2. Omics Technologies and Liquid Biopsy as Emerging Tools for Identifying Endothelial Biomarkers and Therapeutic Targets
In proposing new strategies for preserving the functionality and efficiency of adult endothelium tissue, it is imperative to identify molecules as optimal biomarkers and therapeutic targets. Biomarkers represent crucial indicators of both physiological and pathological processes, such as endothelium dysfunction. Specific changes in molecular and cellular mechanisms of physiological processes result in biochemical alterations at both the tissue and the systemic level, which can give us comprehensive information regarding the health status of tissues (in our case, the endothelium), as well as the nature of any pathological tissue dysfunction and disease. In addition, any disease biomarker should be specific and reliable, able to distinguish between the physiological condition of a tissue, organ, or system and a dysfunctional condition or disease, and diverse across various diseases or their subgroups and phenotypes. Accordingly, biomarkers can predict the risk of disease, facilitate early diagnosis, and enable setting guidelines for the development of new therapies for treating diseases. New-generation omics technologies and their platforms, including next-generation sequencing (NGS), epigenomics, proteomics, metabolomics, and microbiomics [
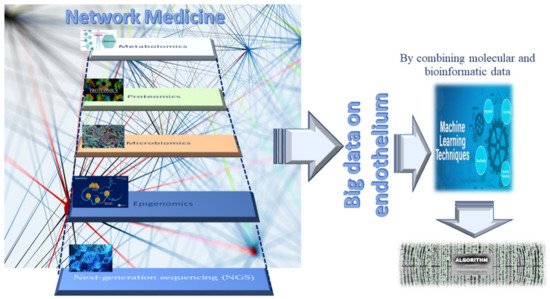
29,
30] (see
Figure 4), can detect molecules involved in the close relationship between genotype and the environment, nutritional habits, and endothelial health or dysfunction, at an unprecedented grade of importance and resolution [
31,
32,
33]. They also allow for obtaining an enormous quantity of data, albeit accompanied by consequent computational difficulties in their interpretation [
31,
32,
33]. Today, such a challenge has been resolved thanks to potent bioinformatics algorithms, which can easily interpret and combine big data, by offering the possibility of both analyzing them at diverse multilevel networks and identifying genes, proteins, metabolites, and microbiomes, as well as their interactions in the complex human interactome, which can be used as emerging biomarkers and drug targets [
31,
32,
33] (see
Figure 4). Such a strategy exemplifies network medicine with the feature of combining molecular and bioinformatic data, which can improve current medical practices in the management of endothelial dysfunction-related pathologies by overcoming the limits of the obsolete points of view regarding endothelial dysfunction, considered the direct consequence of a single molecular alteration. An example of such an approach is machine learning (see
Figure 4). The Abasht group [
34] used this approach to evaluate vascular endothelial dysfunction in wooden breast syndrome, while the Treder group [
35] used this approach to detect graft detachment in Descemet membrane endothelial keratoplasty. Furthermore, it is currently being applied to develop algorithms for vascular endothelial senescence, as recently evidenced by Sun and Feinberg [
36].
Figure 4. Network medicine and its features in endothelium studies.
Furthermore, omics technologies enable evaluating changes at a single-cell level, i.e., the evaluation of gene expression profiles of the endothelium at the transcriptome and epigenome level, when exposed to one or more stressors, such as disturbed blood flow, high-glucose status, or inflammation. Specifically, it is possible to evaluate the transcriptomic and chromatin accessibility variations at the resolution of a single-EC. This allows comparing the effects of stressors in healthy ECs vs. stressed ECs in the same vessel or in diverse vessels, thereby following the changes in physiological status of the endothelium as a result of dysfunction or other conditions, e.g., hyperplasia (see below). A similar study design was recently adopted by Kumar and coworkers [
37] to evaluate the gene expression profile in ECs isolated, via an innovative protocol, from the lumen of carotid arteries from mice subjected to a partial carotid ligation (PCL), which induces disturbed blood flow (d-flow) in the left carotid artery, while maintaining stable laminar flow in the right carotid artery, used as a control. Such a mouse PCL model allows comparing the effects induced in ECs by blood flow under disturbed conditions or with stable laminar flow (s-flow). ECs were obtained from the carotid arteries by dissection 2 days or 2 weeks post PCL surgery, before subjecting their lumen to collagenase digestion to acquire endothelial-enriched single cells or single nuclei. These single-cell and single-nuclei preparations were utilized for RNA preparation, library generation, single-cell RNA sequencing (scRNA-seq), and single-cell transposase-accessible chromatin sequencing (scATACseq), which were used to determine the transcriptomic and chromatin accessibility changes. The data obtained consequently allowed evaluating the genes expressed under the effects induced by d-flow or s-flow at a single-cell level, resulting in the activation of proatherogenic or physiological responses. These proatherogenic genes and their molecules were then suggested as targets for developing therapies. Specifically, it was confirmed that Krüppel-like factors 4 and 2 (KLF4/KLF2) act as s-flow-sensitive transcription factor (TF)-binding sites. The identified TFs sensitive to d-flow were RELA, AP1, STAT1, and TEAD1, which were suggested to induce d-flow EC reprogramming from an athero-protective to a proatherogenic phenotype, including endothelial–mesenchymal transition [
37,
38]. Furthermore, thanks to the integration of diverse omics assays, including the acetylation of histone 3 lysine 27 (H3K27ac) ChIP-seq (chromatin immunoprecipitation followed by high-throughput sequencing), ATAC-seq (an assay for transposase-accessible chromatin sequencing), and RNA-seq (RNA-sequencing), it was possible to detect the genome-wide epigenetic regulations in ECs in response to athero-protective pulsatile shear stress (PS) [
39]. The combined data obtained showed that inositol 1,4,5-trisphosphate receptor 3 (ITPR3) was upregulated under PS conditions via KLF4 or statins, in ECs isolated from mouse aorta, lung ECs isolated from EC-KLF4-transgenic versus EC-KLF4-knoukout mice, and atorvastatin-treated ECs. The use of KLF4 ATAC-qPCR (quantitative polymerase chain reaction) and ChIP-qPCR also led to the identification of a specific locus in the promoter region of the ITPR3 gene indispensable for KLF4 binding, H3K27ac enrichment, chromatin accessibility, RNA polymerase II recruitment, and ITPR3 transcriptional activation [
39]. By evocating the deletion of KLF4 binding locus in EC thanks to the use of a clustered regularly interspaced short palindromic repeats-associated protein 9 (CRISPR/Cas9) system, a blunted calcium influx, reduced expression of endothelial nitric oxide synthase, and diminished nitric oxide bioavailability were evidenced. In the complex, such interesting results obtained thanks to novel multi-omics investigations suggested that KLF4 is crucial for PS-modulated H3K27ac, which allows the transcriptional activation of ITPR3. Such a mechanism contributes to Ca
2+-dependent endothelial nitric oxide synthase (eNOS) activation and EC homeostasis, and these molecules are potential candidates for biomarkers and therapeutic targets [
39].
Another group integrated investigations of in vivo epigenomic mapping with conditional knockout, gene transfer, and pharmacology with the aim of studying, in rodent models, the endothelial hyperplasia/proliferation (IH), which represents the primary etiology of vascular stenosis. The data from injured (IH-prone) rat arteries demonstrated a rise in genome-wide occupancy by histone 3 lysine 27 trimethylation (H3K27me3), a gene-repression indicator. This result was unexpected when considering the traditional point of view on prevailing post-injury gene activation rather than repression. Additional evaluation has also demonstrated a shift in H3K27me3 enrichment to anti-proliferative genes from pro-proliferative genes, characterized by gene activation via H3K27ac (acetylation) accumulation. H3K27ac and its reader BRD4 (bromodomain protein) showed co-enrichment of
Ezh2, while conditional BRD4 knockout in injured mouse arteries resulted in a decrease in H3K27me3 and its writer EZH2, which positively regulates another pro-IH chromatin modulator, UHRF1. Thus, these results reveal an injury-induced locus-specific H3K27me3 redistribution in the epigenomic landscape involving BRD4 → EZH2 → UHRF1 hierarchical regulation, while suggesting that these players may be pharmaceutical targets [
40].
Another interesting study was conducted in 2021 by a group in Napoli examining the glucose-dependent and dose-responsive alterations in endothelial DNA methylation to detect a putative epigenetic mechanism underlying diabetic vasculopathy. Specifically, they discovered the disproportionate glucose-dependent methylation and gene expression of VEGF and NO signaling cascades, constituting a physiological imbalance known to evocate endothelial dysfunction in diabetes. Consequently, they assumed that epigenetic mechanisms encode glycemic memory within EC, and the pathways involved can be used as therapeutic targets [
41]. Specifically, their novel evidence demonstrated that hyperglycemia triggers dose-responsive variations in DNA methylation dynamics, affecting key physiological processes involved in the maintenance of endothelial function, including a glucose-dependent physiologic uncoupling of VEGF and NO signaling, which causes endothelial dysfunction.
Additionally, recent advances in molecular genetics and omics technologies also showed significant molecular heterogeneity within brain endothelial and perivascular cell types [
42]. The blend of these conventional and modern approaches has enabled detecting phenotypical variations between healthy and abnormal conditions at the single-cell level. Accordingly, a deep understanding of brain vascular cell states during physiological, pathological, and aging processes has rapidly emerged, and therapeutic approaches have been developed, including vascularization models of human brain organoids on a chip (widely quoted in [
42]), self-assembling multicellular blood–brain barrier (BBB) spheroids (widely quoted in [
42]), and improved endothelial BBB differentiation protocols using human pluripotent or induced pluripotent stem cells (widely quoted in [
8,
42]). Human organoid and cell reprogramming technologies have rapidly improved, allowing the simulation of human brain development and disease modeling with the construction of a functional vasculature. These in vitro models will become potent instruments for exploring human-specific vascular traits absent in animal models, for investigating drug delivery across the BBB, and for screening drugs targeting BBB dysfunction in neurological diseases. Consequently, this research recently led our group, along with others, to hypothesize a close relationship between endothelial BBB conditions and neurodegenerative diseases (widely quoted in [
8,
42]).
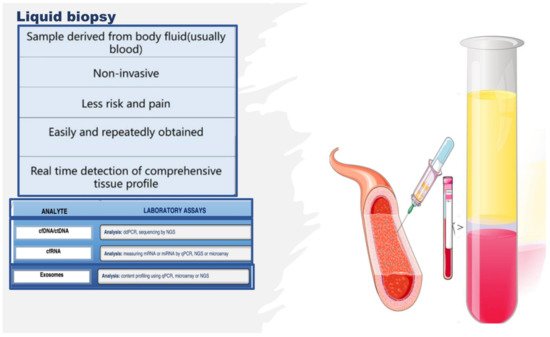
Therefore, the literature is seeing an increase in the number of multi-omics investigations on endothelium, and the studies reported above constitute only some examples. However, many of these studies were complex with regard to their design, often invasive or necessitating the sacrifice of animals, along with an intricate approach to collecting the biological study samples and the related costs. Alternatively, studies on other types of samples, whose collection attracts a minor cost and a simple technique, are growing in number. Indeed, studies aimed at identifying noninvasive indicators of endothelial tissue status, simply using liquid biopsy, are increasing. Liquid biopsy is attractive as it facilitates the collection of an optimal biological sample, thereby enabling the extrapolation of big data via multi-omics technologies [
43,
44,
45]. Liquid biopsy consists of an easy sampling of diverse bodily fluids, principally including blood, saliva, and urine, for the evaluation of various potential circulating noninvasive biomarkers [
43,
44,
45]. As a biopsy, it also enables obtaining circulating intact cells, cell-free nucleic acids, circulating epigenetic-sensitive molecules (methylated DNA, modified histones, and noncoding RNAs), circulating metabolites, and other cell products, such as micro-vesicles and exosomes [
43,
44,
45] (see
Figure 5). However, unlike tissue biopsy, liquid biopsy offers the advantage of colleting heterogeneous cellular phenotypes by giving information on many circulating cell types and their products at specific times, thereby allowing the real-time monitoring of the evolution of a pathological condition or of the switching of the endothelium from physiological to dysfunctional [
46]. In addition, emerging NGS platforms executed on body fluid samples, principally blood, can detect novel circulating noninvasive biomarkers, which may further the development of precision medicine and personalized therapy in the field of endothelium dysfunction-related pathologies, by preserving the health of the endothelium or reverting its alterations [
43,
44,
45,
46]. Furthermore, liquid biopsy shows several benefits compared to tissue biopsy; it is (a) noninvasive with negligible pain and risk, (b) more quickly executed, (c) easier to collect, (d) able to provide a real-time systemic profile, (e) able to give spatiotemporal information, and (f) able to monitor physiological or pathological conditions over time.
Figure 5. Liquid biopsy, describing some components and laboratory analysis techniques. Liquid biopsy involves the collection and analysis of diverse components from peripheral blood samples, including cell-free nucleic acids (cfDNA/ctDNA and cfRNA) and exosomes. cfDNA: circulating free DNA, ctDNA: circulating tumor DNA, cfRNA: cell-free RNA, CTCs: circulating tumor cells, TEPs: tumor-educated platelets, NGS: next-generation sequencing, qPCR: quantitative polymerase chain reaction.
As mentioned above, fluid-based assays may provide noninvasive indicators that indicate the endothelium status or allow sequential monitoring of the evolution of dysfunction. Interesting evidence can be gained by assessing the number, function, and senescence grade of endothelial progenitor cells (EPCs), as well as by evaluating the effects induced by stem-cell therapies (i.e., human umbilical cord-derived mesenchymal stem cells (MSCs)) or by detecting the quantity and quality of circulating metabolites and other cell products, such as micro-vesicles and exosomes or molecules able to modulate the gene expression at transcriptional, post-transcriptional, and translational levels, including circular RNAs (circRNAs) and ADAR enzymes. The next section reports the description of some of these metabolites and cell products, stressing their emerging potential as biomarkers and therapeutic targets of endothelium dysfunction, as well as their related limitations.
3. Endothelial Progenitor Cells (EPCs) as Potential Biomarkers of Endothelium Dysfunction and Therapeutic Agents
Circulating EPCs represent the progenitors of ECs, and they originate from bone marrow (BM)-derived hematopoietic stem cells (HSCs) [
47,
48,
49]. EPCs constitute a real resource of ECs, thus maintaining vascular and tissue homeostasis and appropriate oxygen regulation and transport. Accordingly, EPCs represent a reservoir of circulating cells able to target injury sites, restore endothelium integrity, and enable physiological activities [
47,
48,
49]. The impact of EPCs in the vascularization process has been proven in both animal models and humans. Such evidence has also led to the assumption that reductions in EPC circulating number and/or alterations in their functions related to different causes could impact endothelium function and architecture, as well as the onset and complications of endothelium dysfunction and, consequently, the survival of affected persons. Increased or decreased circulating EPC levels, as well as alterations in their function (for a detailed description, we invite the reader to consult our book, see reference [
48]), have indeed been associated with vascular endothelium aging and diverse endothelium dysfunction pathologies, including coronary artery disease, stroke, diabetes, systemic sclerosis, autoimmune disorders, and aneurysms [
50,
51,
52,
53,
54,
55,
56,
57,
58]. Our group, for instance, recently evidenced that subjects affected by bicuspid aorta valve syndrome show a significant decrease in both the tissue and circulating levels of the Notch pathway, as well as a decrease in blood EPC number, compared to subjects with a tricuspid physiological valve, whether in the presence or absence of aorta aneurysm (AAA) [
51,
59]. In addition, we also evidenced, using both blood and human tissue aorta samples, that the unique independent risk predictors for vascular aging are age and a reduced EPC number, as well as reduced EPC migratory activity and senescence, mediated by high SA-β-Gal activity and high levels of TP53, p21, p16, and inflammatory genes [
59]. Furthermore, we reported in another study [
60] that a decreased circulating number of EPCs, associated with increased RDW values, augmented blood levels of high-sensitivity C-reactive protein, and reduced mean values of both leukocyte telomere length and telomerase activity, can predict both vascular aging and ascending aorta aneurysm (AAA) onset and prognosis. Consequently, we affirm that these factors might be used as an ideal biomarker profile for vascular aging, as well as for the diagnosis and outcome of sporadic AAA.
Such evidence might be of clinical relevance, and possible new recommendations and preventive measures might be applied. Accordingly, Hill and colleagues [
61] already showed that the number of circulating EPCs represents a better predictor of vascular reactivity than conventional cardiovascular risk factors. Furthermore, a significant correlation between in vitro EPC senescence and endothelium dysfunction pathologies risk was also detected in blood donors [
59]. Consequently, EPCs might be considered as an optimal predictive biomarker, as well as a diagnostic and prognostic biomarker of endothelium dysfunction and related pathologies. Certainly, ulterior studies are needed. However, such evidence has also led to the use of EPCs as therapeutic agents in the autologous or heterologous treatment of several endothelium dysfunction diseases, as well as in clinical trials, despite contradictory results being obtained. In particular, a favorable improvement in left-ventricular (LV) function in a rat model of myocardial infarction (MI) after intravenous injection of ex vivo expanded human CD34
+ cells was reported [
47,
48,
49]. Another study examined the effect of catheter-based intramyocardial transplantation in a swine model of MI, providing encouraging outcomes in favoring the application of EPCs as a potential cell therapy in clinical trials [
47,
48,
49]. Naruse and colleagues conducted a study to assess the therapeutic treatment of diabetic neuropathy using in vivo expanded human EPC and streptozotocin-induced diabetic nude rats [
62]. They observed numerous micro-vessels at the site of EPC injection [
63]. Another group evidenced an improvement in neurological functions in chronic cerebral ischemic rats injected with CD34
+ HSC cells, including EPCs [
63]. The ability of EPCs to expand in culture under in vitro conditions represents another barrier for their therapeutic use. Genetically modified and ex vivo expanded EPCs may become promising agents able to appropriately rescue the impaired neovascularization process under disease conditions. In a rhesus model, ex vivo CD34
+ cell transfection with recombinant non-replicative herpes virus vector and subsequent cell transplantation resulted in the expression of vector genes in angiogenic areas of skin autografts of rhesus macaques. Since CD34
+ cells possess a natural angiogenic tropism to the injured endothelium, they may serve as ideal candidates for the delivery of genes into areas of angiogenesis [
64]. These results encouraged the execution of clinical trials for evaluating the potential of EPCs to enhance endothelial integrity and vascularization at ischemia sites in patients with CVDs. Three different strategies were mainly applied: (a) the
administration of granulocyte-colony stimulating factor (G-CSF) for verifying the recruitment of the patient’s own bone morrow resident progenitors, with two preliminary studies demonstrating increased LV function [
65]; (b)
the intracoronary infusion of BM progenitor cells in patients with MI, which demonstrated positive effects on LV function in three smaller studies [
47,
48,
49]. Subsequently, two prospective large trials assessed significant LV function after 4–6 months of administration of BM progenitor cells. Ten recent and large trials confirmed the success and safety of this procedure with a follow-up over 1.5 years (widely quoted in [
47,
48,
49]). In addition, intramyocardial and intracoronary administration has recently been suggested as a suitable strategy for the treatment of patients with refractory angina (widely quoted in [
47,
48,
49]); (c) a more invasive strategy involving
the direct injection of cells into target tissues [
59]. This treatment (specifically,
transepicardial or transendocardial injection of unfractioned BM cells) has been performed in patients with diffuse coronary artery disease and intractable angina with no option of recanalization. Ventricular function and physical capacity were observed to increase, but the small sample size of these studies necessitates confirmation in larger studies (widely quoted in [
47,
48,
49]).
Studies using
autologous cell therapy are also interesting. Accordingly, Yamamoto’s group executed an intramuscular injection of autologous BM-derived mononuclear cells containing 1% CD34
+ cells in patients with chronic limb ischemia [
66]. They quantitatively assessed the expression of EPCs and endothelial markers (i.e., CD133 and VE-cadherin) before the experiment and after the injection. Before investigation, the transcription of these molecules was undetectable. Autologous injection determined an elevation of EPC marker transcription. Thus, they established that autologous BM cells may be used in the therapy of patients with arterial diseases. A replication of these results was obtained by Lenk and colleagues [
67]. Erbs and colleagues also used this autologous treatment in patients who underwent recanalization of chronic coronary total occlusion [
68]. Autologous treatment with EPCs, expanded for 4 days in endothelium growth medium, improves coronary endothelium function and wall motion abnormalities, and it has a beneficial effect on metabolism in the target area in patients with symptomatic coronary atherosclerosis.
Despite these promising data, the clinical application of EPCs in exogenous or autologous cell therapy remains unclear for different reasons. Accordingly, the validity of their results is influenced by different factors: (a) the insignificant number of patients in these studies; (b) their missing randomization; (c) participation of a limited number of centers; (d) the imprecise phenotypic features of EPC utilized in the treatments, (e) the different methods of administration applied, and (f) the security and viability of the treatments [
47,
48,
49]. Teratoma formation, immunoreactivity, or arrhythmias constitute the major unfavorable effects which have been detected after such treatments. In addition, other limitations have not been considered, including the limited number of EPCs in circulation, which usually requires their cultured expansion in a sufficient number of subpopulations from peripheral blood. The in vitro quantification of progenitor cells to obtain a quantity sufficient to utilize in therapeutic treatment can be modulated by phenotypic changes during their differentiation in vitro with the risk of obtaining senescent cells, requiring artificial cell pre-activation or stimulation for this approach. In addition to such evidenced limitations, another aspect influencing the results of this study is the lack of standardized criteria and a consensus for defining, characterizing, and identifying EPCs with well-established surface biomarkers, protocols, and methodologies. Consequently, other investigations are imperative [
47,
48,
49].
Other Candidates for Therapeutic Agents of Endothelium Dysfunction
Promising evidence has recently been reported on the great potential of human umbilical cord-derived MSCs (hucMSCs) in repairing diabetic vascular endothelial damage, by applying assays of resazurin staining, MTT cell viability, wound healing, transwell migration, and Matrigel tube formation on human umbilical vein endothelial cells (HUVECs), and by evaluating how hucMSCs work through the assessment of RNA sequencing (RNA-seq) and molecular experiments [
69]. Specifically, the use of a conditioned medium of hucMSCs (MSC-CM) revealed that hucMSCs improved the cell viability, wound healing, migration, and angiogenesis of HUVECs damaged by high glucose via paracrine signaling, and the altered gene expressions of IL-6, TNF-α, ICAM-1, VCAM-1, BAX, P16, P53, and ET-1 were significantly restored by MSC-CM. RNA-seq incorporated with real-time PCR and Western blot clarified that high glucose activates MAPK/ERK signaling in HUVECs, while MSC-CM reverses the abnormal phosphorylation of ERK and overexpression of MKNK2, ERBB3, MYC, and DUSP5 in the MAPK/ERK signaling pathway [
69]. Certainly, other studies are needed.