Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xiaoyan Yang | -- | 252 | 2022-07-20 13:15:43 | | | |
| 2 | Amina Yu | + 2869 word(s) | 3121 | 2022-07-21 04:27:05 | | | | |
| 3 | Amina Yu | -3 word(s) | 3118 | 2022-07-21 11:06:01 | | | | |
| 4 | Xiaoyan Yang | Meta information modification | 3118 | 2022-07-21 12:15:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wang, Q.; Yang, X.; Zhu, C.; Liu, G.; Sun, Y.; Qian, L. Extraction of Tea Polysaccharides. Encyclopedia. Available online: https://encyclopedia.pub/entry/25340 (accessed on 08 February 2026).
Wang Q, Yang X, Zhu C, Liu G, Sun Y, Qian L. Extraction of Tea Polysaccharides. Encyclopedia. Available at: https://encyclopedia.pub/entry/25340. Accessed February 08, 2026.
Wang, Qian, Xiaoyan Yang, Changwei Zhu, Guodong Liu, Yujun Sun, Lisheng Qian. "Extraction of Tea Polysaccharides" Encyclopedia, https://encyclopedia.pub/entry/25340 (accessed February 08, 2026).
Wang, Q., Yang, X., Zhu, C., Liu, G., Sun, Y., & Qian, L. (2022, July 20). Extraction of Tea Polysaccharides. In Encyclopedia. https://encyclopedia.pub/entry/25340
Wang, Qian, et al. "Extraction of Tea Polysaccharides." Encyclopedia. Web. 20 July, 2022.
Copy Citation
Tea polysaccharide (TPS) is the second most abundant ingredient in tea following tea polyphenols. As a complex polysaccharide, TPS has a complex chemical structure and a variety of bioactivities, such as anti-oxidation, hypoglycemia, hypolipidemic, immune regulation, and anti-tumor. Additionally, it shows excellent development and application prospects in food, cosmetics, and medical and health care products.
tea
polysaccharides
extraction method
1. Introduction
As a traditional drink, tea has been cultivated and consumed for thousands of years, and it is deeply loved by consumers from many countries, such as China, Japan, and South Korea. Tea not only creates a lot of wealth but also generates tea culture and tea ceremony [1]. As a result, tea has become one of the most popular beverages in the world after water [2][3][4].
The unprecedented popularity of tea is due not only to its unique aroma and taste but also to the health benefits of drinking it. The primary bioactivities of tea, including anti-oxidation, hypoglycemic, antibacterial, hypolipidemic, and anti-cancer activities, have been studied and explored. Tea has also been broadly utilized in the food, medical, and health care industries [5][6]. Tea’s biological and pharmacological activities are mainly attributed to the diversity of its chemical components. The chemical features of tea mainly include tea polyphenols (TPPs), tea polysaccharides (TPSs), tea proteins, catechins, theanine, and inorganic elements [4]. Tea polyphenols have long received attention for their excellent antioxidant properties for which accumulating evidence has been presented [7]. Modern pharmacological studies have shown that TPS, an important bioactive component along with TPP, is also the main tea compound that helps lower blood glucose and lipids, resist oxidation, and enhance the body’s immune function [8][9][10]. It also has excellent potential for development and application in the cosmetic industry [11]. In general, the content of TPS decreases with increases in tea quality or grade [12]. Wang et al., reported that the TPS content in low-grade tea was twice that of high-grade tea [13]. Therefore, using low-grade tea as a raw material to extract TPS is conducive to the full utilization of tea resources and has important implication for preventing diseases and promoting human health.
It was conducted that a detailed comparison and summary of the current research on tea polysaccharide’s extraction, preliminary physicochemical properties, and in vitro and in vivo bioactivities in order to provide new insights for the better utilization and development of TPS or TPS-related functional foods.
2. TPS Extraction
Tea leaves, flowers, and seeds are the three primary sources of TPS extraction materials. The current production process of TPS mainly includes hot water extraction, ultrasonic-assisted extraction, microwave-assisted extraction, and enzymolysis extraction (Table 1). Its conventional preparation process is shown in Figure 1.
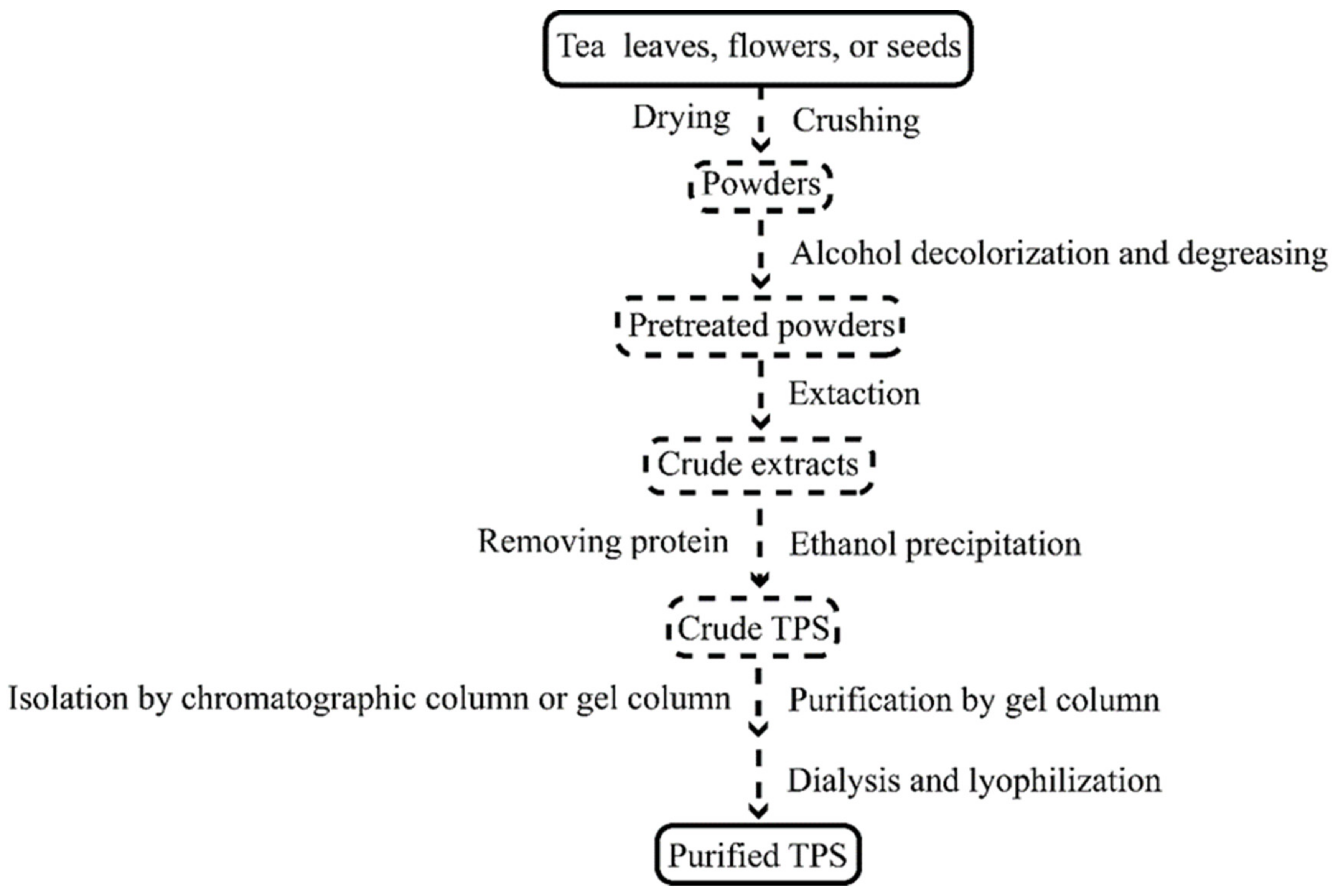
Figure 1. The conventional process of TPS preparation.
Table 1. Comparison of extraction methods of tea polysaccharide (TPS).
| Extraction Method | TPS Origin | Extraction Step | Ref |
|---|---|---|---|
| Hot water extraction | Green tea leaves and flowers | Pre-extraction with 95% ethanol at 40 °C for 2 h, repeated three times; a water bath extraction at 60 °C for 2 h, repeated 3 times | [14] |
| Fuan Baicha and Pingyang Tezaocha | Extraction at 80 °C for 1.5 h, repeated two times | [15] | |
| Fuzhuan tea | 2 h extraction time, 1:20 solid–liquid ratio, and 95 °C extraction temperature; repeated three times | [10] | |
| White tea | 8 min extraction time, 54.1 °C extraction temperature, 12.48 L/g material–water ratio; repeated four times | [16] | |
| Green tea | Heating in a water bath at 90 °C for 2 h with continuous stirring | [17] | |
| Green tea | Pre-extraction with absolute ethanol for 24 h and extraction with deionized water at 60 °C for 90 min | [18] | |
| Chin brick tea | 80% ethanol pretreatment and continuous stirring with distilled water (1:20, w/v) at 90 °C for 2 h | [19] | |
| Liupao tea | 80% ethanol pretreatment for 24 h and extraction with deionized water at 70 °C for 2 h; repeated three times | [20] | |
| Tea flowers | Extraction at 90 °C for 1 h (2 times) | [21] | |
| Green tea | 80% ethanol pretreatment at 70 °C for 1.5 h, extraction with ethanol at 40 °C for 3 h | [22] | |
| Green tea | Pretreatment with two times volume of 95% ethanol at 50 °C for 4 h, 1:8 solid–liquid ratio, and extraction with stirring at 50 °C for 120 min | [23] | |
| Green tea | Pretreatment with 95% alcohol (1:5, w/v) for 2 h, extraction in hot water (1:10, w/v) at 80 °C; repeated 3 times for 1 h each time | [24] | |
| Green tea | 95% ethanol (1:6, w/v) pretreatment at 60 °C for 4 h and extraction with distilled water (1:10, w/v) at 80 °C for 4 h; repeated 3 times | [25] | |
| Keemun black tea | Pretreatment with 95% ethanol (1:6, w/v) at 80 °C for 2 h and immersed in distilled water (1:10, w/v) at 80 °C for 4 h; repeated four times | [26] | |
| Ultrasonic-assisted extraction | Low-grade green tea | 80 °C extraction temperature, 60 min extraction time, 400 W ultrasonic power, and 22 mL:g liquid–solid ratio | [27] |
| Coarse tea | Pretreatment in an ultrasonic bath (50 °C, 200 W) for 30 min followed by extraction in a water bath for 90 min; repeated three times | [23] | |
| Green tea flowers | Ultrasonic power (25 °C, 100, 150, 200, 250, and 300 W) extraction for 5 min; repeated 2 times | [21] | |
| Yellow tea | 95% ethanol pretreatment for 6 h, 90 °C water bath extraction for 55 min (repeated twice), and sonication (20 kHz, 500 W) for 55 min | [21] | |
| Microwave-assisted extraction | Green, black, and oolong teas | 1:20 solid/liquid ratio, 200–230 °C extraction temperature, and 2 min extraction time | [28] |
| Green tea flowers | Extraction at controlled microwave power for 5 min followed by extraction with distilled water for 5 min at the same microwave power | [21] | |
| Green tea | Extraction in a 600 W microwave apparatus for 30 min, followed by stirring in a water bath for 90 min; repeated three times | [29] | |
| Enzymolysis extraction | Green tea | Extraction at 100 °C for 3 h and aqueous extraction with pectinase and tannase at 35 °C for 2 h | [30] |
| Green tea | Extraction with complex enzymes (cellulase:pectinase:glucanase = 1:1:2) at 50 °C for 30 min, boiling at 90 °C for 10 min, and then extraction in a water bath at 50 °C for 80 min | [29] | |
| Green tea leaves and flowers | 95% ethanol pretreatment at 40 °C for 2 h (repeated 3 times), treatment with 0.5% (m/v) pentosan complex enzyme solution (45 °C, pH 5.5) for 2 h, and extraction in 45 °C water bath for 2 h | [14] | |
| Green tea | Heating in a water bath at 90 °C for 2–4 h, repeated twice; incubating with 0.5% pectinase (260,001 PGU/mL, v/w) at 40 °C for 30 min; and heating at 90 °C for 1 h to inactivate the enzyme | [31] | |
| Hydro/solvothermal extraction | Chinese tea Zhongcha 108 | Extraction at 120 °C for 1 h | [1] |
| Alkali-assisted extraction | Fuzhuan brick tea | Extraction with 0.1 M NaOH solution (pH = 10.0) at 60 °C, repeated 3 times | [32] |
| Supercritical fluid extraction | Green tea | 380 μm particle size, 20% absolute ethanol, 35 MPa extraction pressure, 45 °C extraction temperature, and 2 h extraction time | [33] |
| Anionic reverse micelle extraction | Green tea | pH = 4.6, 0.06 M guanidine hydrochloride, 7% methanol, and 0.05 M NaCl; forward extraction | [34] |
2.1. Hot Water Extraction
Most bioactive polysaccharides are polar, so polar solvents such as hot water or alkaline solutions are usually used for polysaccharide extraction [33]. Hot water extraction is a classic method widely used to prepare polysaccharides in food, medicine, and other industries [34]. Chen et al., used water bath heating (70 °C, 60 min) to extract three kinds of crude TPSs from black, oolong, and green tea leaves [35]. Xu et al., prepared TPS from Pu-erh tea three times for 180 min in hot water at 70 °C [36]. Fan et al., extracted TPS twice in Fuan Baicha and Pingyang Tezaocha by adding double-distilled water and heating in a water bath at 80 °C for 1.5 h [37]. Zhu et al., used the response surface methodology to explore the extraction process of Fuzhuan tea crude polysaccharide (CDTPS) and found that the optimal extraction conditions (repeated four times) were as follows: an extraction time of 2 h, a solid–liquid ratio of 1:20, and an extraction temperature of 95 °C. Under these conditions, the yield of CDTPS was 6.07% [10]. The response surface methodology used by Jin et al., predicted the optimal extraction conditions of TPS via repetition four times in white tea: the optimal extraction time was 97.8 min, the extraction temperature was 54.1 °C, and the material–water ratio was 12.48 L/g [14]. Wang et al., pretreated dried green tea leaves and flowers in 95% ethanol and 40 °C for 2 h, then repeated the process three times to remove pigments and other substances. Then, 2 L of distilled water was added to the filtered tea samples for extraction in a water bath at 60 °C for 2 h. After filtration, 2.5 L of distilled water was added, and the hot water extraction was repeated again (60 °C, 2 h) [38]. Similarly, Cai et al., pretreated green tea leaves with absolute ethanol for 24 h to remove some small-molecular pigments and polyphenols, and then they dried the tea samples with deionized water for 90 min at 60 °C [16]. Li et al., also pretreated Chin brick tea powder with 80% ethanol, centrifuged it, and then continuously stirred it with distilled water (1:20, w/v) for 2 h at 90 °C to extract TPS [17]. Qin et al., pretreated Liupao tea samples with 80% ethanol for 24 h. After filtration and drying, the samples were extracted with deionized water at 70 °C for 2 h, and the process was repeated three times [18]. Wei et al., performed the hot water extraction of dried tea flower polysaccharides (TFPSs) and then extracted TFPSs twice with distilled water (1 h each). They found that the yield of TFPS increased with the extraction temperature, and 90 °C was the optimal extraction temperature for TFPS. The yield at this condition was close to 35% [19]. Though hot water extraction is a commonly used method for TPS extraction, conventional hot water extraction has disadvantages such as a low extraction efficiency, long extraction time, and high extraction temperature, all of which limit its availability [33][39]. For example, Wang et al., further compared the yields of hot water extraction, boiling water extraction, and enzymolysis extraction for TFPS, and they found that the yield of TFPS obtained with enzymolysis extraction was the highest (2.01%), followed by boiling water extraction (1.91%) and finally hot water extraction (1.83%) [20]. Zhu et al., compared the yields of crude green tea polysaccharides (CTPSs) under hot water extraction (WE), enzymatic extraction (EE), microwave-assisted extraction (MAE) and ultrasonic-assisted extraction (UAE), and they found that the four yields of CTPS under these extraction methods were 3.98%, 4.17%, 4.31%, and 4.52%, respectively [21]. Numerous studies have verified that although hot water extraction has strong practicability, its obtained TPS yield is relatively low and easily leads to the unnecessary waste of raw materials. Therefore, many researchers have also improved the technology on the basis of hot water extraction and developed other auxiliary extraction methods, such as ultrasonic-assisted extraction, microwave-assisted extraction, and enzyme-assisted extraction, to improve the extraction efficiency of TPS [40].
2.2. Ultrasonic-Assisted Extraction (UAE)
UAE can accelerate the rupture of plant cell walls by the high-speed movement of molecules in samples caused by high-frequency ultrasonic vibration, thereby dissolving and releasing intracellular substances. Karadag et al., used UAE to extract low-grade green tea polysaccharides (GTPSs) and then reported the optimal extraction parameters through response surface optimization as follows: 80 °C for extraction temperature, 60 min for extraction time, 400 W for ultrasonic power, and 22 mL/g for liquid–solid ratio. Under these conditions, the yield of GTPS was 4.65%, which was higher than that of the hot water extraction method (1.83%) without ultrasound [25]. In addition, they also found that the Mw of GTPS obtained with ultrasonic-assisted extraction was lower, which may have been due to the partial degradation of TPS caused by the ultrasonic process. Zhu et al., prepared TPS from coarse green tea leaves, placing the tea leaves in an ultrasonic bath (50 °C, 200 W) for pretreatment for 30 min and then performing extraction in a water bath for 90 min. The TPS yield obtained with this method was higher than other tested methods [21]. To explore the effects of ultrasound on the structure and activity of yellow tea polysaccharide (YTPS), Wang et al., treated a YTPS fraction obtained after hot water extraction and deproteinization with ultrasound (20 kHz, 500 W) for 55 min. The results showed that ultrasonic treatment basically did not change the main chemical composition of YTPS but did cause it to degrade [26]. Wei et al., mixed dried green tea flower blocks with distilled water and extracted them for 5 min at 25 °C under ultrasonic powers of 100, 150, 200, 250, and 300 W. This process was repeated twice to obtain crude TFPS [19]. Overall, the UAE method has the advantages of saved time, simple operation, experimental safety, low cost, and high extraction rate. Still, it may degrade soluble TPS and affect its bioactivity.
2.3. Microwave-Assisted Extraction (MAE)
Recently, microwave-assisted extraction (MAE) technology has become widely used to analyze and extract active components in plants. MAE is a new extraction technology that uses high-frequency electromagnetic waves (0.3–300 GHZ) with strong penetrability and heating effect to extract active plant components. High-energy microwaves can penetrate solvents and plant cell walls, transfer energy to the cytoplasm, and interact with polar components to generate heat, which increases the temperature and pressure inside cells. When the pressure reaches a certain level, the cell wall expands and ruptures, releasing intracellular polysaccharides and other substances [41]. Shuntaro et al., used MAE technology to extract TPS from tea residues (green tea, black tea, and oolong tea). When the extraction conditions were a solid/liquid ratio of 1:20, an extraction temperature of 200–230 °C, and an extraction time of 2 min, the yield of tea residue TPS was 40–50% [27]. Wei et al., used MAE equipment to extract TFPS twice, for 5 min each time. They found that the yield of TFPS changed irregularly with the increase in microwave power. In addition, with increases in microwave power, the content of neutral sugars in TFPS increased while the content of acidic sugars increased and then decreased [19]. Li et al., used a 600 W microwave instrument to extract coarse green tea crude TPS (CTPS), and the extraction process was repeated three times. After extraction with MAE, the content of soluble protein in CTPS was the highest of all tested methods, reaching 5.93%. Furthermore, they found that MAE treatment had little effect on CTPS chains with high Mw but resulted in the drastic degradation of small-Mw CTPS. According to related reports, small-Mw polysaccharides tend to have better bioactivities than their high-Mw counterparts [42]. The subsequent in vitro activity test of CTPS prepared by the MAE method by Zhu et al., also confirmed this conclusion [21]. Compared to other extraction methods, the MAE method has the advantages of high extraction efficiency, high purity, non-degradable active ingredients, convenient operation, saved time, and environmental friendliness. It is a “green extraction process”, which has made it popular. Although MAE has favorable prospects in TPS extraction, it also has disadvantages such as complex extract components, difficult separation and purification in the later stages, and the necessity of polar solvents [43]. Therefore, in addition to the basic closed and open systems, several improved microwave extraction technologies, such as vacuum microwave-assisted extraction, nitrogen-protected microwave-assisted extraction, ultrasonic microwave-assisted extraction, and dynamic microwave-assisted extraction, have been developed [41].
2.4. Enzymolysis Extraction
The enzymolysis method refers to the destruction of plant cell walls with enzymatic hydrolysis. The cell wall is decomposed into small molecular substances readily soluble in the extraction solvent, thereby accelerating the dissolution of active ingredients. The yield of TPS extracted with enzymatic hydrolysis is usually higher and the effect of mixed enzymes is better than that of a single enzyme. However, the enzyme’s activity is easily affected by the reaction temperature, pH, and concentration, so the requirements for experimental conditions and costs are usually higher. Baik et al., investigated the effect of the simultaneous treatment of pectinase and tannase on TPS extraction from green tea. They found that the concurrent treatment of the two enzymes was an effective method for TPS extraction and could significantly improve TPS’s free radical scavenging activity [28]. Chang et al., used pectinase-assisted extraction to obtain green tea TPS, and the primary extraction process was as follows: the ground tea powder was heated in a water bath at 90 °C for 2–4 h, 0.5% pectinase (260,001 PGU/mL, v/w) was added and incubated at 40 °C for 30 min, and then the enzyme was inactivated by heating at 90 °C for 1 h. The prepared TPS presented excellent immune stimulation and protection against immune cells [30]. In addition to bioactivity, yield is also a concern for enzymolysis extraction. Zhu et al., used mixed enzymes (cellulase:pectinase:glucanase = 1:1:2) for crude green tea polysaccharide (CTPS) extraction at 50 °C (30 min), followed by boiling to inactivate the enzyme (10 min) and extracting in a water bath at 50 °C for 80 min. The whole process was repeated three times. The CTPS obtained with this method had a high total sugar content (71.83%), which could mainly be attributed to the gentle and efficient destruction of the cell walls by mixed enzymes [21][44]. Wang et al., used a 0.5% (m/v) pentosan complex enzyme solution (45 °C, pH 5.5) to extract TPS from green tea leaves and flowers pretreated with 95% ethanol for 2 h. After filtration, the same extraction process at the same temperature was repeated. The yields of two TPSs obtained with this method were 4.08% and 6.88%, respectively, which were much higher than those obtained with hot water extraction under the same conditions (1.28% and 2.93%, respectively) [38]. Compared to the conventional solvent extraction method, the enzymolysis extraction method has the advantages of a high extraction efficiency, strong specificity, and high extraction rate. In addition, it can reduce the environmental pollution caused by using a large amount of solvent and thus has broad application prospects. However, since the price of the enzyme is relatively high and its activity is affected by various factors, the extraction conditions for enzymolysis extraction must be strictly controlled to effectively obtain a higher extraction rate.
2.5. Other Extraction Methods
Some new methods for TPS extraction in addition to the above-mentioned common extraction methods have also been reported. For example, Xu et al., optimized extraction conditions using a hydro/solvothermal method. They used high temperature and pressure (120 °C, 0.1 MPa) to infiltrate water into the tea leaves of Zhongcha 108 to destroy the cell structure, thereby separating TPS [1]. The extraction rate of crude polysaccharides obtained with this method was 4.7%, which was much higher than that of TPS obtained with ordinary hot water extraction, such as Ziyang green tea (3.46%) [22], Huangshan Maofeng tea (2.3%) [23], and Keemun black tea (3.2%) [24]. Sun et al., used alkali-assisted extraction to extract Fuzhuan brick tea polysaccharide (FBTPS); the extraction conditions were a 60 °C extraction temperature and a 0.1 mol/L NaOH solution (pH = 10.0). Compared to hot water extraction, the yield of FBTPS by alkaline extraction was found to have a greater impact on the monosaccharide composition and yield [30]. In addition, emerging extraction technology supercritical fluid extraction (SFE) has also been used to extract polysaccharides in recent years. Many researchers have used SFE to extract various plant-derived polysaccharides, though there are still few applications of this process for TPS extraction. Chen et al., extracted TPS with a CO2-based SFE method, and they determined the optimum parameters of this method in TPS extraction as a particle size of 380 μm, 20% absolute ethanol, an extraction pressure of 35 MPa, an extraction temperature of 45 °C, an extraction time of 2 h, which enabled a TPS extraction rate of up to 92.5%. Moreover, the TPS obtained with this method was significantly bioactive [31]. Although the SFE method is impressive, manageable, efficient, and environmentally-friendly, it is still not as common as other extraction methods in practical applications due to its expensive and time-consuming equipment. In addition, Li et al., found that extraction via a anionic reverse micelle system exhibited the advantages of a fast mass transfer, high selectivity, and low cost [32]. In short, various auxiliary methods for TPS extraction are able to improve the bioactivity of polysaccharides, shorten extraction times, and improve extraction yields.
References
- Gao, Y.; Zhou, Y.; Zhang, Q.; Zhang, K.; Peng, P.; Chen, L.; Xiao, B. Hydrothermal extraction, structural characterization, and inhibition HeLa cells proliferation of functional polysaccharides from Chinese tea Zhongcha 108. J. Funct. Foods 2017, 39, 1–8.
- Ye, X.; Tang, X.; Li, F.; Zhu, J.; Wu, M.; Wei, X.; Wang, Y. Green and Oolong Tea Extracts with Different Phytochemical Compositions Prevent Hypertension and Modulate the Intestinal Flora in a High-Salt Diet Fed Wistar Rats. Front. Nutr. 2022, 9, 892801.
- Zhu, J.; Wu, M.; Zhou, H.; Cheng, L.; Wei, X.; Wang, Y. Liubao brick tea activates the PI3K-Akt signaling pathway to lower blood glucose, metabolic disorders and insulin resistance via altering the intestinal flora. Food Res. Int. 2021, 148, 110594.
- Zhu, J.; Yu, C.; Zhou, H.; Wei, X.; Wang, Y. Comparative evaluation for phytochemical composition and regulation of blood glucose, hepatic oxidative stress and insulin resistance in mice and HepG2 models of four typical Chinese dark teas. J. Sci. Food Agric. 2021, 101, 6563–6577.
- Ding, Y.; Pu, L.; Kan, J. Hypolipidemic effects of lipid-lowering granulated tea preparation from Monascus -fermented grains (adlay and barley bran) mixed with lotus leaves on Sprague–Dawley rats fed a high-fat diet. J. Funct. Foods 2017, 32, 80–89.
- Orem, A.; Alasalvar, C.; Kural, B.V.; Yaman, S.; Orem, C.; Karadag, A.; Pelvan, E.; Zawistowski, J. Cardio-protective effects of phytosterol-enriched functional black tea in mild hypercholesterolemia subjects. J. Funct. Foods 2017, 31, 311–319.
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39.
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Dai, Z.; Ye, H.; Hu, B.; Zeng, X.; Liu, Z. Fuzhuan Brick Tea Polysaccharides Attenuate Metabolic Syndrome in High-Fat Diet Induced Mice in Association with Modulation in the Gut Microbiota. J. Agric. Food Chem. 2018, 66, 2783–2795.
- Yuan, C.; Li, Z.; Peng, F.; Xiao, F.; Ren, D.; Xue, H.; Chen, T.; Mushtaq, G.; Kamal, M.A. Combination of selenium-enriched green tea polysaccharides and Huo-ji polysaccharides synergistically enhances antioxidant and immune activity in mice. J. Sci. Food Agric. 2015, 95, 3211–3217.
- Zhu, J.; Zhou, H.; Zhang, J.; Li, F.; Wei, K.; Wei, X.; Wang, Y. Valorization of polysaccharides obtained from dark tea: Preparation, physicochemical, antioxidant, and hypoglycemic properties. Foods 2021, 10, 2276.
- Wei, X.; Liu, Y.; Xiao, J.; Wang, Y. Protective effects of tea polysaccharides and polyphenols on skin. J. Agric. Food Chem. 2009, 57, 7757–7762.
- Wang, Y.; Wei, X.; Jin, Z. Structure analysis of an acidic polysaccharide isolated from green tea. Nat. Prod. Res. 2009, 23, 678–687.
- Xiao, J.; Huo, J.; Jiang, H.; Yang, F. Chemical compositions and bioactivities of crude polysaccharides from tea leaves beyond their useful date. Int. J. Biol. Macromol. 2011, 49, 1143–1151.
- Jin, F.; He, J.; Jia, L.-Y.; Tu, Y.-Y. Optimizing conditions for the extraction of polysaccharides of white tea. Biotechnol. Biotechnol. Equip. 2015, 29, 921–925.
- Chen, X.; Zhi, L.; Yang, Y.; Rui, Z.; Yin, J.; Jiang, Y.; Wan, H. Suppression of diabetes in non-obese diabetic (NOD) mice by oral administration of water-soluble and alkali-soluble polysaccharide conjugates prepared from green tea. Carbohydr. Polym. 2010, 82, 28–33.
- Cai, W.; Xie, L.; Chen, Y.; Zhang, H. Purification, characterization and anticoagulant activity of the polysaccharides from green tea. Carbohydr. Polym. 2013, 92, 1086–1090.
- Li, Q.; Shi, J.; Li, J.; Liu, L.; Zhao, T.; McClements, D.J.; Fu, Y.; Wu, Z.; Duan, M.; Chen, X. Influence of thermal treatment on the physicochemical and functional properties of tea polysaccharide conjugates. LWT-Food Sci. Technol. 2021, 150, 111967.
- Qin, H.; Huang, L.; Teng, J.; Wei, B.; Xia, N.; Ye, Y. Purification, characterization, and bioactivity of Liupao tea polysaccharides before and after fermentation. Food Chem. 2021, 353, 129419.
- Wei, X.; Chen, M.; Xiao, J.; Ying, L.; Lan, Y.; Zhang, H.; Wang, Y. Composition and bioactivity of tea flower polysaccharides obtained by different methods. Carbohydr. Polym. 2010, 79, 418–422.
- Wang, Y.; Peng, Y.; Wei, X.; Yang, Z.; Xiao, J.; Jin, Z. Sulfation of tea polysaccharides: Synthesis, characterization and hypoglycemic activity. Int. J. Biol. Macromol. 2010, 46, 270–274.
- Zhu, J.; Chen, Z.; Zhou, H.; Yu, C.; Han, Z.; Shao, S.; Hu, X.; Wei, X.; Wang, Y. Effects of extraction methods on physicochemical properties and hypoglycemic activities of polysaccharides from coarse green tea. Glycoconj. J. 2020, 37, 241–250.
- Chi, A.; Li, H.; Kang, C.; Guo, H.; Wang, Y.; Guo, F.; Tang, L. Anti-fatigue activity of a novel polysaccharide conjugates from Ziyang green tea. Int. J. Biol. Macromol. 2015, 80, 566–572.
- Lu, X.; Zhao, Y.; Sun, Y.; Yang, S.; Yang, X. Characterisation of polysaccharides from green tea of Huangshan Maofeng with antioxidant and hepatoprotective effects. Food Chem. 2013, 141, 3415–3423.
- Sun, Y.; Yang, X.; Lu, X.; Wang, D.; Zhao, Y. Protective effects of Keemun black tea polysaccharides on acute carbon tetrachloride-caused oxidative hepatotoxicity in mice. Food Chem. Toxicol. 2013, 58, 184–192.
- Karadag, A.; Pelvan, E.; Dogan, K.; Celik, N.; Ozturk, D.; Akaln, K.; Alasalvar, C. Optimisation of green tea polysaccharides by ultrasound-assisted extraction and their in vitro antidiabetic activities. Qual. Assur. Saf. Crops Foods 2019, 11, 479–490.
- Wang, H.; Chen, J.; Ren, P.; Zhang, Y.; Omondi Onyango, S. Ultrasound irradiation alters the spatial structure and improves the antioxidant activity of the yellow tea polysaccharide. Ultrason. Sonochem. 2021, 70, 105355.
- Tsubaki, S.; Iida, H.; Sakamoto, M.; Azuma, J. Microwave heating of tea residue yields polysaccharides, polyphenols, and plant biopolyester. J. Agric. Food Chem. 2008, 56, 11293–11299.
- Baik, J.H.; Shin, K.S.; Park, Y.; Yu, K.W.; Suh, H.J.; Choi, H.S. Biotransformation of catechin and extraction of active polysaccharide from green tea leaves via simultaneous treatment with tannase and pectinase. J. Sci. Food Agric. 2015, 95, 2337–2344.
- Chang, B.Y.; Kim, T.Y.; Kim, S.Y. Polysaccharides from pectinase digests of green tea enhances host immune defence through toll-like receptor 4. Food Agric. Immunol. 2018, 29, 870–885.
- Sun, Y.; Wang, F.; Liu, Y.; An, Y.; Chang, D.; Wang, J.; Xia, F.; Liu, N.; Chen, X.; Cao, Y. Comparison of water- and alkali-extracted polysaccharides from Fuzhuan brick tea and their immunomodulatory effects in vitro and in vivo. Food Funct. 2022, 13, 806–824.
- Chen, M.; Xiong, L.Y. Supercritical extraction technology in tea polysaccharide extracting application. Adv. Mater. Res. 2012, 347–353, 1683–1688.
- Li, S.; Cao, X. Extraction of tea polysaccharides (TPS) using anionic reverse micellar system. Sep. Purif. Technol. 2014, 122, 306–314.
- Shashidhar, G.M.; Giridhar, P.; Manohar, B. Functional polysaccharides from medicinal mushroom Cordyceps sinensis as a potent food supplement: Extraction, characterization and therapeutic potentials—A systematic review. RSC Adv. 2015, 5, 16050–16066.
- Jin, M.; Zhao, K.; Huang, Q.; Xu, C.; Shang, P. Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (Oliv.) Diels: A review. Carbohydr. Polym. 2012, 89, 713–722.
- Chen, H.; Qu, Z.; Fu, L.; Dong, P.; Zhang, X. Physicochemical properties and antioxidant capacity of 3 polysaccharides from green tea, oolong tea, and black tea. J. Food Sci. 2009, 74, 469–474.
- Xu, P.; Wu, J.; Zhang, Y.; Chen, H.; Wang, Y. Physicochemical characterization of puerh tea polysaccharides and their antioxidant and α-glycosidase inhibition. J. Funct. Foods 2014, 6, 545–554.
- Fan, M.; Sun, X.; Qian, Y.; Xu, Y.; Wang, D.; Cao, Y. Effects of metal ions in tea polysaccharides on their in vitro antioxidant activity and hypoglycemic activity. Int. J. Biol. Macromol. 2018, 113, 418–426.
- Wang, Y.; Yang, Z.; Wei, X. Sugar compositions, α-glucosidase inhibitory and amylase inhibitory activities of polysaccharides from leaves and flowers of Camellia sinensis obtained by different extraction methods. Int. J. Biol. Macromol. 2010, 47, 534–539.
- Yan, J.K.; Wang, W.Q.; Wu, J.Y. Recent advances in Cordyceps sinensis polysaccharides: Mycelial fermentation, isolation, structure, and bioactivities: A review. J. Funct. Foods 2014, 6, 33–47.
- Chen, G.; Yuan, Q.; Saeeduddin, M.; Ou, S.; Zeng, X.; Ye, H. Recent advances in tea polysaccharides: Extraction, purification, physicochemical characterization and bioactivities. Carbohydr. Polym. 2016, 153, 663–678.
- Chan, C.H.; Yusoff, R.; Ngoh, G.C.; Kung, F.W. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225.
- Li, X.; Wang, L. Effect of extraction method on structure and antioxidant activity of Hohenbuehelia serotina polysaccharides. Int. J. Biol. Macromol. 2016, 83, 270–276.
- Dean, J.R.; Xiong, G. Extraction of organic pollutants from environmental matrices: Selection of extraction technique. TrAC Trends Anal. Chem. 2000, 19, 553–564.
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
4 times
(View History)
Update Date:
21 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




