Oliver, D.J.; Coutu, J.; Shulaev, V.; Schlauch, K.; Mittler, R. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 2005, 17, 268–281.
- Wang, J.; Zhang, H.; Allen, R.D. Overexpression of an Arabidopsis peroxisomal ascorbate peroxidase gene in tobacco increases protection against oxidative stress. Plant Cell Physiol. 1999, 40, 725–732.
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410.
- Dixon, D.P.; Cummins, L.; Cole, D.J.; Edwards, R. Glutathione-mediated detoxification systems in plants. Curr. Opin. Plant Biol. 1998, 1, 258–266.
- Gueta-Dahan, Y.; Yaniv, Z.; Zilinskas, B.A.; Ben-Hayyim, G. Salt and oxidative stress: Similar and specific responses and their relation to salt tolerance in citrus. Planta 1997, 203, 460–469.
- Willekens, H.; Chamnongpol, S.; Davey, M.; Schraudner, M.; Langebartels, C.; Van Montagu, M.; Inzé, D.; Van Camp, W. Catalase is a sink for H2 O2 and is indispensable for stress defence in C3 plants. EMBO J. 1997, 16, 4806–4816.
- Asada, K. The water–water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639.
- Bhargava, S.; Sawant, K. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32.
- Forni, C.; Duca, D.; Glick, B.B. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356.
- Lata, C.; Muthamilarasan, M.; Prasad, M. Drought stress responses and signal transduction in plants. In Elucidation of Abiotic Stress Signaling in Plants; Pandey, G.K., Ed.; Springer: New York, NY, USA, 2015; pp. 195–224.
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 2014, 21, 133–139.
- Parida, A.K.; Das, A.B.; Mohanty, P. Investigations on the antioxidative defense responses to NaCl stress in a mangrove, Bruguiera parviflora: Differential regulations of isoforms of some antioxidative enzymes. Plant Growth Regul. 2004, 42, 213–226.
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681.
- Zhang, J.; Li, X.-M.; Lin, H.-X.; Chong, K. Crop improvement through temperature resilience. Annu. Rev. Plant Biol. 2019, 70, 753–780.
- Soma, F.; Takahashi, F.; Yamagushi-Shinozaki, K.; Shinozaki, K. Cellular phosphorylation, signaling and gene expression in drought stress responses: ABA-dependent and ABA-independent regulation systems. Plants 2021, 10, 756.
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global Plant Stress Signaling: Reactive Oxygen Species at the Cross-Road. Front. Plant Sci. 2016, 7, 187.
- Finkelstein, R. Abscisic Acid synthesis and response. Arabidopsis Book 2013, 11, e0166.
- Lan Thi Hoang, X.; Du Nhi, N.H.; Binh Anh Thu, N.; Phuong Thao, N.; Phan Tran, L.S. Transcription factors and their roles in signal transduction in plants under abiotic stresses. Curr. Genom. 2017, 18, 483–497.
- Foyer, C.H.; Noctor, G. Oxygen processing in photosynthesis: Regulation and signalling. New Phytol. 2000, 146, 359–388.
- Chugh, V.; Kaur, N.; Grewal, M.S.; Gupta, A.K. Differential antioxidative response of tolerant and sensitive maize (Zea mays L.) genotypes to drought stress at reproductive stage. Indian J. Biochem. Biophys. 2013, 5, 150–158.
- Terzi, R.; Kadioglu, A. Drought stress tolerance and the antioxidant enzyme system in Ctenanthe setosa. Acta Crocoviensia-Ser. Bot. 2006, 48, 89–96.




