
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sumiko Mochida | -- | 3222 | 2022-07-13 01:42:40 | | | |
| 2 | Vivi Li | Meta information modification | 3222 | 2022-07-13 03:57:53 | | |
Video Upload Options
Within 1 millisecond of action potential arrival at presynaptic terminals voltage–gated Ca2+ channels open. The Ca2+ channels are linked to synaptic vesicles which are tethered by active zone proteins. Ca2+ entrance into the active zone triggers: (1) the fusion of the vesicle and exocytosis, (2) the replenishment of the active zone with vesicles for incoming exocytosis, and (3) various types of endocytosis for vesicle reuse, dependent on the pattern of firing. These time-dependent vesicle dynamics are controlled by presynaptic Ca2+ sensor proteins, regulating active zone scaffold proteins, fusion machinery proteins, motor proteins, endocytic proteins, several enzymes, and even Ca2+ channels, following the decay of Ca2+ concentration after the action potential.
1. Introduction
2. Structure and Function of Presynaptic Neurotransmitter Release Sites
2.1. AZ Structure
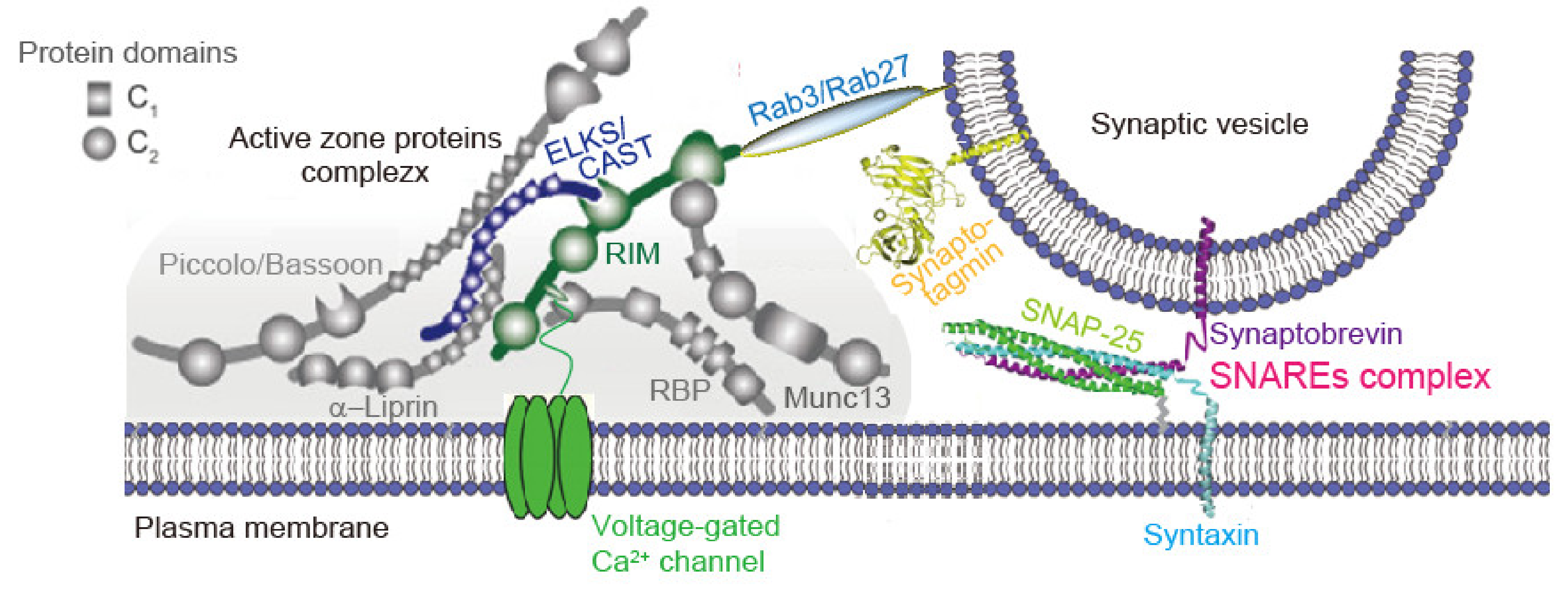
2.2. AZ Proteins Control SV States
2.3. AZ Protein Complex Formation
2.4. A Possible Model for SV Pools Formation
2.5. AZ Assembly Stability
3. Synaptic Vesicle Exocytosis
3.1. SV Fusion Complex—A Model where Ca2+ Releases Inhibition of SV Fusion
3.2. Asynchronous SV Fusion
3.3. Regulation of the Prefusion Complex
3.4. Disassembly of the Postfusion SNAREs
4. Replenishment of Release Site with Synaptic Vesicles
4.1. SV Dynamics after AP
4.2. AZ Proteins
References
- Südhof, T.C. The presynaptic active zone. Neuron 2012, 75, 11–25.
- Biederer, T.; Kaeser, P.S.; Blanpied, T.A. Transcellular Nanoalignment of Synaptic Function. Neuron 2017, 96, 680–696.
- Wang, S.S.; Held, R.G.; Wong, M.Y.; Liu, C.; Karakhanyan, A.; Kaeser, P.S. Fusion Competent Synaptic Vesicles Persist upon Active Zone Disruption and Loss of Vesicle Docking. Neuron 2016, 91, 777–791.
- Mochida, S. Stable and Flexible Synaptic Transmission Controlled by the Active Zone Protein Interactions. Int. J. Mol. Sci. 2021, 22, 1775.
- Chanaday, N.L.; Cousin, M.A.; Milosevic, I.; Watanabe, S.; Morgan, J.R. The Synaptic Vesicle Cycle Revisited: New Insights into the Modes and Mechanisms. J. Neurosci. 2019, 39, 8209–8216.
- Kononenko, N.L.; Puchkov, D.; Classen, G.A.; Walter, A.M.; Pechstein, A.; Sawade, L.; Kaempf, N.; Trimbuch, T.; Lorenz, D.; Rosenmund, C.; et al. Clathrin/AP-2 mediate synaptic vesicle reformation from endosome-like vacuoles but are not essential for membrane retrieval at central synapses. Neuron 2014, 82, 981–988.
- Mochida, S. Neurotransmitter Release Site Replenishment and Presynaptic Plasticity. Int. J. Mol. Sci. 2020, 22, 327.
- Emperador-Melero, J.; Kaeser, P.S. Assembly of the presynaptic active zone. Curr. Opin. Neurobiol. 2020, 63, 95–103.
- Ikeda, S.R. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature 1996, 380, 255–258.
- Wang, C.C.; Weyrer, C.; Fioravante, D.; Kaeser, P.S.; Regehr, W.G. Presynaptic Short-Term Plasticity Persists in the Absence of PKC Phosphorylation of Munc18-1. J. Neurosci. 2021, 41, 7329–7339.
- Brose, N.; Hofmann, K.; Hata, Y.; Südhof, T.C. Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C2-domain proteins. J. Biol. Chem. 1995, 270, 25273–25280.
- Fenster, S.D.; Chung, W.J.; Zhai, R.; Cases-Langhoff, C.; Voss, B.; Garner, A.M.; Kaempf, U.; Kindler, S.; Gundelfinger, E.D.; Garner, C.C. Piccolo, a presynaptic zinc finger protein structurally related to bassoon. Neuron 2000, 25, 203–214.
- Ohtsuka, T.; Takao-Rikitsu, E.; Inoue, E.; Inoue, M.; Takeuchi, M.; Matsubara, K.; Deguchi-Tawarada, M.; Satoh, K.; Morimoto, K.; Nakanishi, H.; et al. Cast: A novel protein of the cytomatrix at the active zone of synapses that forms a ternary complex with RIM1 and munc13-1. J. Cell Biol. 2002, 158, 577–590.
- Takao-Rikitsu, E.; Mochida, S.; Inoue, E.; Deguchi-Tawarada, M.; Inoue, M.; Ohtsuka, T.; Takai, Y. Physical and functional interaction of the active zone proteins, CAST, RIM1, and Bassoon, in neurotransmitter release. J. Cell Biol. 2004, 164, 301–311.
- tom Dieck, S.; Sanmartí-Vila, L.; Langnaese, K.; Richter, K.; Kindler, S.; Soyke, A.; Wex, H.; Smalla, K.H.; Kämpf, U.; Fränzer, J.T.; et al. Bassoon, a novel zinc-finger CAG/glutamine-repeat protein selectively localized at the active zone of presynaptic nerve terminals. J. Cell Biol. 1998, 142, 499–509.
- Wang, Y.; Liu, X.; Biederer, T.; Südhof, T.C. A family of RIM-binding proteins regulated by alternative splicing: Implications for the genesis of synaptic active zones. Proc. Natl. Acad. Sci. USA 2002, 99, 14464–14469.
- Wang, Y.; Okamoto, M.; Schmitz, F.; Hofmann, K.; Südhof, T.C. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature 1997, 388, 593–598.
- Hibino, H.; Pironkova, R.; Onwumere, O.; Vologodskaia, M.; Hudspeth, A.J.; Lesage, F. RIM binding proteins (RBPs) couple Rab3-interacting molecules (RIMs) to voltage-gated Ca2+ channels. Neuron 2002, 34, 411–423.
- Kaeser, P.S.; Deng, L.; Wang, Y.; Dulubova, I.; Liu, X.; Rizo, J.; Südhof, T.C. RIM Proteins Tether Ca2+ Channels to Presynaptic Active Zones via a Direct PDZ-Domain Interaction. Cell 2011, 144, 282–295.
- Butola, T.; Alvanos, T.; Hintze, A.; Koppensteiner, P.; Kleindienst, D.; Shigemoto, R.; Wichmann, C.; Moser, T. RIM-Binding Protein 2 Organizes Ca2+ Channel Topography and Regulates Release Probability and Vesicle Replenishment at a Fast Central Synapse. J. Neurosci. 2021, 41, 7742–7767.
- Kaeser, P.S.; Deng, L.; Chávez, A.E.; Liu, X.; Castillo, P.E.; Südhof, T.C. ELKS2alpha/CAST deletion selectively increases neurotransmitter release at inhibitory synapses. Neuron 2009, 64, 227–239.
- Dong, W.; Radulovic, T.; Goral, R.O.; Thomas, C.; Suarez Montesinos, M.; Guerrero-Given, D.; Hagiwara, A.; Putzke, T.; Hida, Y.; Abe, M.; et al. CAST/ELKS Proteins Control Voltage-Gated Ca2+ Channel Density and Synaptic Release Probability at a Mammalian Central Synapse. Cell Rep. 2018, 24, 284–293.e286.
- tom Dieck, S.; Specht, D.; Strenzke, N.; Hida, Y.; Krishnamoorthy, V.; Schmidt, K.F.; Inoue, E.; Ishizaki, H.; Tanaka-Okamoto, M.; Miyoshi, J.; et al. Deletion of the presynaptic scaffold CAST reduces active zone size in rod photoreceptors and impairs visual processing. J. Neurosci. 2012, 32, 12192–12203.
- Radulovic, T.; Dong, W.; Goral, R.O.; Thomas, C.I.; Veeraraghavan, P.; Montesinos, M.S.; Guerrero-Given, D.; Goff, K.; Lübbert, M.; Kamasawa, N.; et al. Presynaptic development is controlled by the core active zone proteins CAST/ELKS. J. Physiol. 2020, 598, 2431–2452.
- Mochida, S.; Hida, Y.; Tanifuji, S.; Hagiwara, A.; Hamada, S.; Abe, M.; Ma, H.; Yasumura, M.; Kitajima, I.; Sakimura, K.; et al. SAD-B Phosphorylation of CAST Controls Active Zone Vesicle Recycling for Synaptic Depression. Cell Rep. 2016, 16, 2901–2913.
- Emperador-Melero, J.; Wong, M.Y.; Wang, S.S.H.; de Nola, G.; Nyitrai, H.; Kirchhausen, T.; Kaeser, P.S. PKC-phosphorylation of Liprin-α3 triggers phase separation and controls presynaptic active zone structure. Nat. Commun. 2021, 12, 3057.
- Wu, X.; Ganzella, M.; Zhou, J.; Zhu, S.; Jahn, R.; Zhang, M. Vesicle Tethering on the Surface of Phase-Separated Active Zone Condensates. Mol. Cell 2021, 81, 13–24.e17.
- Brunger, A.T.; Leitz, J.; Zhou, Q.; Choi, U.B.; Lai, Y. Ca2+-Triggered Synaptic Vesicle Fusion Initiated by Release of Inhibition. Trends Cell Biol. 2018, 28, 631–645.
- Brunger, A.T.; Choi, U.B.; Lai, Y.; Leitz, J.; White, K.I.; Zhou, Q. The pre-synaptic fusion machinery. Curr. Opin. Struct. Biol. 2019, 54, 179–188.
- Südhof, T.C. Neurotransmitter release: The last millisecond in the life of a synaptic vesicle. Neuron 2013, 80, 675–690.
- Hallermann, S.; Fejtova, A.; Schmidt, H.; Weyhersmüller, A.; Silver, R.A.; Gundelfinger, E.D.; Eilers, J. Bassoon Speeds Vesicle Reloading at a Central Excitatory Synapse. Neuron 2010, 68, 710–723.
- Parthier, D.; Kuner, T.; Körber, C. The presynaptic scaffolding protein Piccolo organizes the readily releasable pool at the calyx of Held. J. Physiol. 2018, 596, 1485–1499.
- Butola, T.; Wichmann, C.; Moser, T. Piccolo Promotes Vesicle Replenishment at a Fast Central Auditory Synapse. Front. Synaptic Neurosci. 2017, 9, 14.
- Wojcik, S.M.; Brose, N. Regulation of membrane fusion in synaptic excitation-secretion coupling: Speed and accuracy matter. Neuron 2007, 55, 11–24.
- Betz, A.; Thakur, P.; Junge, H.J.; Ashery, U.; Rhee, J.S.; Scheuss, V.; Rosenmund, C.; Rettig, J.; Brose, N. Functional interaction of the active zone proteins Munc13-1 and RIM1 in synaptic vesicle priming. Neuron 2001, 30, 183–196.
- Dulubova, I.; Lou, X.; Lu, J.; Huryeva, I.; Alam, A.; Schneggenburger, R.; Südhof, T.C.; Rizo, J. A Munc13/RIM/Rab3 tripartite complex: From priming to plasticity? EMBO J. 2005, 24, 2839–2850.
- Mahoney, T.R.; Liu, Q.; Itoh, T.; Luo, S.; Hadwiger, G.; Vincent, R.; Wang, Z.W.; Fukuda, M.; Nonet, M.L. Regulation of synaptic transmission by RAB-3 and RAB-27 in Caenorhabditis elegans. Mol. Biol. Cell 2006, 17, 2617–2625.
- Han, Y.; Kaeser, P.S.; Südhof, T.C.; Schneggenburger, R. RIM determines Ca²+ channel density and vesicle docking at the presynaptic active zone. Neuron 2011, 69, 304–316.
- Brockmann, M.M.; Zarebidaki, F.; Camacho, M.; Grauel, M.K.; Trimbuch, T.; Südhof, T.C.; Rosenmund, C. A Trio of Active Zone Proteins Comprised of RIM-BPs, RIMs, and Munc13s Governs Neurotransmitter Release. Cell Rep. 2020, 32, 107960.
- Deng, L.; Kaeser, P.S.; Xu, W.; Südhof, T.C. RIM Proteins Activate Vesicle Priming by Reversing Autoinhibitory Homodimerization of Munc13. Neuron 2011, 69, 317–331.
- Lai, Y.; Choi, U.B.; Leitz, J.; Rhee, H.J.; Lee, C.; Altas, B.; Zhao, M.; Pfuetzner, R.A.; Wang, A.L.; Brose, N.; et al. Molecular Mechanisms of Synaptic Vesicle Priming by Munc13 and Munc18. Neuron 2017, 95, 591–607.e510.
- Held, R.G.; Liu, C.; Kaeser, P.S. ELKS controls the pool of readily releasable vesicles at excitatory synapses through its N-terminal coiled-coil domains. eLife 2016, 5, e14862.
- Pofantis, E.; Neher, E.; Dresbach, T. Regulation of a subset of release-ready vesicles by the presynaptic protein Mover. Proc. Natl. Acad. Sci. USA 2021, 118, e2022551118.
- Augustin, I.; Rosenmund, C.; Südhof, T.C.; Brose, N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature 1999, 400, 457–461.
- Yang, X.; Wang, S.; Sheng, Y.; Zhang, M.; Zou, W.; Wu, L.; Kang, L.; Rizo, J.; Zhang, R.; Xu, T.; et al. Syntaxin opening by the MUN domain underlies the function of Munc13 in synaptic-vesicle priming. Nat. Struct. Mol. Biol. 2015, 22, 547–554.
- Wang, S.; Li, Y.; Gong, J.; Ye, S.; Yang, X.; Zhang, R.; Ma, C. Munc18 and Munc13 serve as a functional template to orchestrate neuronal SNARE complex assembly. Nat. Commun. 2019, 10, 69.
- Shu, T.; Jin, H.; Rothman, J.E.; Zhang, Y. Munc13-1 MUN domain and Munc18-1 cooperatively chaperone SNARE assembly through a tetrameric complex. Proc. Natl. Acad. Sci. USA 2020, 117, 1036–1041.
- Shen, J.; Tareste, D.C.; Paumet, F.; Rothman, J.E.; Melia, T.J. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell 2007, 128, 183–195.
- Liu, K.S.; Siebert, M.; Mertel, S.; Knoche, E.; Wegener, S.; Wichmann, C.; Matkovic, T.; Muhammad, K.; Depner, H.; Mettke, C.; et al. RIM-binding protein, a central part of the active zone, is essential for neurotransmitter release. Science 2011, 334, 1565–1569.
- Wu, X.; Cai, Q.; Shen, Z.; Chen, X.; Zeng, M.; Du, S.; Zhang, M. RIM and RIM-BP Form Presynaptic Active-Zone-like Condensates via Phase Separation. Mol. Cell 2019, 73, 971–984.e975.
- Milovanovic, D.; Wu, Y.; Bian, X.; De Camilli, P. A liquid phase of synapsin and lipid vesicles. Science 2018, 361, 604–607.
- Park, D.; Wu, Y.; Lee, S.E.; Kim, G.; Jeong, S.; Milovanovic, D.; De Camilli, P.; Chang, S. Cooperative function of synaptophysin and synapsin in the generation of synaptic vesicle-like clusters in non-neuronal cells. Nat. Commun. 2021, 12, 263.
- Zeng, M.; Shang, Y.; Araki, Y.; Guo, T.; Huganir, R.L.; Zhang, M. Phase Transition in Postsynaptic Densities Underlies Formation of Synaptic Complexes and Synaptic Plasticity. Cell 2016, 166, 1163–1175.e1112.
- Denker, A.; Rizzoli, S.O. Synaptic vesicle pools: An update. Front. Synaptic Neurosci. 2010, 2, 135.
- Waites, C.L.; Leal-Ortiz, S.A.; Okerlund, N.; Dalke, H.; Fejtova, A.; Altrock, W.D.; Gundelfinger, E.D.; Garner, C.C. Bassoon and Piccolo maintain synapse integrity by regulating protein ubiquitination and degradation. EMBO J. 2013, 32, 954–969.
- Zhou, Q.; Zhou, P.; Wang, A.L.; Wu, D.; Zhao, M.; Südhof, T.C.; Brunger, A.T. The primed SNARE-complexin-synaptotagmin complex for neuronal exocytosis. Nature 2017, 548, 420–425.
- Mohrmann, R.; Dhara, M.; Bruns, D. Complexins: Small but capable. Cell Mol. Life Sci. 2015, 72, 4221–4235.
- Trimbuch, T.; Rosenmund, C. Should I stop or should I go? The role of complexin in neurotransmitter release. Nat. Rev. Neurosci. 2016, 17, 118–125.
- Perin, M.S.; Fried, V.A.; Mignery, G.A.; Jahn, R.; Südhof, T.C. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature 1990, 345, 260–263.
- Perin, M.S.; Johnston, P.A.; Ozcelik, T.; Jahn, R.; Francke, U.; Südhof, T.C. Structural and functional conservation of synaptotagmin (p65) in Drosophila and humans. J. Biol. Chem. 1991, 266, 615–622.
- Zhou, Q.; Lai, Y.; Bacaj, T.; Zhao, M.; Lyubimov, A.Y.; Uervirojnangkoorn, M.; Zeldin, O.B.; Brewster, A.S.; Sauter, N.K.; Cohen, A.E.; et al. Architecture of the synaptotagmin-SNARE machinery for neuronal exocytosis. Nature 2015, 525, 62–67.
- Pang, Z.P.; Sun, J.; Rizo, J.; Maximov, A.; Südhof, T.C. Genetic analysis of synaptotagmin 2 in spontaneous and Ca2+-triggered neurotransmitter release. EMBO J. 2006, 25, 2039–2050.
- Chen, C.; Arai, I.; Satterfield, R.; Young, S.M., Jr.; Jonas, P. Synaptotagmin 2 Is the Fast Ca2+ Sensor at a Central Inhibitory Synapse. Cell Rep. 2017, 18, 723–736.
- Bacaj, T.; Wu, D.; Yang, X.; Morishita, W.; Zhou, P.; Xu, W.; Malenka, R.C.; Südhof, T.C. Synaptotagmin-1 and synaptotagmin-7 trigger synchronous and asynchronous phases of neurotransmitter release. Neuron 2013, 80, 947–959.
- Bouazza-Arostegui, B.; Camacho, M.; Brockmann, M.M.; Zobel, S.; Rosenmund, C. Deconstructing Synaptotagmin-1’s Distinct Roles in Synaptic Vesicle Priming and Neurotransmitter Release. J. Neurosci. 2022, 42, 2856–2871.
- Sabatini, B.L.; Regehr, W.G. Timing of synaptic transmission. Annu. Rev. Physiol. 1999, 61, 521–542.
- Goda, Y.; Stevens, C.F. Two components of transmitter release at a central synapse. Proc. Natl. Acad. Sci. USA 1994, 91, 12942–12946.
- Kaeser, P.S.; Regehr, W.G. Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu. Rev. Physiol. 2014, 76, 333–363.
- Otsu, Y.; Shahrezaei, V.; Li, B.; Raymond, L.A.; Delaney, K.R.; Murphy, T.H. Competition between phasic and asynchronous release for recovered synaptic vesicles at developing hippocampal autaptic synapses. J. Neurosci. 2004, 24, 420–433.
- Iremonger, K.J.; Bains, J.S. Asynchronous presynaptic glutamate release enhances neuronal excitability during the post-spike refractory period. J. Physiol. 2016, 594, 1005–1015.
- Luo, F.; Südhof, T.C. Synaptotagmin-7-Mediated Asynchronous Release Boosts High-Fidelity Synchronous Transmission at a Central Synapse. Neuron 2017, 94, 826–839.e823.
- Yao, J.; Gaffaney, J.D.; Kwon, S.E.; Chapman, E.R. Doc2 is a Ca2+ sensor required for asynchronous neurotransmitter release. Cell 2011, 147, 666–677.
- Groffen, A.J.; Martens, S.; Díez Arazola, R.; Cornelisse, L.N.; Lozovaya, N.; de Jong, A.P.; Goriounova, N.A.; Habets, R.L.; Takai, Y.; Borst, J.G.; et al. Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science 2010, 327, 1614–1618.
- Hui, E.; Bai, J.; Wang, P.; Sugimori, M.; Llinas, R.R.; Chapman, E.R. Three distinct kinetic groupings of the synaptotagmin family: Candidate sensors for rapid and delayed exocytosis. Proc. Natl. Acad. Sci. USA 2005, 102, 5210–5214.
- Xue, R.; Gaffaney, J.D.; Chapman, E.R. Structural elements that underlie Doc2β function during asynchronous synaptic transmission. Proc. Natl. Acad. Sci. USA 2015, 112, E4316–E4325.
- Chen, C.; Satterfield, R.; Young, S.M., Jr.; Jonas, P. Triple Function of Synaptotagmin 7 Ensures Efficiency of High-Frequency Transmission at Central GABAergic Synapses. Cell Rep. 2017, 21, 2082–2089.
- Turecek, J.; Regehr, W.G. Synaptotagmin 7 Mediates Both Facilitation and Asynchronous Release at Granule Cell Synapses. J. Neurosci. 2018, 38, 3240–3251.
- Deng, S.; Li, J.; He, Q.; Zhang, X.; Zhu, J.; Li, L.; Mi, Z.; Yang, X.; Jiang, M.; Dong, Q.; et al. Regulation of Recurrent Inhibition by Asynchronous Glutamate Release in Neocortex. Neuron 2020, 105, 522–533.e524.
- Li, L.; Liu, H.; Krout, M.; Richmond, J.E.; Wang, Y.; Bai, J.; Weeratunga, S.; Collins, B.M.; Ventimiglia, D.; Yu, Y.; et al. A novel dual Ca2+ sensor system regulates Ca2+-dependent neurotransmitter release. J. Cell Biol. 2021, 220, e202008121.
- Hikima, T.; Witkovsky, P.; Khatri, L.; Chao, M.V.; Rice, M.E. Synaptotagmins 1 and 7 Play Complementary Roles in Somatodendritic Dopamine Release. J. Neurosci. 2022, 42, 3919–3930.
- Gaffaney, J.D.; Xue, R.; Chapman, E.R. Mutations that disrupt Ca2+-binding activity endow Doc2β with novel functional properties during synaptic transmission. Mol. Biol. Cell 2014, 25, 481–494.
- Xue, R.; Ruhl, D.A.; Briguglio, J.S.; Figueroa, A.G.; Pearce, R.A.; Chapman, E.R. Doc2-mediated superpriming supports synaptic augmentation. Proc. Natl. Acad. Sci. USA 2018, 115, E5605–E5613.
- Khan, M.M.; Regehr, W.G. Loss of Doc2b does not influence transmission at Purkinje cell to deep nuclei synapses under physiological conditions. eLife 2020, 9, e55165.
- Wu, Z.; Kusick, G.F.; Raychaudhuri, S.; Itoh, K.; Chapman, E.R.; Watanabe, S. Synaptotagmin 7 docks synaptic vesicles for Doc2α-triggered asynchronous neurotransmitter release. bioRxiv 2022, 489101.
- Misura, K.M.; Scheller, R.H.; Weis, W.I. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature 2000, 404, 355–362.
- Burkhardt, P.; Hattendorf, D.A.; Weis, W.I.; Fasshauer, D. Munc18a controls SNARE assembly through its interaction with the syntaxin N-peptide. EMBO J. 2008, 27, 923–933.
- Basu, J.; Shen, N.; Dulubova, I.; Lu, J.; Guan, R.; Guryev, O.; Grishin, N.V.; Rosenmund, C.; Rizo, J. A minimal domain responsible for Munc13 activity. Nat. Struct. Mol. Biol. 2005, 12, 1017–1018.
- Ma, C.; Su, L.; Seven, A.B.; Xu, Y.; Rizo, J. Reconstitution of the vital functions of Munc18 and Munc13 in neurotransmitter release. Science 2013, 339, 421–425.
- Ma, C.; Li, W.; Xu, Y.; Rizo, J. Munc13 mediates the transition from the closed syntaxin-Munc18 complex to the SNARE complex. Nat. Struct. Mol. Biol. 2011, 18, 542–549.
- Sitarska, E.; Xu, J.; Park, S.; Liu, X.; Quade, B.; Stepien, K.; Sugita, K.; Brautigam, C.A.; Sugita, S.; Rizo, J. Autoinhibition of Munc18-1 modulates synaptobrevin binding and helps to enable Munc13-dependent regulation of membrane fusion. eLife 2017, 6, e24278.
- Söllner, T.; Bennett, M.K.; Whiteheart, S.W.; Scheller, R.H.; Rothman, J.E. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 1993, 75, 409–418.
- Mayer, A.; Wickner, W.; Haas, A. Sec18p (NSF)-driven release of Sec17p (alpha-SNAP) can precede docking and fusion of yeast vacuoles. Cell 1996, 85, 83–94.
- Hanson, P.I.; Roth, R.; Morisaki, H.; Jahn, R.; Heuser, J.E. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell 1997, 90, 523–535.
- White, K.I.; Zhao, M.; Choi, U.B.; Pfuetzner, R.A.; Brunger, A.T. Structural principles of SNARE complex recognition by the AAA+ protein NSF. eLife 2018, 7, e38888.
- Kusick, G.F.; Chin, M.; Raychaudhuri, S.; Lippmann, K.; Adula, K.P.; Hujber, E.J.; Vu, T.; Davis, M.W.; Jorgensen, E.M.; Watanabe, S. Synaptic vesicles transiently dock to refill release sites. Nat. Neurosci. 2020, 23, 1329–1338.
- Lipstein, N.; Sakaba, T.; Cooper, B.H.; Lin, K.H.; Strenzke, N.; Ashery, U.; Rhee, J.S.; Taschenberger, H.; Neher, E.; Brose, N. Dynamic control of synaptic vesicle replenishment and short-term plasticity by Ca2+-calmodulin-Munc13-1 signaling. Neuron 2013, 79, 82–96.
- Müller, M.; Genç, Ö.; Davis, G.W. RIM-binding protein links synaptic homeostasis to the stabilization and replenishment of high release probability vesicles. Neuron 2015, 85, 1056–1069.
- Davydova, D.; Marini, C.; King, C.; Klueva, J.; Bischof, F.; Romorini, S.; Montenegro-Venegas, C.; Heine, M.; Schneider, R.; Schröder, M.S.; et al. Bassoon specifically controls presynaptic P/Q-type Ca2+ channels via RIM-binding protein. Neuron 2014, 82, 181–194.
- Zarebidaki, F.; Camacho, M.; Brockmann, M.M.; Trimbuch, T.; Herman, M.A.; Rosenmund, C. Disentangling the Roles of RIM and Munc13 in Synaptic Vesicle Localization and Neurotransmission. J. Neurosci. 2020, 40, 9372–9385.
- Mendoza Schulz, A.; Jing, Z.; Sánchez Caro, J.M.; Wetzel, F.; Dresbach, T.; Strenzke, N.; Wichmann, C.; Moser, T. Bassoon-disruption slows vesicle replenishment and induces homeostatic plasticity at a CNS synapse. EMBO J. 2014, 33, 512–527.




