Environmental factors, such as high light intensity, adverse temperature, drought, or soil salinity, are summarized as abiotic stresses and discriminated from biotic stresses that are exerted by pathogens and herbivores, for instance. It was an unexpected observation that overproduction of reactive oxygen species (ROS) is a common response to all kinds of stress investigated so far. ROS are important messengers in cell signaling, but exceeding a concentration threshold causes damage. This requires fine-tuning of ROS production and degradation rates. In general, there are two options to control cellular ROS levels, (I) ROS scavenging at the expense of antioxidant consumption and (II) enzyme-controlled degradation of ROS. As antioxidants are limited in quantity, the first strategy only allows temporarily buffering of a certain cellular ROS level. This way, it prevents spells of eventually damaging ROS concentrations.
- stress response
- ROS
- antioxidants
1. Introduction
2. ROS Production
ROS-producing reactions can be categorized into three groups: (1) ROS production as a side reaction in metabolism, (2) ROS production as a response to pathogen attack (biotic stress response), and (3) ROS production during the abiotic stress response. In any of these cases, ROS (H2O2) functions as signaling compounds in addition to potentially damaging effects. Thus, ROS control cellular homeostasis and, this way, may stimulate adaptation to adverse growth conditions (contributing to the development of stress tolerance).2.1. ROS Production in Metabolism
ROS are byproducts of metabolic activity. They are important regulators of cellular homeostasis, but their synthesis and storage have to be controlled to prevent ROS from eventually reaching toxic concentrations. In all cell compartments, non-enzymatic and enzymatic antioxidants are present to protect cell contents from ROS damage. ROS results in H2O2 production and is the most important component in signaling [4][19]. In peroxisomes and glyoxysomes, we find oxidase activities were found. In leaves, the glycolate oxidase is among the most active enzymes [5][20]. It is involved in the photorespiration pathway (Figure 1). Further oxidase activities are linked to fatty acid oxidation (acyl-CoA oxidase) and polyamide and purine metabolism, for instance. While photorespiration-dependent oxidase activity is dominant in green tissues in the light, high rates of H2O2 production can be found in glyoxysomes of germinating oil seeds.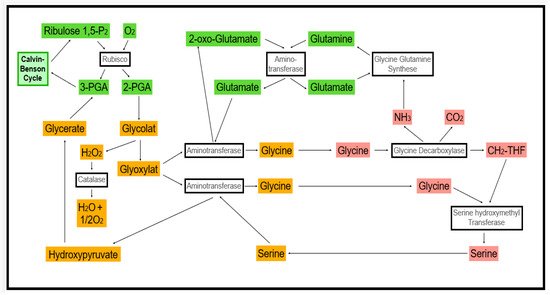
2.2. ROS Production as a Response to Pathogen Attack
2.3. Abiotic Stress-Induced ROS Production
The metabolic flux, i.e., the consumption of assimilates released from chloroplasts in the light, is inhibited under stress, and the probability of light-induced ROS production increases (Figure 1) [17][18][35,36]. Sink regulation of photosynthetic activity is dependent on the physiological state of the plant. In other words, the plant is integrating adverse stress effects occurring at the same time on the basis of assimilating supply to sink organs [19][37]. Feedback inhibition of photosynthesis increases the probability of ROS production. ROS functions as messengers in retrograde gene regulation in the nucleus, but increasing ROS concentrations causes damage to cell components.3. Components Controlling the ROS Level in Plants
3.1. Major Antioxidants Involved
3.1.1. Ascorbic Acid (Vitamin C)
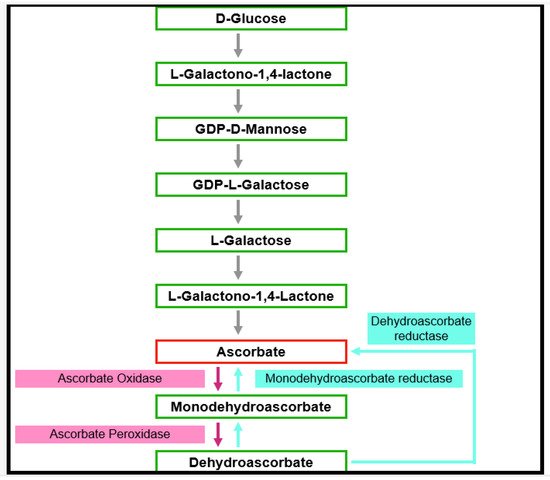
Ascorbate is thought to be the most abundant antioxidant [20][38]. It interacts with other antioxidants, thus forming an antioxidant network (Figure 3) [21][39]. Phosphorylated sugars and nucleotide-linked sugars of the cytosolic carbohydrate pool are substrates for ascorbate synthesis, while the last steps of ascorbate synthesis take place inside the mitochondria [22][40]. Several pathways leading to ascorbate formation were identified, but it is generally agreed that the Smirnow–Wheeler pathway is the most important one for ascorbate biosynthesis in plants (Figure 4) [23][41].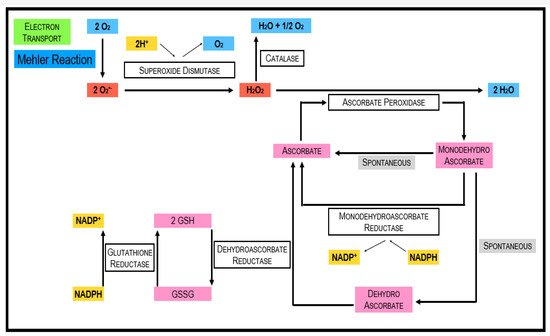
3.1.2. α-Tocopherol
3.1.3. Glutathione
Glutathione is a tripeptide (γ-glutamyl-cysteinyl glycine), which was found in essentially all cell compartments such as cytosol, vacuoles, chloroplasts, mitochondria, and endoplasmic reticulum [33][52]. It is engaged with a wide scope of processes such as cell differentiation, cell development/division, cell death, senescence, detoxification of xenobiotics, formation of metabolites, regulation of enzymatic action, synthesis of proteins, and nucleotides, lastly, articulation of stress-responsive genes [34][53]. The reactivity of the thiol group of glutathione makes it especially appropriate to serve a wide scope of biochemical capacities in all living beings. The nucleophilic nature of the thiol is significant in the development of mercaptide bonds with metals and for reacting with individual electrophiles [35][54]. For both enzymatically and non-enzymatically reduction of DHA (dehydroascorbate), GSH is used. In these reactions, two GSH molecules are oxidized to GSSG (oxidized glutathione). Glutathione reductase and NADPH are utilized for the regeneration of GSSG to recycle 2 GSH.3.1.4. Carotenoids
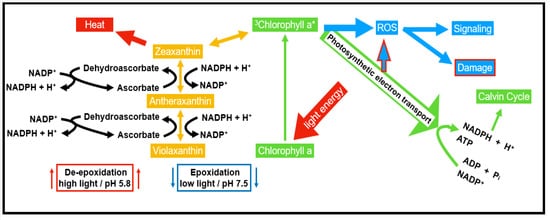
Carotenoids have a place within a group of lipophilic antioxidants, which are confined in the plastids of both photosynthetic and non-photosynthetic plant tissues. They are tracked down not just in plants but also found in microorganisms. Carotenoids show their antioxidative property by securing the photosynthetic apparatus in four ways, (a) responding with LPO items to end the radical chain reaction, (b) interacting with 1O2 and creating heat as a byproduct, (c) preventing light-dependent production of 1O2 by reacting with 3Chl* and exciting chlorophyll (Chl*), and (d) interacting with xanthophylls to allow transfer of excitation energy. This reaction allows the release of surplus energy as heat through the xanthophyll cycle [36][12].3.1.5. Flavonoids
Flavonoids are generally found in the plant kingdom preferentially in the leaves, flower organs, and pollen grains. Flavonoids can be ordered into four classes based on their structure, flavonols, flavones, isoflavones, and anthocyanins. They were considered a secondary ROS scavenging system in plants. They likewise interact with 1O2, and this way reduces the risk of peroxidation of membrane lipids [37][55]. Flavonoids are the only antioxidant biomolecules that possess the capacity to absorb UV radiation. Absorbed energy quanta result in a generation of ROS. The ROS generation from certain flavonoids was studied using fluorescence probes. Flavonoids generate three ROS types: the superoxide anion radical (O2•− ), the hydroxyl radical (•OH), and singlet oxygen (1O2). This is based on the presence of the 2,3 double bond found in all flavonoids.3.2. Enzymes Catalyzing ROS Removal
3.2.1. Superoxide Dismutase (SOD; EC.1.15.1)
The superoxide dismutase enzyme family is arranged into three categories Cu/Zn-SOD, Fe-SOD, and Mn-SOD. They are protecting from damage by dismutating O2•− into O2 and H2O2 and lessening the probability of •OH formation [38][57]. Cu/ZnSOD is present in chloroplasts and the cytosol of the plant cell, and MnSOD is present in peroxisomes and the mitochondrial matrix. The upregulation of SODs is part of the oxidative stress response and is crucial for the survival of plants.3.2.2. Catalases (CAT; EC 1.11.1.6)
Catalases are preferentially found in peroxisomes. They are tetrameric heme-containing enzymes that convert 2 H2O2 to O2 + 2H2O [24][43]. Many plants have different catalase isozymes. Six were found in Arabidopsis, two in castor bean [39][58]. They can dismutate H2O2 or, on the other hand, can oxidize substrates such as ethanol, methanol, formic acid, and formaldehyde. Plant catalases can be grouped into three classes: class I catalases are generally noticeable in photosynthetic tissues and are associated with the expulsion of H2O2 delivered during photorespiration; class II catalases are produced in vascular tissues and may assume a part in lignification, and their accurate biological function stays obscure; class III catalases are profoundly plentiful in seeds, and young plants and their function connect with the removal of excessive H2O2 delivered during unsaturated fat degradation in the glyoxylate cycle in glyoxysomes [40][59].3.2.3. Ascorbate Peroxidase (APX; E.C. 1.1.11.1)
APX is an essential part of the Ascorbate–Glutathione (ASC-GSH) cycle. While CAT preferentially scavenges H2O2 in the peroxisomes, APX fills a similar role in the chloroplast and cytosol. Ascorbic acid is used as a reducing agent to reduce H2O2 to H2O and also DHA. The APX family comprises at minimum five distinct isoforms, including thylakoid and microsomal membrane-bound structures, just as dissolvable stromal, cytosolic, and also apoplastic enzymes [41][42][43][66,67,68]. APX is a more efficient scavenger of H2O2 in times of stress because it is widely distributed and has a better affinity for H2O2 than CAT. The isoform that is more responsive to light-mediated oxidative stress is APX1. This is due to the suppression of tylEX. An enhanced stress tolerance could be observed when the expression of tylAPX was stimulated [43][68].3.2.4. Monodehydroascorbate Reductase (MDHAR; E.C. 1.6.5.4)
MDHAR is liable for recovering ascorbic acid (AA) from the fleeting MDHA, involving NADPH as a reducing agent, ultimately renewing the cell AA pool. Since it recovers AA, it is co-localized with the APX in the mitochondria and peroxisomes, where APX rummages H2O2 and oxidizes AA in the process [44][69]. MDHAR has a few isozymes that are bound in chloroplast, glyoxysomes, mitochondria, cytosol, and peroxisomes.3.2.5. Glutathione Peroxidases (GPX, EC 1.11.1.9)
Glutathione peroxidases are a group of numerous isozymes which catalyze the reduction in H2O2 [45][46][47][70,71,72]. It assumes an essential part in the biosynthesis of lignin, just as guards against biotic stress by debasing indole acetic acid (IAA) and using H2O2 all the while. GPX favors fragrant aromatic compounds such as guaiacol and pyrogallol [48][73] as electron donors. GPxs in plants are characterized into three kinds: selenium-subordinate (GPx, EC 1.11.1.19), the non-selenium-subordinate phospholipids hydroperoxide GPx (PHGPX), and glutathione transferases (GST, EC 2.5.1.18) showing GPx movement (GST-GPx). Due to its presence in cytosol and vacuole, it is considered a vital enzyme in the evacuation of H2O2. PHGPx was displayed to react to salt stress [46][71], and this increment in action was seen in catalase deficient tobacco plants [45][70]. In citrus, PHGPx protein and the gene encoding were isolated and characterized.4. Regulating ROS Concentrations in Plant Cells
4.1. Stress Perception and Signaling
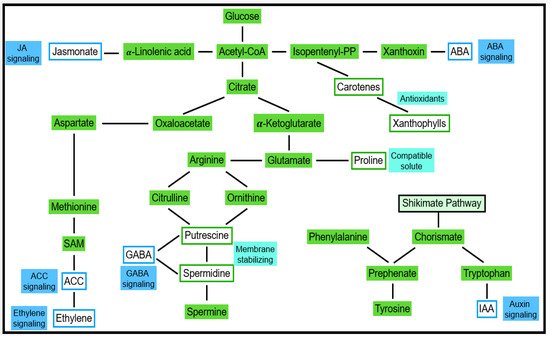
Several forms of abiotic stress are indirectly linked to water deficit stress. This applies to ionic stresses (salt/sodic stress, high nutrient stress, etc.) and cold stress, for instance [49][50][76,77]. As a result, responses of cell turgor are observed, and the cellular concentration of ABA increases [51][78]. ABA-dependent and ABA-independent responses were described [52][79]. Due to ABA transport, respective effects can be observed in the whole plant. Stomata closure is among the most obvious ABA responses. By using molecular methods, the expression of ABA-responsive genes was observed [41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80]. Gene products can be categorized into compatible solutes and compounds conferring protection from osmotic and ionic damage. As shown in Figure 5, metabolites of primary carbohydrate and amino acid pathways function as substrates for the respective catalysis of these compounds. Among the second type of products are signaling compounds and transcription factors regulating further genes [57][58][59][81,82,83].