
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hui Yin Lim | -- | 1693 | 2022-07-19 03:44:37 | | | |
| 2 | Jessie Wu | + 35 word(s) | 1728 | 2022-07-19 04:32:12 | | | | |
| 3 | Jessie Wu | Meta information modification | 1728 | 2022-07-19 04:33:24 | | | | |
| 4 | Jessie Wu | -35 word(s) | 1693 | 2022-07-19 05:38:07 | | | | |
| 5 | Jessie Wu | + 35 word(s) | 1728 | 2022-07-19 07:57:07 | | | | |
| 6 | Jessie Wu | Meta information modification | 1728 | 2022-07-19 07:57:26 | | |
Video Upload Options
Cardiovascular disease is a major global cause of death, with an estimated 17.9 million cardiovascular disease-related deaths in 2019, representing nearly one third of global deaths. Atherosclerosis, which can begin in childhood, is a multifactorial, chronic condition that contributes to cardiovascular disease. Characterized by lipid deposition in the blood vessel intima, atherosclerosis is associated with inflammation and calcification and can cause vessel stenosis with thrombotic occlusion and/or embolism. A more precise and sophisticated tool that can reliably predict the thrombosis and bleeding risks at an individual level is required in order for clinicians to confidently recommend early interventions with a favorable risk–benefit profile. Critical to the development of this tool is the assessment and understanding of Virchow’s triad and its complex interactions between hypercoagulability, endothelial dysfunction and vessel flow, a fundamental concept to the development of thrombosis.
1. Traditional Risk Factors for Cardiovascular Disease
Advancing age confers a major risk for cardiovascular. There are several mechanisms for the dominant effect of age including increased exposure time to age-dependent risk factors that may co-vary in number and severity with aging as well as changes in cardiovascular structure and function with age and how these changes interact with pathophysiological disease mechanisms [4]. In an attempt to limit the changes, the cardiovascular cells generate an inflammatory defense, through the activation of renin–angiotensin–aldosterone endothelin signaling cascades. Advancing age is also associated with the increased prevalence of salt-sensitive hypertension, Over time, the age-associated inflammation in the heart and arteries exceeds a threshold and together with aging-related hemodynamic alterations contributes to fibrosis and arterial stiffness, resulting in cardiovascular diseases [5].
2. Current Available Risk Assessment Tools
3. Virchow's Triad and Its Association with Thrombosis and Cardiovascular Disease
Crucial to the development of thrombosis is the concept of Virchow’s triad. The complex interplay between three elements—the presence of hypercoagulable state, flow stasis and endothelial injury or dysfunction—provides the perfect storm for thrombogenesis. In atherosclerosis, the triad are representative of abnormalities in the endothelium, platelets and coagulation and fibrinolytic pathways, as well as in hemorheology and turbulence at bifurcation and stenotic regions. Plaque rupture exposes prothrombotic proteins including collagen and tissue factor to the coagulation system resulting in thrombus formation, restricted blood flow and onset of cardiovascular disease. The activated platelets also interact with the atherosclerotic endothelium, resulting in the delivery of pro-inflammatory chemokines, further propagating the inflammatory process.
Coagulation also plays an important role in atherogenesis. It is a dynamic but tightly regulated process. While conventional coagulation assays are not useful in predicting thrombosis risks, global coagulation assays provide a more comprehensive assessment of coagulation including the evaluation of the final products of the coagulation cascade, thrombin and fibrin [14][15]. Although limited in their clinical use in thrombosis medicine in the current state, these assays appear promising, particularly in detecting hypercoagulable states. Some examples of these assays include viscoelastic testing such as thromboelastography and rotational thromboelastometry, thrombin generation assays with calibrated automated thrombogram, and fibrin generation with overall haemostatic potential assays.
The endothelium is highly plastic and diverse and is very amenable in both health and disease. The vascular endothelial cells play critical roles in regulating the crosstalk of the various components required for the maintenance of cardiovascular homeostasis. Endothelial dysfunction is associated with increased vascular permeability and the decreased production of nitric oxide as well as increased reactive oxygen species (ROS) and proinflammatory factors, and it is induced by oxidized LDL [1][16]. Several chronic metabolic conditions such as the previously listed traditional risk factors of cardiovascular disease can lead to a loss of this equilibrium, shifting towards endothelial dysfunction, a key pathophysiology in atherosclerosis. There are several ways to measure endothelial function which the use of biomarkers including those that are lipid-related, inflammation-based or components of renin-angiotensin system such as angiotensin-converting enzyme 2 (ACE2).
The third contributing factor in Virchow’s triad is flow stasis. Imaging of vessel flow to measure the burden of atherosclerosis may provide better risk assessment than risk assessment calculators. Coronary artery calcium (CAC) scoring can be helpful for determining the extent of coronary artery plaque, while coronary computed tomography angiography (CCTA) provides very high sensitivity for the detection of coronary artery stenosis and nonobstructive plaques. Retinal vascular imaging is a relatively new non-invasive technology for assessing cardiovascular disease risk. The changes in retinal microvasculature have been associated with early-life cardiovascular risk factors including lower birth weight, lesser physical activity, parental hypertension history, childhood obesity and type 1 diabetes [17]. Figure 1 shows some key biomarkers that can be used to assess the components of Virchow’s triad.
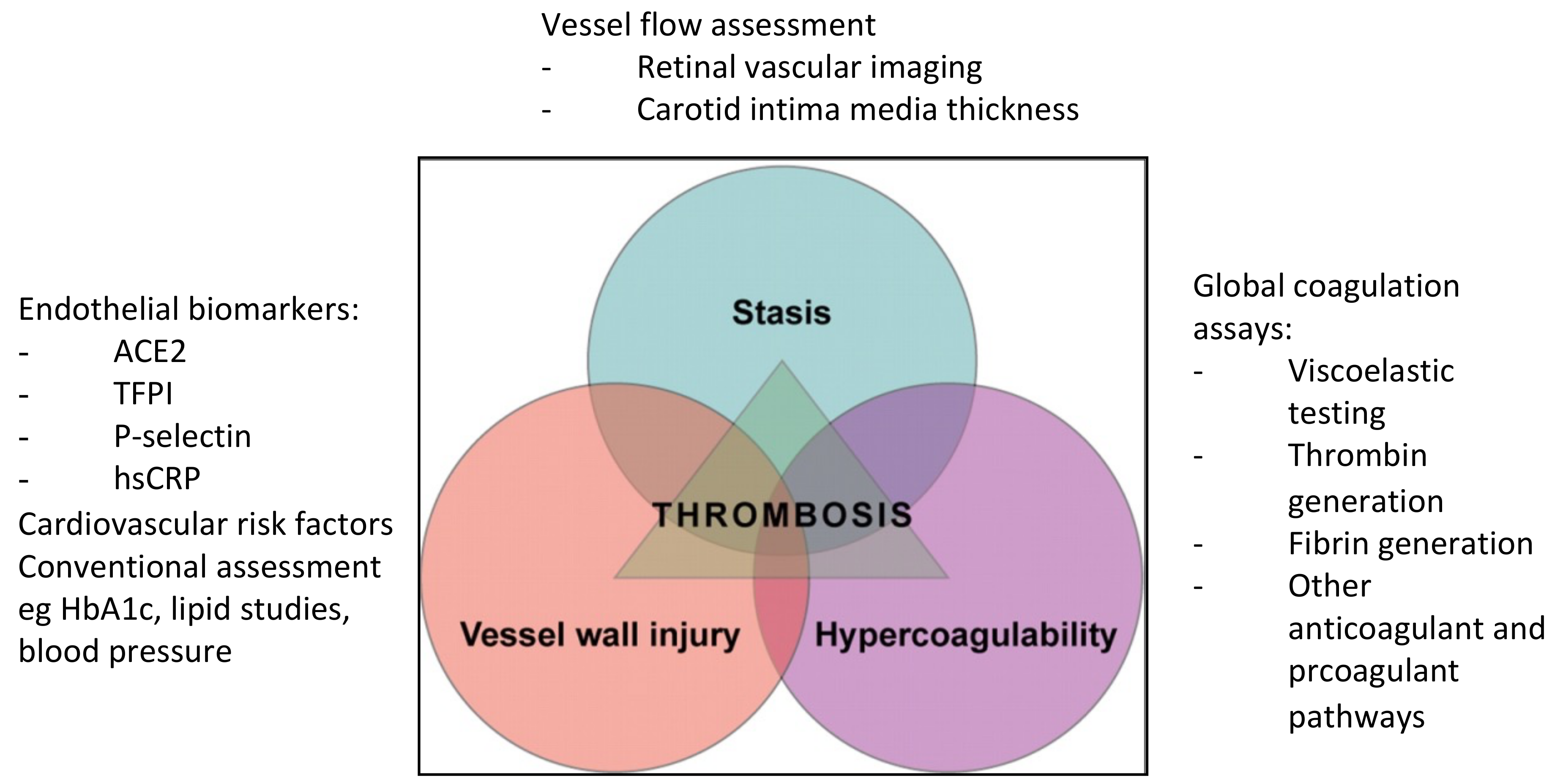
Figure 1. Some of the key biomarkers used in the assessment of the various components of Virchow’s triad. (Abbreviations: ACE2 angiotensin converting enzyme 2; TFPI tissue factor pathway inhibitor; hsCRP high-sensitivity C-reactive protein; HbA1c glycated hemoglobin).
4. Genetic Insights into Cardiovascular Risk and Disease
5. Conclusions
A more specific and sensitive risk assessment model is required to allow clinicians to confidently recommend early interventions in thrombosis and cardiovascular disease management with favorable risk–benefit profiles. Critical to the development of this model is the assessment and understanding of Virchow’s triad and its complex interactions between hypercoagulability, endothelial dysfunction and vessel flow. To achieve this, individualized risk assessment can be improved through an integrated, multimodal approach using a combination of tools such as global coagulation assays, endothelial biomarkers and vessel flow assessment in tandem with clinical surrogate markers. The addition of new tools such as GWAS may further refine the risk stratification
References
- Seidman, M.A.; Mitchell, R.N.; Stone, J.R. Pathophysiology of atherosclerosis. In Cellular and Molecular Pathobiology of Cardiovascular Disease; Willis, M., Homeister, J., Stone, J., Eds.; Elsevier: San Diego, CA, USA, 2014; pp. 221–237. ISBN 978-0-12-405206-2.
- Singh, R.B.; Mengi, S.A.; Xu, Y.; Arneja, A.S.; Dhalla, N.S. Pathogenesis of atherosclerosis: A multifactorial process. Exp. Clin. Cardiol. 2002, 7, 40–53.
- Bergheany, S.C.; Bodde, M.C.; Jukema, J.W. Pathophysiology and treatment of atherosclerosis: Current view and future perspective on lipoprotein modification treatment. Neth. Heart J. 2017, 25, 231–242.
- Lakatta, E.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises. Circulation 2003, 107, 139–146.
- Lakatta, E. So! What’s aging? Is cardiovascular aging a disease? J. Mol. Cell. Cardiol. 2015, 83, 1–13.
- Li, X.; Weber, N.C.; Cohn, D.M.; Hollmann, M.W.; Hans DeVries, J.; Hermanides, J.; Preckel, B. Effects of hyperglycemia and diabetes mellitus on coagulation and hemostasis. J. Clin. Med. 2021, 10, 2419.
- Carr, M.E. Diabetes mellitus: A hypercoagulable state. J. Diabetes Complicat. 2001, 15, 44–54.
- Alzahrani, S.H.; Ajjan, R.A. Coagulation and fibrinolysis in diabetes. Diabetes Vasc. Dis. Res. 2010, 7, 260–273.
- Dokken, B.B. The pathophysiology of cardiovascular disease and diabetes: Beyond blood pressure and lipids. Diabetes Spectr. 2008, 21, 160–165.
- Tabas, I. Consequences of cellular cholesterol accumulation: Basic concepts and physiological implications. J. Clin. Investig. 2002, 110, 905–911.
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021, 143, e984–e1010.
- Khambhati, J.; Allard-Ratick, M.; Dhindsa, D.; Lee, S.; Chen, J.; Sandesara, P.B.; O’Neal, W.; Quyyumi, A.A.; Wong, N.D.; Blumenthal, R.S.; et al. The art of cardiovascular risk assessment. Clin. Cardiol. 2018, 41, 677–684.
- DeFilippis, A.P.; Young, R.; Carrubba, C.J.; McEvoy, J.W.; Budoff, M.J.; Blumenthal, R.S.; Kronmal, R.A.; McClelland, R.L.; Nasir, K.; Blaha, M.J. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann. Intern. Med. 2015, 162, 266–275.
- Lancé, M.D. A general review of major global coagulation assays: Thrombelastography, thrombin generation test and clot waveform analysis. Thromb. J. 2015, 13, 1.
- Lim, H.Y.; O’Malley, C.; Donnan, G.; Nandurkar, H.; Ho, P. A review of global coagulation assays—Is there a role in thrombosis risk prediction? Thromb. Res. 2019, 179, 45–55.
- Kitta, Y.; Obata, J.; Nakamura, T.; Hirano, M.; Kodama, Y.; Fujioka, D.; Saito, Y.; Kawabata, K.; Sano, K.; Kobayashi, T.; et al. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J. Am. Coll. Cardiol. 2009, 53, 323–330.
- Li, L.; Ikram, M.K.; Wong, T.Y. Retinal vascular imaging in early life: Insights into processes and risk of cardiovascular disease. J. Physiol. 2016, 594, 2175–2203.
- O’Donnell, C.J.; Nabel, E.G. Genomics of cardiovascular disease. N. Engl. J. Med. 2011, 365, 2098–2109.
- Vrablik, M.; Dlouha, D.; Todorovova, V.; Stefler, D.; Hubacek, J.A. Genetics of cardiovascular disease: How far are we from personalized CVD risk prediction and management? Int. J. Mol. Sci. 2021, 22, 4182.
- Jaiswal, S.; Fontanillas, P.; Flannick, J.; Manning, A.; Grauman, P.V.; Mar, B.G.; Lindsley, R.C.; Mermel, C.H.; Burtt, N.; Chavez, A.; et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014, 371, 2488–2498.
- Jaiswal, S.; Natarajan, P.; Silver, A.J.; Gibson, C.J.; Bick, A.G.; Shvartz, E.; McConkey, M.; Gupta, N.; Gabriel, S.; Ardissino, D.; et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 2017, 377, 111–121.
- Senguttuvan, N.B.; Subramanian, V.; Venkatesan, V.; Muralidharan, T.R.; Sankaranarayanan, K. Clonal hematopoiesis of indeterminate potential (CHIP) and cardiovascular diseases—an updated systematic review. J. Genet. Eng. Biotechnol. 2021, 19, 105.
- Papa, V.; Marracino, L.; Fortini, F.; Rizzo, P.; Campo, G.; Vaccarezza, M.; Vieceli Dalla Sega, F. Translating evidence from Clonal hematopoiesis to cardiovascular disease: A systematic review. J. Clin. Med. 2020, 9, 2480.




