
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carmela Balistreri | -- | 4377 | 2022-07-14 02:13:37 | | | |
| 2 | Conner Chen | -15 word(s) | 4362 | 2022-07-14 03:11:18 | | |
Video Upload Options
The endothelium has multiple functions, ranging from maintaining vascular homeostasis and providing nutrition and oxygen to tissues to evocating inflammation under adverse conditions and determining endothelial barrier disruption, resulting in dysfunction. Endothelial dysfunction represents a common condition associated with the pathogenesis of all diseases of the cardiovascular system, as well as of diseases of all of the other systems of the human body, including sepsis, acute respiratory distress syndrome, and COVID-19 respiratory distress. Such evidence is leading to the identification of potential biomarkers and therapeutic targets for preserving, reverting, or restoring endothelium integrity and functionality by promptly treating its dysfunction.
1. Introduction
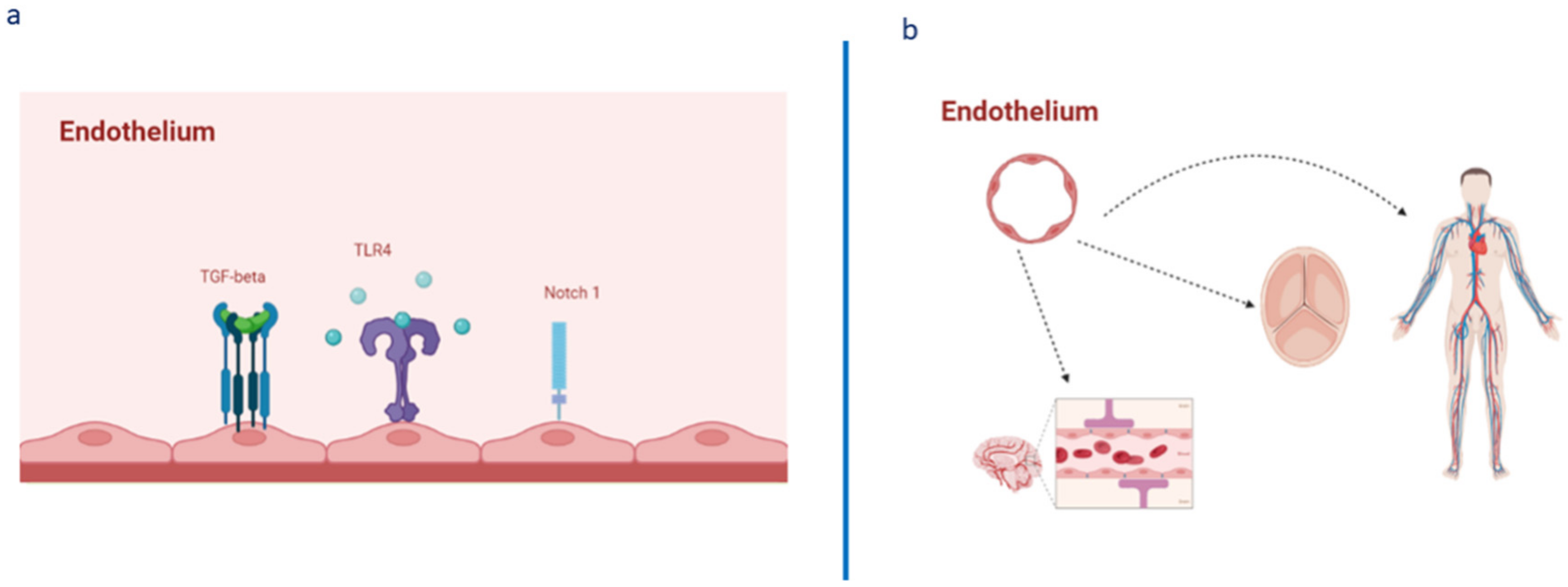
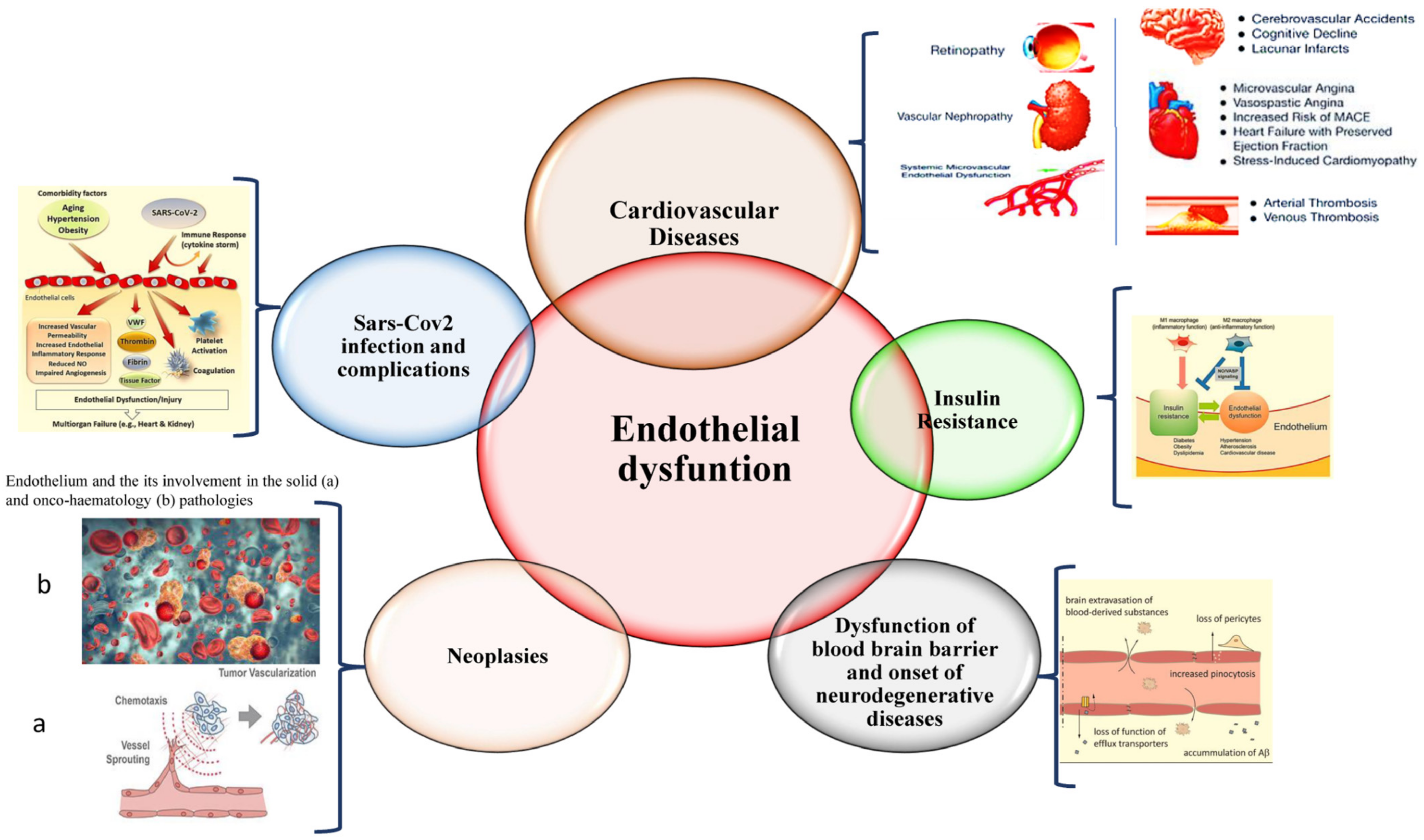
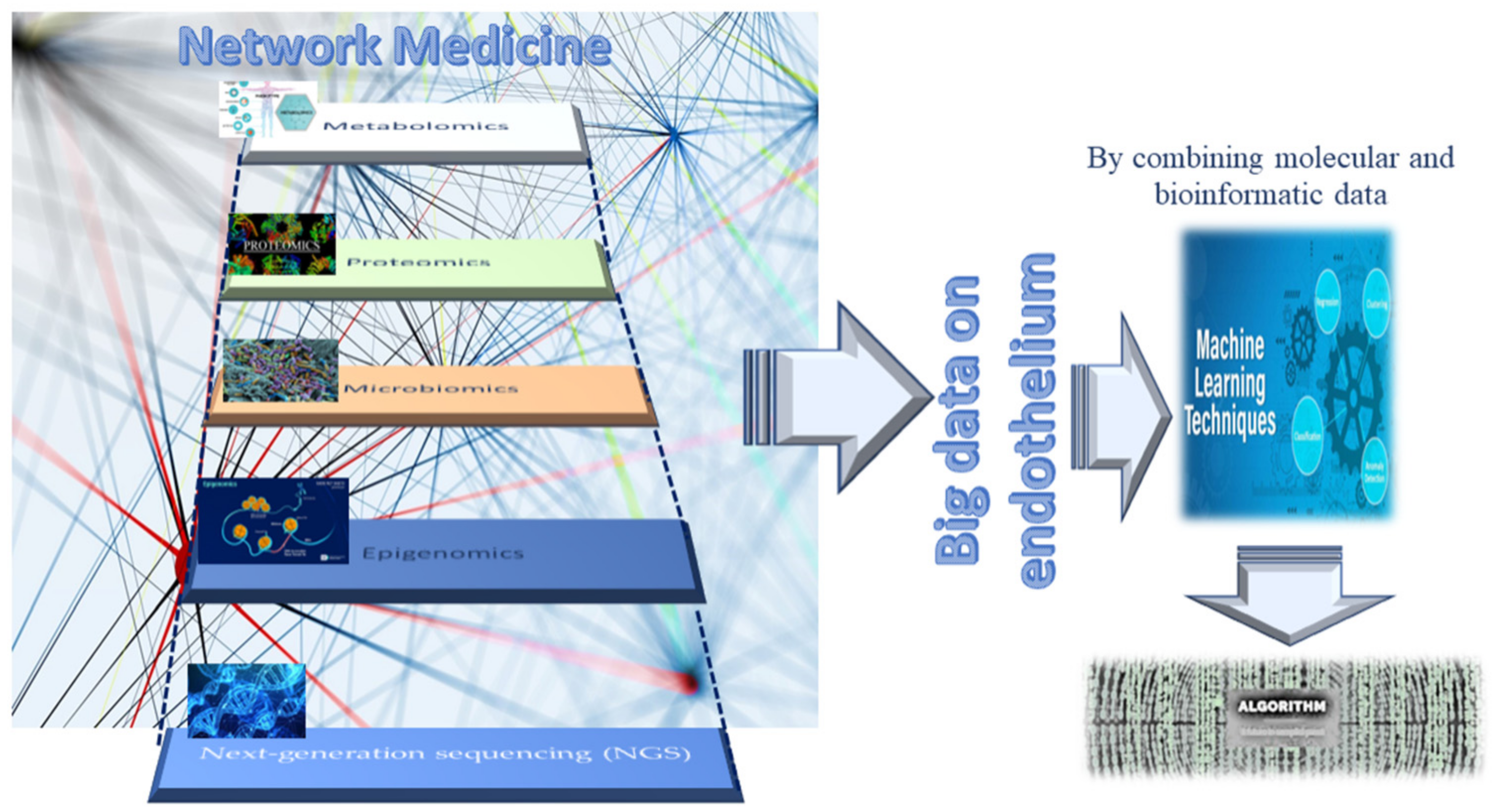
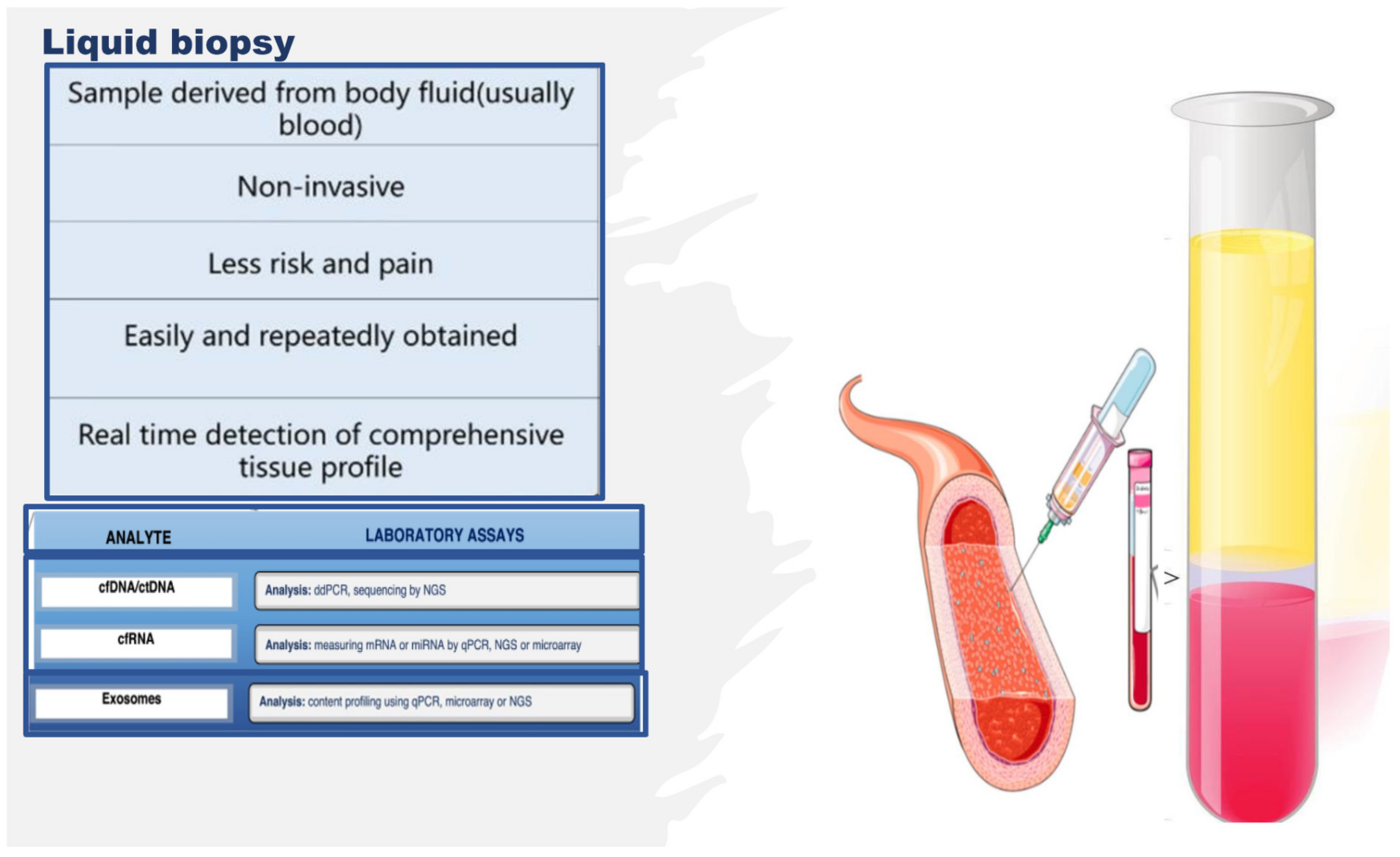
2. Omics Technologies and Liquid Biopsy as Emerging Tools for Identifying Endothelial Biomarkers and Therapeutic Targets
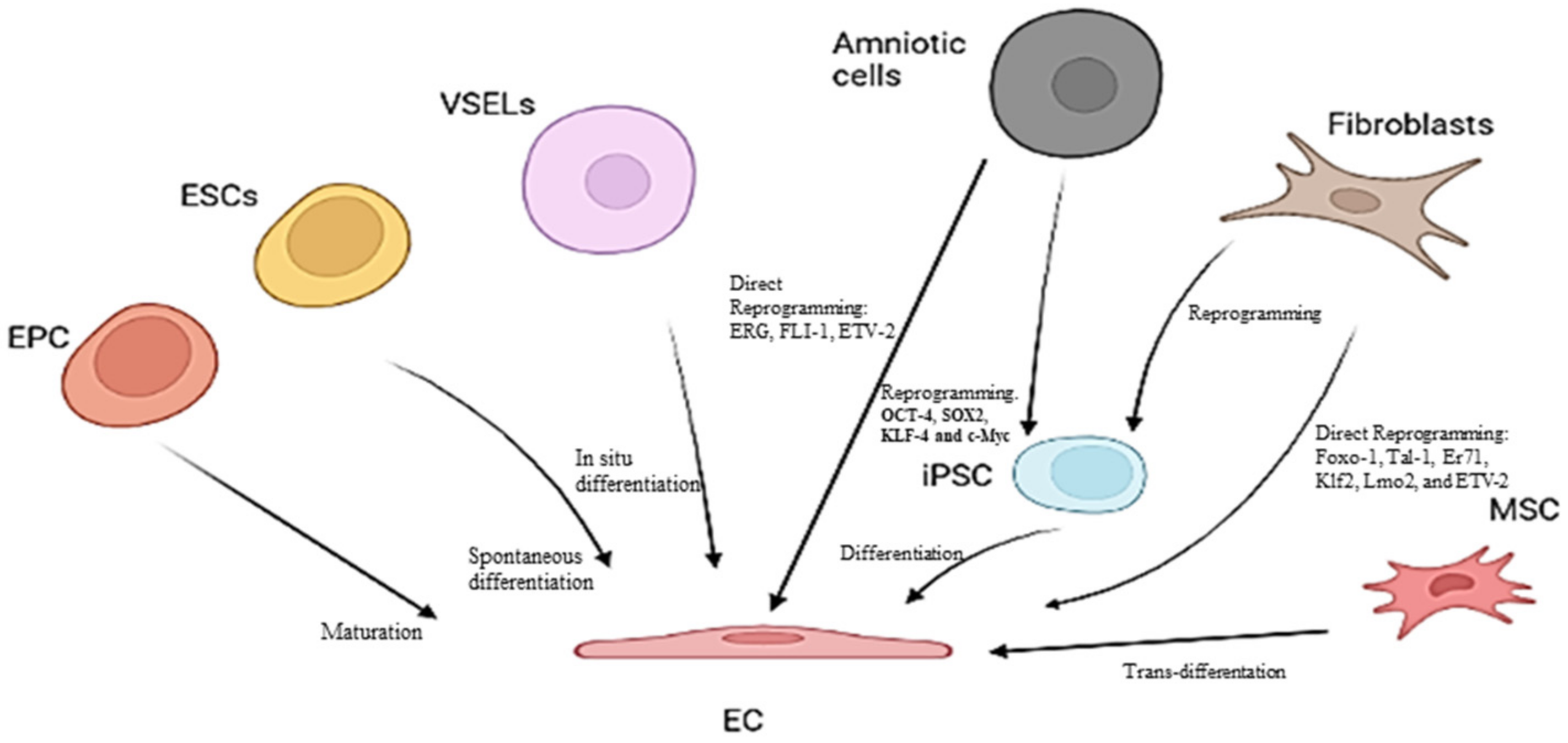
3. Endothelial Progenitor Cells (EPCs) as Potential Biomarkers of Endothelium Dysfunction and Therapeutic Agents
Other Candidates for Therapeutic Agents of Endothelium Dysfunction
References
- Inai, K. Chapter 17: Atrioventricular Valve Abnormalities: From Molecular Mechanisms Underlying Morphogenesis to Clinical Perspective. In Etiology and Morphogenesis of Congenital Heart Disease: From Gene Function and Cellular Interaction to Morphology ; Nakanishi, T., Markwald, R.R., Baldwin, H.S., Keller, B.B., Srivastava, D., Yamagishi, H., Eds.; Springer: Tokyo, Japan, 2016.
- Vita, J.A. Endothelial Function. Circulation 2011, 124, e906–e912.
- Sabe, S.A.; Feng, J.; Sellke, F.W.; Abid, M.R. Mechanisms and clinical implications of endothelium-dependent vasomotor dysfunction in coronary microvasculature. Am. J. Physiol. Circ. Physiol. 2022, 322, H819–H841.
- Boulanger, C.M. Endothelium. Arterioscler. Thromb. Vasc. Biol. 2016, 36, e26–e31.
- Balistreri, C.R.; Madonna, R.; Melino, G.; Caruso, C. The emerging role of Notch pathway in ageing: Focus on the related mechanisms in age-related diseases. Ageing Res. Rev. 2016, 29, 50–65.
- Balistreri, C.R.; Garagnani, P.; Madonna, R.; Vaiserman, A.; Melino, G. Developmental programming of adult haematopoiesis system. Ageing Res. Rev. 2019, 54, 100918.
- Allegra, A.; Giarratana, R.M.; Scola, L.; Balistreri, C.R. The close link between the fetal programming imprinting and neurodegeneration in adulthood: The key role of “hemogenic endothelium” programming. Mech. Ageing Dev. 2021, 195, 111461.
- Schirò, G.; Balistreri, C.R. The close link between brain vascular pathological conditions and neurodegenerative diseases: Focus on some examples and potential treatments. Vasc. Pharmacol. 2021, 142, 106951.
- Pober, J.S.; Min, W.; Bradley, J.R. Mechanisms of Endothelial Dysfunction, Injury, and Death. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 71–95.
- Regina, C.; Panatta, E.; Candi, E.; Melino, G.; Amelio, I.; Balistreri, C.R.; Annicchiarico-Petruzzelli, M.; Di Daniele, N.; Ruvolo, G. Vascular ageing and endothelial cell senescence: Molecular mechanisms of physiology and diseases. Mech. Ageing Dev. 2016, 159, 14–21.
- Madonna, R.; Novo, G.; Balistreri, C.R. Cellular and molecular basis of the imbalance between vascular damage and repair in ageing and age-related diseases: As biomarkers and targets for new treatments. Mech. Ageing Dev. 2016, 159, 22–30.
- Wang, Y.-F.; Hsu, Y.-J.; Wu, H.-F.; Lee, G.-L.; Yang, Y.-S.; Wu, J.-Y.; Yet, S.-F.; Wu, K.K.; Kuo, C.-C. Endothelium-Derived 5-Methoxytryptophan Is a Circulating Anti-Inflammatory Molecule That Blocks Systemic Inflammation. Circ. Res. 2016, 119, 222–236.
- Miura, S.; Morimoto, Y.; Furihata, T.; Takeuchi, S. Functional analysis of human brain endothelium using a microfluidic device integrating a cell culture insert. APL Bioeng. 2022, 6, 016103.
- Jalnapurkar, S.; Landes, S.; Wei, J.; Mehta, P.K.; Shufelt, C.; Minissian, M.; Pepine, C.J.; Handberg, E.; Cook-Wiens, G.; Sopko, G.; et al. Coronary endothelial dysfunction appears to be a manifestation of a systemic process: A report from the Women’s Ischemia Syndrome Evaluation—Coronary Vascular Dysfunction (WISE-CVD) study. PLoS ONE 2021, 16, e0257184.
- Meilhac, A.; Cautela, J.; Thuny, F. Cancer Therapies and Vascular Toxicities. Curr. Treat. Options Oncol. 2022, 23, 333–347.
- Camilli, M.; La Vecchia, G.; Lillo, R.; Iannaccone, G.; Lamendola, P.; Montone, R.A.; Hohaus, S.; Aspromonte, N.; Massetti, M.; Lanza, G.A.; et al. Cardiovascular involvement in patients affected by multiple myeloma: A comprehensive review of recent advances. Expert Rev. Hematol. 2021, 14, 1115–1128.
- Love, K.M.; Barrett, E.J.; Malin, S.K.; Reusch, J.E.B.; Regensteiner, J.G.; Liu, Z. Diabetes pathogenesis and management: The endothelium comes of age. J. Mol. Cell Biol. 2021, 13, 500–512.
- Trejo-Lopez, J.A.; Yachnis, A.T.; Prokop, S. Neuropathology of Alzheimer’s Disease. Neurotherapeutics 2021, 19, 173–185.
- Olivieri, F.; Prattichizzo, F.; Grillari, J.; Balistreri, C.R. Cellular Senescence and Inflammaging in Age-Related Diseases. Mediat. Inflamm. 2018, 2018, 9076485.
- Peng, H.; Wang, X.; Du, J.; Cui, Q.; Huang, Y.; Jin, H. Metabolic Reprogramming of Vascular Endothelial Cells: Basic Research and Clinical Applications. Front. Cell Dev. Biol. 2021, 9, 626047.
- Jaul, E.; Barron, J. Characterizing the Heterogeneity of Aging: A Vision for a Staging System for Aging. Front. Public Health 2021, 9, 513557.
- Pisano, C.; Polisano, D.; Balistreri, C.; Altieri, C.; Nardi, P.; Bertoldo, F.; Trombetti, D.; Asta, L.; Ferrante, M.; Buioni, D.; et al. Role of Cachexia and Fragility in the Patient Candidate for Cardiac Surgery. Nutrients 2021, 13, 517.
- Iglesias, M.J.; Kruse, L.D.; Sanchez-Rivera, L.; Enge, L.; Dusart, P.; Hong, M.-G.; Uhlén, M.; Renné, T.; Schwenk, J.M.; Bergstrom, G.; et al. Identification of Endothelial Proteins in Plasma Associated with Cardiovascular Risk Factors. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2990–3004.
- Amraei, R.; Rahimi, N. COVID-19, Renin-Angiotensin System and Endothelial Dysfunction. Cells 2020, 9, 1652.
- Pons, S.; Fodil, S.; Azoulay, E.; Zafrani, L. The vascular endothelium: The cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit. Care 2020, 24, 353.
- Zhang, J.; Tecson, K.M.; McCullough, P.A. Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Rev. Cardiovasc. Med. 2020, 21, 315–319.
- Lio, D.; Scola, L.; Giarratana, R.M.; Candore, G.; Colonna-Romano, G.; Caruso, C.; Balistreri, C.R. SARS CoV2 infection _The longevity study perspectives. Ageing Res. Rev. 2021, 67, 101299.
- Wingo, M.; Rafii, S. Endothelial reprogramming for vascular regeneration: Past milestones and future directions. Semin. Cell Dev. Biol. 2021, 122, 50–55.
- León, P.; Wang, J.; Huertas-Vazquez, A. Relevance of Multi-Omics Studies in Cardiovascular Diseases. Front. Cardiovasc. Med. 2019, 6, 91.
- Scola, L.; Giarratana, R.M.; Torre, S.; Argano, V.; Lio, D.; Balistreri, C.R. On the Road to Accurate Biomarkers for Cardiometabolic Diseases by Integrating Precision and Gender Medicine Approaches. Int. J. Mol. Sci. 2019, 20, 6015.
- Greene, J.A.; Loscalzo, J. Putting the Patient Back Together—Social Medicine, Network Medicine, and the Limits of Reductionism. N. Engl. J. Med. 2017, 377, 2493–2499.
- Cheng, F.; Lu, W.; Liu, C.; Fang, J.; Hou, Y.; Handy, D.E.; Wang, R.; Zhao, Y.; Yang, Y.; Huang, J.; et al. A genome-wide positioning systems network algorithm for in silico drug repurposing. Nat. Commun. 2019, 10, 3476.
- Lee, L.Y.-H.; Loscalzo, J. Network Medicine in Pathobiology. Am. J. Pathol. 2019, 189, 1311–1326.
- Abasht, B.; Papah, M.B.; Qiu, J. Evidence of vascular endothelial dysfunction in Wooden Breast disorder in chickens: Insights through gene expression analysis, ultra-structural evaluation and supervised machine learning methods. PLoS ONE 2021, 16, e0243983.
- Treder, M.; Lauermann, J.L.; Alnawaiseh, M.; Eter, N. Using Deep Learning in Automated Detection of Graft Detachment in Descemet Membrane Endothelial Keratoplasty: A Pilot Study. Cornea 2018, 38, 157–161.
- Sun, X.; Feinberg, M.W. Vascular Endothelial Senescence: Pathobiological Insights, Emerging Long Noncoding RNA Targets, Challenges and Therapeutic Opportunities. Front. Physiol. 2021, 12, 693067.
- Kumar, S.; Andueza, A.; Kim, J.; Kang, D.-W.; Jo, H. Isolation of Endothelial Cells from the Lumen of Mouse Carotid Arteries for Single-cell Multi-omics Experiments. J. Vis. Exp. 2021, 176, e63128.
- Andueza, A.; Kumar, S.; Kim, J.; Kang, D.-W.; Mumme, H.L.; Perez, J.I.; Villa-Roel, N.; Jo, H. Endothelial Reprogramming by Disturbed Flow Revealed by Single-Cell RNA and Chromatin Accessibility Study. Cell Rep. 2020, 33, 108491.
- He, M.; Huang, T.-S.; Li, S.; Hong, H.-C.; Chen, Z.; Martin, M.; Zhou, X.; Huang, H.-Y.; Su, S.-H.; Zhang, J.; et al. Atheroprotective Flow Upregulates ITPR3 (Inositol 1,4,5-Trisphosphate Receptor 3) in Vascular Endothelium via KLF4 (Krüppel-Like Factor 4)-Mediated Histone Modifications. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 902–914.
- Zhang, M.; Urabe, G.; Ozer, H.G.; Xie, X.; Webb, A.; Shirasu, T.; Li, J.; Han, R.; Kent, K.C.; Wang, B.; et al. Angioplasty induces epigenomic remodeling in injured arteries. Life Sci. Alliance 2022, 5, e202101114.
- Pepin, M.E.; Schiano, C.; Miceli, M.; Benincasa, G.; Mansueto, G.; Grimaldi, V.; Soricelli, A.; Wende, A.R.; Napoli, C. The human aortic endothelium undergoes dose-dependent DNA methylation in response to transient hyperglycemia. Exp. Cell Res. 2021, 400, 112485.
- Matsuoka, R.L.; Buck, L.D.; Vajrala, K.P.; Quick, R.E.; Card, O.A. Historical and current perspectives on blood endothelial cell heterogeneity in the brain. Experientia 2022, 79, 372.
- Benincasa, G.; Mansueto, G.; Napoli, C. Fluid-based assays and precision medicine of cardiovascular diseases: The ‘hope’ for Pandora’s box? J. Clin. Pathol. 2019, 72, 785–799.
- Mattox, A.K.; Bettegowda, C.; Zhou, S.; Papadopoulos, N.; Kinzler, K.W.; Vogelstein, B. Applications of liquid biopsies for cancer. Sci. Transl. Med. 2019, 11, eaay1984.
- Rubis, G.; Krishnan, S.; Bebawy, M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol. Sci. 2019, 40, 172–186.
- Perakis, S.; Speicher, M.R. Emerging concepts in liquid biopsies. BMC Med. 2017, 15, 75.
- Balistreri, C.R.; Buffa, S.; Pisano, C.; Lio, D.; Ruvolo, G.; Mazzesi, G. Are Endothelial Progenitor Cells the Real Solution for Cardiovascular Diseases? Focus on Controversies and Perspectives. BioMed Res. Int. 2015, 2015, 835934.
- Balistrieri, C. Endothelial Progenitor Cells: A New Real Hope? Springer International Publishing: Dordrecht, The Netherlands, 2017; pp. 1–80.
- Olivieri, F.; Pompilio, G.; Balistreri, C.R. Endothelial progenitor cells in ageing. Mech. Ageing Dev. 2016, 159, 1–3.
- Altabas, V.; Altabas, K.; Kirigin, L. Endothelial progenitor cells (EPCs) in ageing and age-related diseases: How currently available treatment modalities affect EPC biology, atherosclerosis, and cardiovascular outcomes. Mech. Ageing Dev. 2016, 159, 49–62.
- Balistreri, C.R.; Crapanzano, F.; Schirone, L.; Allegra, A.; Pisano, C.; Ruvolo, G.; Forte, M.; Greco, E.; Cavarretta, E.; Marullo, A.G.M.; et al. Deregulation of Notch1 pathway and circulating endothelial progenitor cell (EPC) number in patients with bicuspid aortic valve with and without ascending aorta aneurysm. Sci. Rep. 2018, 8, 13834.
- Balistreri, C.R.; Pisano, C.; Bertoldo, F.; Massoud, R.; Dolci, S.; Ruvolo, G. Red Blood Cell Distribution Width, Vascular Aging Biomarkers, and Endothelial Progenitor Cells for Predicting Vascular Aging and Diagnosing/Prognosing Age-Related Degenerative Arterial Diseases. Rejuvenation Res. 2019, 22, 399–408.
- Ferrante, A.; Guggino, G.; Di Liberto, D.; Ciccia, F.; Cipriani, P.; Balistreri, C.R.; Sireci, G.; Giacomelli, R.; Triolo, G. Endothelial progenitor cells: Are they displaying a function in autoimmune disorders? Mech. Ageing Dev. 2016, 159, 44–48.
- Loiola, R.A.; García-Gabilondo, M.; Grayston, A.; Bugno, P.; Kowalska, A.; Duban-Deweer, S.; Rizzi, E.; Hachani, J.; Sano, Y.; Shimizu, F.; et al. Secretome of endothelial progenitor cells from stroke patients promotes endothelial barrier tightness and protects against hypoxia-induced vascular leakage. Stem Cell Res. Ther. 2021, 12, 552.
- Morrone, D.; Picoi, M.E.L.; Felice, F.; De Martino, A.; Scatena, C.; Spontoni, P.; Naccarato, A.G.; Di Stefano, R.; Bortolotti, U.; Monte, M.D.; et al. Endothelial Progenitor Cells: An Appraisal of Relevant Data from Bench to Bedside. Int. J. Mol. Sci. 2021, 22, 12874.
- Ota, Y.; Kuwana, M. Endothelial cells and endothelial progenitor cells in the pathogenesis of systemic sclerosis. Eur. J. Rheumatol. 2020, 7, 139–146.
- Pulito-Cueto, V.; Remuzgo-Martínez, S.; Genre, F.; Atienza-Mateo, B.; Mora-Cuesta, V.; Iturbe-Fernández, D.; Lera-Gómez, L.; Pérez-Fernández, R.; Prieto-Peña, D.; Portilla, V.; et al. Endothelial Progenitor Cells: Relevant Players in the Vasculopathy and Lung Fibrosis Associated with the Presence of Interstitial Lung Disease in Systemic Sclerosis Patients. Biomedicines 2021, 9, 847.
- Endothelial progenitor cells and coronary artery disease: Current concepts and future research directions. World J. Clin. Cases 2021, 9, 8953–8966.
- Buffa, S.; Borzì, D.; Chiarelli, R.; Crapanzano, F.; Lena, A.M.; Nania, M.; Candi, E.; Triolo, F.; Ruvolo, G.; Melino, G.; et al. Biomarkers for vascular ageing in aorta tissues and blood samples. Exp. Gerontol. 2019, 128, 110741.
- Poz, D.; De Falco, E.; Pisano, C.; Madonna, R.; Ferdinandy, P.; Balistreri, C.R. Diagnostic and Prognostic Relevance of Red Blood Cell Distribution Width for Vascular Aging and Cardiovascular Diseases. Rejuvenation Res. 2019, 22, 146–162.
- Hill, J.M.; Zalos, G.; Halcox, J.P.J.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating Endothelial Progenitor Cells, Vascular Function, and Cardiovascular Risk. N. Engl. J. Med. 2003, 348, 593–600.
- Naruse, K.; Hamada, Y.; Nakashima, E.; Kato, K.; Mizubayashi, R.; Kamiya, H.; Yuzawa, Y.; Matsuo, S.; Murohara, T.; Matsubara, T.; et al. Therapeutic Neovascularization Using Cord Blood–Derived Endothelial Progenitor Cells for Diabetic Neuropathy. Diabetes 2005, 54, 1823–1828, Erratum in Diabetes 2006, 55, 1534.
- Shyu, W.-C.; Lin, S.-Z.; Chiang, M.-F.; Su, C.-Y.; Li, H. Intracerebral Peripheral Blood Stem Cell (CD34+) Implantation Induces Neuroplasticity by Enhancing beta1 Integrin-Mediated Angiogenesis in Chronic Stroke Rats. J. Neurosci. 2006, 26, 3444–3453.
- Gómez-Navarro, J.; Contreras, J.L.; Arafat, W.; Jiang, X.L.; Krisky, D.; Oligino, T.; Marconi, P.; Hubbard, B.; Glorioso, J.C.; Curiel, D.T.; et al. Genetically modified CD34+ cells as cellular vehicles for gene delivery into areas of angiogenesis in a rhesus model. Gene Ther. 2000, 7, 43–52.
- Ohtsuka, M.; Takano, H.; Zou, Y.; Toko, H.; Akazawa, H.; Qin, Y.; Suzuki, M.; Hasegawa, H.; Nakaya, H.; Komuro, I. Cytokine therapy prevents left ventricular remodeling and dysfunction after myocardial infarction through neovascularization. FASEB J. 2004, 18, 851–853.
- Yamamoto, K.; Kondo, T.; Suzuki, S.; Izawa, H.; Kobayashi, M.; Emi, N.; Komori, K.; Naoe, T.; Takamatsu, J.; Murohara, T. Molecular Evaluation of Endothelial Progenitor Cells in Patients with Ischemic Limbs. Arterioscler. Thromb. Vasc. Biol. 2004, 24, e192–e196.
- Lenk, K.; Adams, V.; Lurz, P.; Erbs, S.; Linke, A.; Gielen, S.; Schmidt, A.; Scheinert, D.; Biamino, G.; Emmrich, F.; et al. Therapeutical potential of blood-derived progenitor cells in patients with peripheral arterial occlusive disease and critical limb ischaemia. Eur. Heart J. 2005, 26, 1903–1909.
- Erbs, S.; Linke, A.; Adams, V.; Lenk, K.; Thiele, H.; Diederich, K.-W.; Emmrich, F.; Kluge, R.; Kendziorra, K.; Sabri, O.; et al. Transplantation of Blood-Derived Progenitor Cells After Recanalization of Chronic Coronary Artery Occlusion. Circ. Res. 2005, 97, 756–762.
- Liu, Y.; Chen, J.; Liang, H.; Cai, Y.; Li, X.; Yan, L.; Zhou, L.; Shan, L.; Wang, H. Human umbilical cord-derived mesenchymal stem cells not only ameliorate blood glucose but also protect vascular endothelium from diabetic damage through a paracrine mechanism mediated by MAPK/ERK signaling. Stem Cell Res. Ther. 2022, 13, 258.




