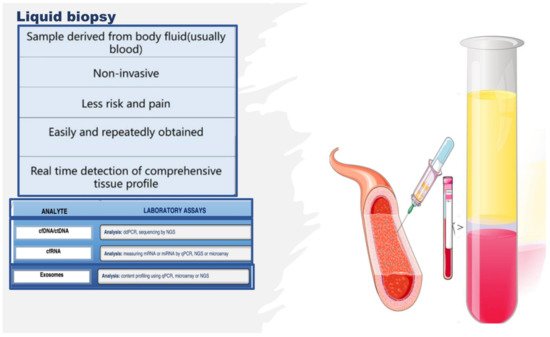
Figure 5. Liquid biopsy, describing some components and laboratory analysis techniques. Liquid biopsy involves the collection and analysis of diverse components from peripheral blood samples, including cell-free nucleic acids (cfDNA/ctDNA and cfRNA) and exosomes. cfDNA: circulating free DNA, ctDNA: circulating tumor DNA, cfRNA: cell-free RNA, CTCs: circulating tumor cells, TEPs: tumor-educated platelets, NGS: next-generation sequencing, qPCR: quantitative polymerase chain reaction.
As mentioned above, fluid-based assays may provide noninvasive indicators that indicate the endothelium status or allow sequential monitoring of the evolution of dysfunction. Interesting evidence can be gained by assessing the number, function, and senescence grade of endothelial progenitor cells (EPCs), as well as by evaluating the effects induced by stem-cell therapies (i.e., human umbilical cord-derived mesenchymal stem cells (MSCs)) or by detecting the quantity and quality of circulating metabolites and other cell products, such as micro-vesicles and exosomes or molecules able to modulate the gene expression at transcriptional, post-transcriptional, and translational levels, including circular RNAs (circRNAs) and ADAR enzymes. The next section reports the description of some of these metabolites and cell products, stressing their emerging potential as biomarkers and therapeutic targets of endothelium dysfunction, as well as their related limitations.
3. Endothelial Progenitor Cells (EPCs) as Potential Biomarkers of Endothelium Dysfunction and Therapeutic Agents
Circulating EPCs represent the progenitors of ECs, and they originate from bone marrow (BM)-derived hematopoietic stem cells (HSCs)
[47][48][49][47,48,49]. EPCs constitute a real resource of ECs, thus maintaining vascular and tissue homeostasis and appropriate oxygen regulation and transport. Accordingly, EPCs represent a reservoir of circulating cells able to target injury sites, restore endothelium integrity, and enable physiological activities
[47][48][49][47,48,49]. The impact of EPCs in the vascularization process has been proven in both animal models and humans. Such evidence has also led to the assumption that reductions in EPC circulating number and/or alterations in their functions related to different causes could impact endothelium function and architecture, as well as the onset and complications of endothelium dysfunction and, consequently, the survival of affected persons. Increased or decreased circulating EPC levels, as well as alterations in their function (for a detailed description,
we invite the reader to cons
ult our book, see reference
[48]), have indeed been associated with vascular endothelium aging and diverse endothelium dysfunction pathologies, including coronary artery disease, stroke, diabetes, systemic sclerosis, autoimmune disorders, and aneurysms
[50][51][52][53][54][55][56][57][58][50,51,52,53,54,55,56,57,58].
AOur group, for instance, recently evidenced that subjects affected by bicuspid aorta valve syndrome show a significant decrease in both the tissue and circulating levels of the Notch pathway, as well as a decrease in blood EPC number, compared to subjects with a tricuspid physiological valve, whether in the presence or absence of aorta aneurysm (AAA)
[51][59][51,59]. In addition,
we also evidenced, using both blood and human tissue aorta samples, that the unique independent risk predictors for vascular aging are age and a reduced EPC number, as well as reduced EPC migratory activity and senescence, mediated by high SA-β-Gal activity and high levels of TP53, p21, p16, and inflammatory genes
[59]. Furthermore,
it has bewe
n reported in another study
[60] that a decreased circulating number of EPCs, associated with increased RDW values, augmented blood levels of high-sensitivity C-reactive protein, and reduced mean values of both leukocyte telomere length and telomerase activity, can predict both vascular aging and ascending aorta aneurysm (AAA) onset and prognosis. Consequently,
it can bwe affirm
ed that these factors might be used as an ideal biomarker profile for vascular aging, as well as for the diagnosis and outcome of sporadic AAA.
Such evidence might be of clinical relevance, and possible new recommendations and preventive measures might be applied. Accordingly, Hill and colleagues
[61] already showed that the number of circulating EPCs represents a better predictor of vascular reactivity than conventional cardiovascular risk factors. Furthermore, a significant correlation between in vitro EPC senescence and endothelium dysfunction pathologies risk was also detected in blood donors
[59]. Consequently, EPCs might be considered as an optimal predictive biomarker, as well as a diagnostic and prognostic biomarker of endothelium dysfunction and related pathologies. Certainly, ulterior studies are needed. However, such evidence has also led to the use of EPCs as therapeutic agents in the autologous or heterologous treatment of several endothelium dysfunction diseases, as well as in clinical trials, despite contradictory results being obtained. In particular, a favorable improvement in left-ventricular (LV) function in a rat model of myocardial infarction (MI) after intravenous injection of ex vivo expanded human CD34
+ cells was reported
[47][48][49][47,48,49]. Another study examined the effect of catheter-based intramyocardial transplantation in a swine model of MI, providing encouraging outcomes in favoring the application of EPCs as a potential cell therapy in clinical trials
[47][48][49][47,48,49]. Naruse and colleagues conducted a study to assess the therapeutic treatment of diabetic neuropathy using in vivo expanded human EPC and streptozotocin-induced diabetic nude rats
[62]. They observed numerous micro-vessels at the site of EPC injection
[63]. Another group evidenced an improvement in neurological functions in chronic cerebral ischemic rats injected with CD34
+ HSC cells, including EPCs
[63]. The ability of EPCs to expand in culture under in vitro conditions represents another barrier for their therapeutic use. Genetically modified and ex vivo expanded EPCs may become promising agents able to appropriately rescue the impaired neovascularization process under disease conditions. In a rhesus model, ex vivo CD34
+ cell transfection with recombinant non-replicative herpes virus vector and subsequent cell transplantation resulted in the expression of vector genes in angiogenic areas of skin autografts of rhesus macaques. Since CD34
+ cells possess a natural angiogenic tropism to the injured endothelium, they may serve as ideal candidates for the delivery of genes into areas of angiogenesis
[64]. These results encouraged the execution of clinical trials for evaluating the potential of EPCs to enhance endothelial integrity and vascularization at ischemia sites in patients with CVDs. Three different strategies were mainly applied: (a) the
administration of granulocyte-colony stimulating factor (G-CSF) for verifying the recruitment of the patient’s own bone morrow resident progenitors, with two preliminary studies demonstrating increased LV function
[65]; (b)
the intracoronary infusion of BM progenitor cells in patients with MI, which demonstrated positive effects on LV function in three smaller studies
[47][48][49][47,48,49]. Subsequently, two prospective large trials assessed significant LV function after 4–6 months of administration of BM progenitor cells. Ten recent and large trials confirmed the success and safety of this procedure with a follow-up over 1.5 years (widely quoted in
[47][48][49][47,48,49]). In addition, intramyocardial and intracoronary administration has recently been suggested as a suitable strategy for the treatment of patients with refractory angina (widely quoted in
[47][48][49][47,48,49]); (c) a more invasive strategy involving
the direct injection of cells into target tissues [59]. This treatment (specifically,
transepicardial or transendocardial injection of unfractioned BM cells) has been performed in patients with diffuse coronary artery disease and intractable angina with no option of recanalization. Ventricular function and physical capacity were observed to increase, but the small sample size of these studies necessitates confirmation in larger studies (widely quoted in
[47][48][49][47,48,49]).
Studies using
autologous cell therapy are also interesting. Accordingly, Yamamoto’s group executed an intramuscular injection of autologous BM-derived mononuclear cells containing 1% CD34
+ cells in patients with chronic limb ischemia
[66]. They quantitatively assessed the expression of EPCs and endothelial markers (i.e., CD133 and VE-cadherin) before the experiment and after the injection. Before investigation, the transcription of these molecules was undetectable. Autologous injection determined an elevation of EPC marker transcription. Thus, they established that autologous BM cells may be used in the therapy of patients with arterial diseases. A replication of these results was obtained by Lenk and colleagues
[67]. Erbs and colleagues also used this autologous treatment in patients who underwent recanalization of chronic coronary total occlusion
[68]. Autologous treatment with EPCs, expanded for 4 days in endothelium growth medium, improves coronary endothelium function and wall motion abnormalities, and it has a beneficial effect on metabolism in the target area in patients with symptomatic coronary atherosclerosis.
Despite these promising data, the clinical application of EPCs in exogenous or autologous cell therapy remains unclear for different reasons. Accordingly, the validity of their results is influenced by different factors: (a) the insignificant number of patients in these studies; (b) their missing randomization; (c) participation of a limited number of centers; (d) the imprecise phenotypic features of EPC utilized in the treatments, (e) the different methods of administration applied, and (f) the security and viability of the treatments
[47][48][49][47,48,49]. Teratoma formation, immunoreactivity, or arrhythmias constitute the major unfavorable effects which have been detected after such treatments. In addition, other limitations have not been considered, including the limited number of EPCs in circulation, which usually requires their cultured expansion in a sufficient number of subpopulations from peripheral blood. The in vitro quantification of progenitor cells to obtain a quantity sufficient to utilize in therapeutic treatment can be modulated by phenotypic changes during their differentiation in vitro with the risk of obtaining senescent cells, requiring artificial cell pre-activation or stimulation for this approach. In addition to such evidenced limitations, another aspect influencing the results of this study is the lack of standardized criteria and a consensus for defining, characterizing, and identifying EPCs with well-established surface biomarkers, protocols, and methodologies. Consequently, other investigations are imperative
[47][48][49][47,48,49].
Other Candidates for Therapeutic Agents of Endothelium Dysfunction
Promising evidence has recently been reported on the great potential of human umbilical cord-derived MSCs (hucMSCs) in repairing diabetic vascular endothelial damage, by applying assays of resazurin staining, MTT cell viability, wound healing, transwell migration, and Matrigel tube formation on human umbilical vein endothelial cells (HUVECs), and by evaluating how hucMSCs work through the assessment of RNA sequencing (RNA-seq) and molecular experiments
[69]. Specifically, the use of a conditioned medium of hucMSCs (MSC-CM) revealed that hucMSCs improved the cell viability, wound healing, migration, and angiogenesis of HUVECs damaged by high glucose via paracrine signaling, and the altered gene expressions of IL-6, TNF-α, ICAM-1, VCAM-1, BAX, P16, P53, and ET-1 were significantly restored by MSC-CM. RNA-seq incorporated with real-time PCR and Western blot clarified that high glucose activates MAPK/ERK signaling in HUVECs, while MSC-CM reverses the abnormal phosphorylation of ERK and overexpression of MKNK2, ERBB3, MYC, and DUSP5 in the MAPK/ERK signaling pathway
[69]. Certainly, other studies are needed.