Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chen, Z.; Lu, Q.; Zong, W.; Zhang, M.; Yang, Z. Regulationary Factors of the Peroxisomal β-Oxidation. Encyclopedia. Available online: https://encyclopedia.pub/entry/24897 (accessed on 07 February 2026).
Chen Z, Lu Q, Zong W, Zhang M, Yang Z. Regulationary Factors of the Peroxisomal β-Oxidation. Encyclopedia. Available at: https://encyclopedia.pub/entry/24897. Accessed February 07, 2026.
Chen, Zhi, Qinyue Lu, Weicheng Zong, Mingyixing Zhang, Zhangping Yang. "Regulationary Factors of the Peroxisomal β-Oxidation" Encyclopedia, https://encyclopedia.pub/entry/24897 (accessed February 07, 2026).
Chen, Z., Lu, Q., Zong, W., Zhang, M., & Yang, Z. (2022, July 07). Regulationary Factors of the Peroxisomal β-Oxidation. In Encyclopedia. https://encyclopedia.pub/entry/24897
Chen, Zhi, et al. "Regulationary Factors of the Peroxisomal β-Oxidation." Encyclopedia. Web. 07 July, 2022.
Copy Citation
Beta-oxidation(β-oxidation) is an important metabolic process involving multiple steps by which fatty acid molecules are broken down to produce energy. The very long-chain fatty acids (VLCFAs), a type of fatty acid (FA), are usually highly toxic when free in vivo, and their oxidative metabolism depends on the peroxisomal β-oxidation. Although peroxisomal β-Oxidation attracts less research than mitochondria, the importance of the peroxisomal β-oxidation molecular mechanism can still be spotted from some mechanisms involved in upstream regulation.
peroxisome
very long-chain fatty acids
beta-oxidation
1. PPAR
Peroxisome proliferation activating nuclear receptors (PPARs), members of the steroid hormone nuclear receptor ligand-dependent transcription factor superfamily, can regulate peroxisome proliferation by regulating the peroxisome proliferation β-oxidation process [1]. PPARs have three subunits, namely PPARα, PPARβ/δ, and PPARγ (Figure 1), which differ in tissue distribution, ligand affinity, and target genes. Surprisingly, they are all related to peroxisomal β-oxidative metabolism [2]. Activation of PPARα promotes fatty acid entry into peroxisomes, and PPARα participates in the regulation of energy homeostasis. By contrast, activation of PPARγ causes insulin sensitization and enhances glucose metabolism, whereas activation of PPARβ/δ enhances fatty acid oxidation (FAO).
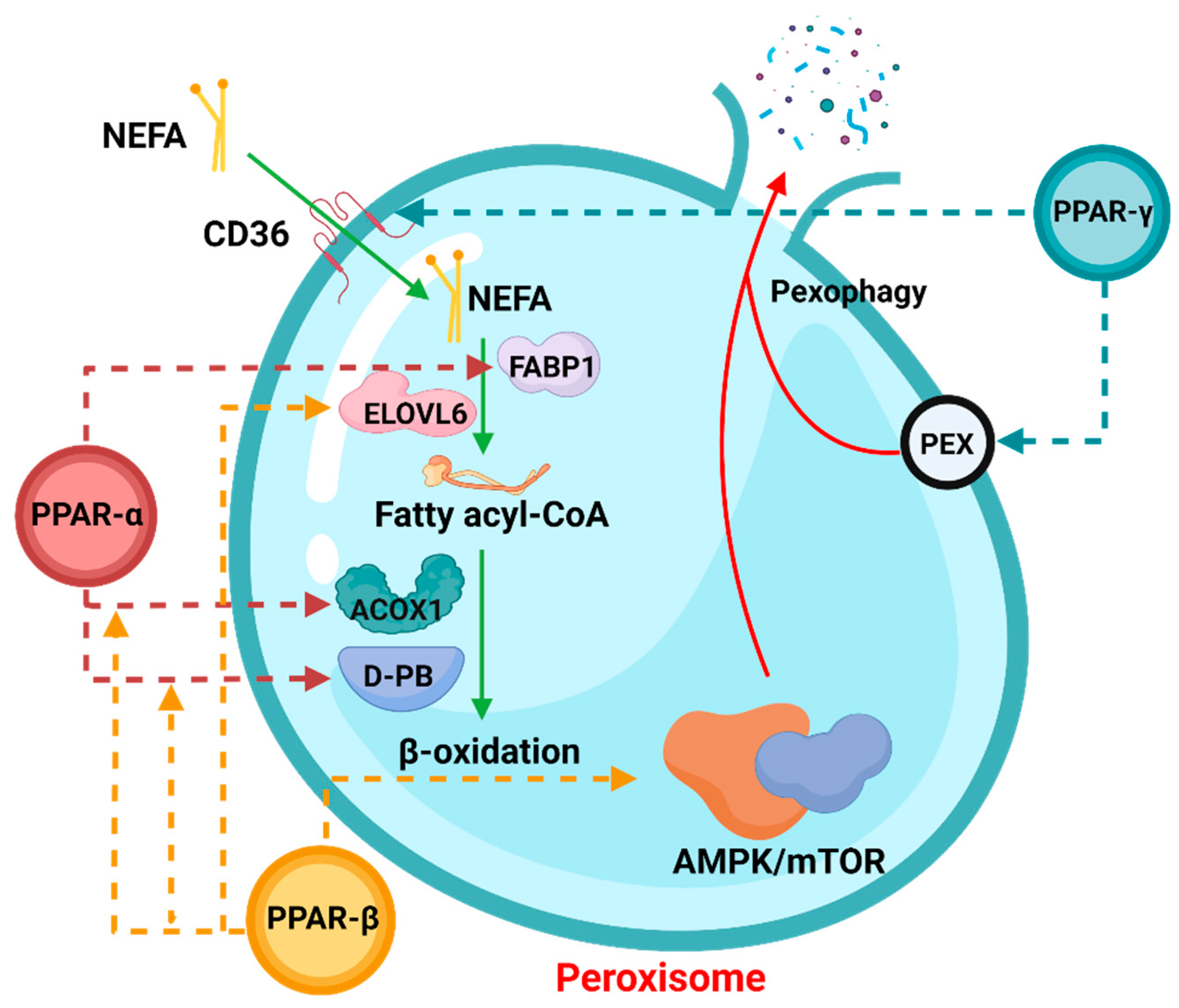
Figure 1. The role of PPARs in peroxisomal β-oxidation.
In the process of peroxisomal β-oxidation, PPARs are actively involved in various processes. The dotted arrows in the figure indicate that the corresponding PPAR members participate in the regulation of the process. Among them, NEFA: non-esterified fatty acid, FABP1: fatty acid binding protein 1, ELOVL6: ELOVL Fatty Acid Elongase 6, ACOX1: Acyl-CoA Oxidase 1. The process involving pexophagy was complicated, and “PEX” was only a general reference.
1.1. PPARα
The effect of peroxisome proliferators on the synergistic induction of the peroxisomal β-oxidation system enzymes has been well established, which is regulated by PPARα. As a lipid sensor, PPARα can coordinate and promote the expression of a large number of target genes in the process of FAO, especially in starvation or high-fat diet conditions [3]. Studies have shown that PPARα induces the translation of downstream genes under the stimulation of long-chain fatty acids (VLCFAs): it promotes the proliferation of peroxisomes, and it specifically upregulates the expression of peroxisomal β-oxidative proteins and coenzymes [4]. At present, research on PPARα mainly focuses on its targeted ligands. For example, synthetic lipid-lowering drugs have been shown to activate PPARα and then regulate the peroxisomal β-oxidation [5]. And in the ruminants, some studies have found that PPARα has different expression levels in the mammary between the dry and lactation period [6][7]. Further, the study also showed that PPARα plays an important role in regulating milk fat synthesis in ruminants [8]. In addition to this, PPARα is also involved in the regulation of fat in the liver [9][10]. Interestingly, PPARα also plays an important role in ruminant reproduction [11][12], but it is not known whether this role is related to peroxisomal β-oxidation, which is a direction worth exploring. Simply put, regardless of whether the drugs are endogenous or exogenous ligands, their activation mechanism to bind the PPARα further increases transcriptional activity.
1.2. PPARγ
As one of the key transcriptional regulators of adipocyte differentiation, PPARγ also plays an important role in mediating peroxisomal β-oxidation and lipid metabolisms [13]. Studies have shown that a variety of genes involved in fatty acid (FA) transport and metabolism are regulated by PPARγ at the transcriptional level, such as FA translocases, implying that PPARγ can stimulate peroxidase by increasing the expression of FA transporters and FA transportases [14][15]. The initiation of β-oxidation in vivo has also been proven. By knocking out PPARγ in mice, it was found that they had obvious lipid metabolism disorders [16].
In dairy cows, PPARγ plays an important role which is the critical mediator of lipogenesis [17]. One of the significant roles it plays in dairy cows from the point of view of economic interest is controlling the synthesis of milk fat in dairy cows, not only in mitochondria but also with ELVOL participating in the regulation of peroxisomal β-oxidation. PPARγ expression increases during changes in the lactation period [18][19], thereby regulating peroxisomal β-oxidation to prevent metabolic stress. Research further confirmed the importance of PPARγ in regulating milk fat production [20][21]. Similarly, as milk-producing ruminants, goats and sheep are also regulated by PPARγ. Many studies indicate that PPARγ is involved in adipocyte differentiation and adipogenesis in sheep/goats [22][23][24][25][26], though, in addition to fat regulation, PPARγ was previously thought to be involved in the regulation of hormones in goats and sheep [27][28]. However, there has been some relevant research in recent years. Some studies have pointed out that the role of PPARγ and hormones is only a servo-assist mechanism [29]. Up to now, there is no obvious evidence to prove either statement; thus, this is also a worthy question.
1.3. PPARβ/δ
Compared with PPARα and PPARγ, PPARβ/δ is more widely distributed in various tissues in vivo. Aline et al. showed that PPARβ/δ could participate in the activation of FAO in BATs, but genes involved in processes such as lipogenesis were not significantly correlated [30]. This demonstrates that PPARβ/δ is a thermogenic transcription factor in vivo, which bears great resemblance to the purpose of peroxisomal β-oxidation. Furthermore, Tong et al. studied PPARβ/δ-induced autophagy, although the study assessed expression changes in mitochondria and revealed the PPARβ/δ-AMPK/mTOR pathway by searching for a signaling pathway (mTOR, one of the important factors of peroxisomal β-oxidation), which further demonstrated that PPARβ/δ has a regulatory effect on peroxisomal β-oxidation [31]. Recent studies have shown that PPARβ/δ is indispensable for the upregulation of autophagic behavior [5][32][33][34][35], making the relationship between PPARβ/δ and peroxisomal β-oxidation more explicit. When the body is under certain conditions, PPARβ/δ frees more VLCFAs by upregulating cellular autophagy, which in turn regulates the initiation and enhancement of peroxisomal β-oxidation. However, a lot of research is still needed to confirm what certain conditions are and whether this speculation is correct.
Regardless of which subunit of PPARs is involved in the regulation of peroxisomal β-oxidation, as PPAR is often reported to be associated with the occurrence of diseases like type 2 diabetes, PPAR-associated peroxisomal β-oxidation changes may also be involved. A study pointed out that inhibition of peroxisome biosynthesis can interrupt β-oxidation through the action of PPAR, thereby effectively preventing the occurrence of type 2 diabetes [36]. Nevertheless, the molecular mechanism underlying this phenomenon has not been elucidated. In addition, the prevalence of some diseases is different by gender [37], and many experiments also show that PPARs are different in function by gender. PPARα expression is more abundant in native and activated male T cells than in female cells [38][39], suggesting that PPARα has a more substantial role in male T cells than in female T cells. Likewise, multiple studies have shown that the male hormone androgen has been suggested to influence the expression of PPARα in male T cells [40][41][42]. A study of sex-specific differences in the role of PPARγ in T cell survival has shown that male PPARγ-deficient T cells have increased apoptosis and contain a greater proportion of apoptotic cells than female PPARγ-deficient T cells [43]. Some studies have also validated similar conclusions, suggesting that PPARγ plays an important role in T cell survival [44][45][46][47]. Although more convincing data are needed to resolve this discrepancy, PPARγ may act as a survival factor in female T cells. Unlike others, the regulatory role of PPARβ/δ in T cells has not been well studied, but sex-specific differences in PPARβ/δ regulation should be considered in future studies.
2. PGC-1α
Peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α) is a powerful transcriptional coactivator that regulates a broad range of physiological and energy homeostasis responses at the transcriptional level in diverse mammalian tissues. In the past, scholars focused more on the regulatory mechanism of PGC-1α in controlling mitochondrial function [48][49]. In 2010, Bagattin et al. found that PGC-1α coordinates not only mitochondrial remodeling but also peroxisome specialization and biogenesis [50], which stimulated an upsurge in the function study of peroxisome PGC-1α. Moreover, Huang et al. studied peroxisomes in human skeletal muscle cells. They found that overexpression of PGC-1α induced the expression of AOCX1, and that the levels of some proteins associated with peroxisome activity also substantially increased [51]. In addition, some studies have also demonstrated the importance of PGC-1α in peroxisomes [52][53].
In conclusion, the regulatory relationship between PGC-1α and peroxisomal β-oxidation deserves further research because of the critical functionality of PGC-1α, and it is wondered whether PGC-1α could be used as a novel chemical modulator for the treatment of Zellweger syndrome symptoms and other diseases. PGC-1α has not been studied much in ruminants and has mostly focused on regulating fatty acids [54][55]. But there is one direction worth mentioning. Zhou et al. found that PGC-1α seems to play a role in the skeletal muscles of goats, and it can also maintain metabolic rhythm through the phosphorylation of upstream regulators [56]. It was speculated that this is related to peroxisomal β-oxidation.
3. PEX
In 1996, the term peroxin was coined for proteins in peroxisome biogenesis, including peroxisomal matrix protein import, membrane biogenesis, peroxisome proliferation, and peroxisome inheritance [57]. Peroxins are encoded by PEX genes, also known as PEX proteins. To date, 37 PEX proteins have been discovered and studied. Some are highly conserved, while others only occur in a limited number of species, such as PEX17, which only distributes in Fungi, and PEX35, which only distributes in Saccharomycetaceae [58]. Most PEX proteins have been shown to play a significant role in peroxisomal β-oxidation (Figure 2).
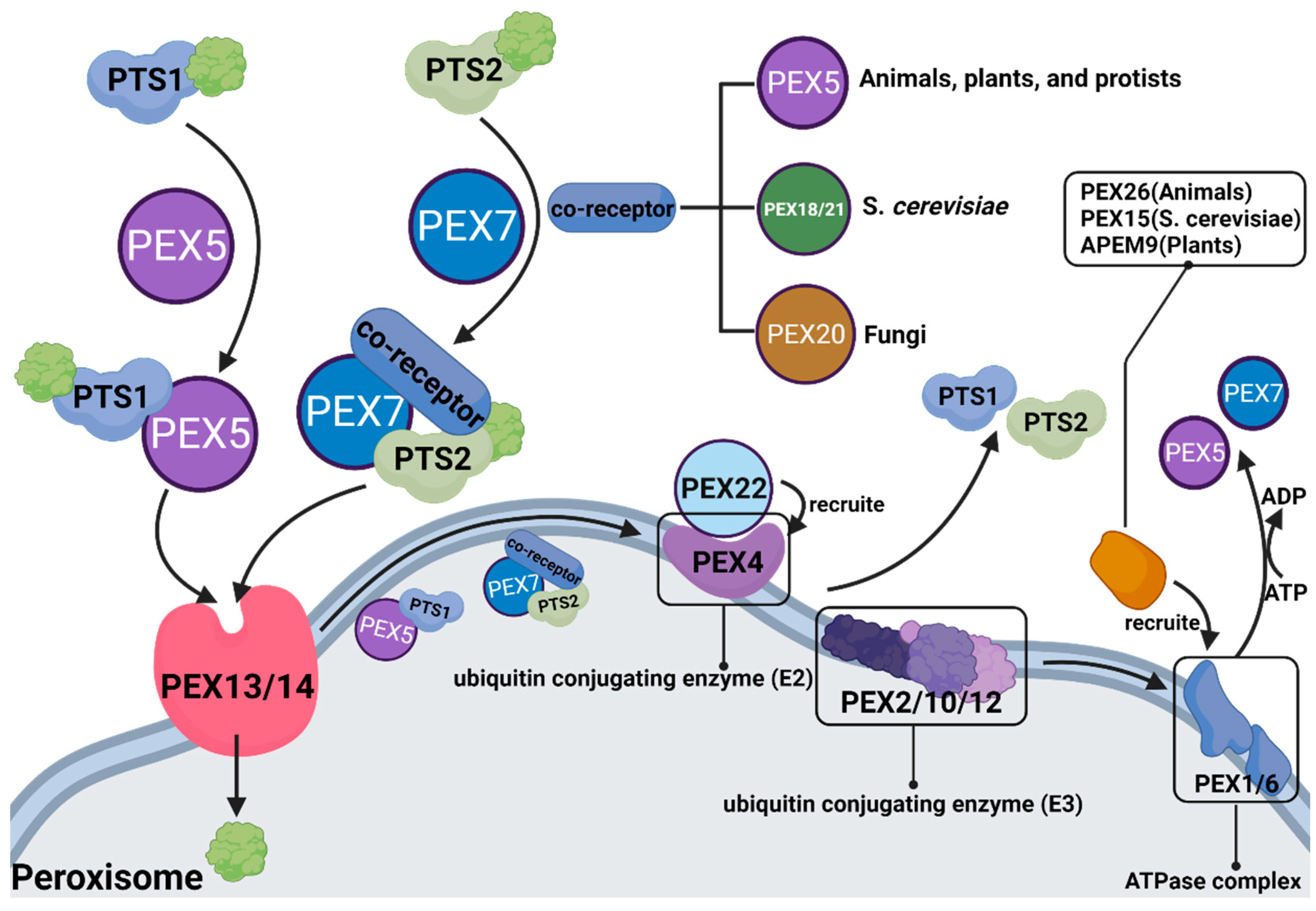
Figure 2. The role of different PEX proteins in peroxisomal β-oxidation.
PEX5 is the most commonly studied object in eukaryotes. As mentioned above, peroxisomes are very sensitive to reactive oxygen species (ROS), whereas multiple organelles in the internal environment can produce ROS. Consequently, peroxisomes themselves are susceptible to the influences of other metabolic processes and become dysfunctional. A study found that PEX5 can respond to the expression of ROS and induce cell autophagy after ubiquitination to prevent damage from excessive or defective peroxisomes from cellular β-oxidation [59].
More research on PEX has focused on diseases caused by mutation or dysfunction of the PEX gene, including peroxisome biogenesis disorders in the Zellweger spectrum (PBD-ZSD) and rhizomelic chondrodysplasia punctata (RCDP). Studies have pointed out that PBD-ZSD is caused by PEX gene mutation that results in insufficient peroxisomal β-oxidation, which increases levels of VLCFAs in plasma and cells. However, so far, there is no treatment for PBD-ZSD, and researchers have tried to treat it from the perspective of autophagy, but the effect is not ideal [60]. Does this imply that there are unknown mechanisms other than autophagy regulating the relationship between PEX and β-oxidation? It reminds that long-term neglect makes it impossible to have precise treatment for diseases caused by peroxisomal β-oxidation disorders, that it can’t be found that the therapeutic target, and thus further exploration is urgently needed.
Substances entering peroxisomes for β-oxidation need to form receptor cargo complexes with PTS (PTS: peroxisome targeting sequence, whose role is to locate peroxisomes to ensure accurate transport of carried substances) and PEX, in which PTS1 is transported by PEX5, and PTS2 is transported by PEX7 and coreceptors (coreceptors are PEX5, PEX18/21 and PEX20, depending on the species). After the substance enters the peroxisome, PTS and PEX must be ubiquitinated and recovered.
4. ATP-Binding Cassette (ABC)
In peroxisomes, VLCFAs are mainly introduced as CoA through ABCD1–3 (Figure 3). Studies have shown that peroxisomal β-oxidation is dysfunctional after the deletion of ABCD1/ALDP in vivo, resulting in the expression levels of VLCFAs in both plasma and tissues being increased [61][62]. ABCD1 and ABCD2 share a high degree of sequence homology, except that ABCD2 plays a central role in the metabolism of monounsaturated and polyunsaturated VLCFAs, rather than saturated VLCFAs, and may be involved in the regulation of oxidative stress and DHA synthesis [63]. ABCD3/PMP70 plays a major role in transporting 2-methylacyl-CoA esters [64]. In addition, despite the distinct functions of peroxisomal ABC transporters, in vitro and in vivo studies have clearly identified that there is at least partial functional redundancy between these transporters [65][66]. In ruminants, ABC is equally important. Ahmad et al. found that the expression of ABC varies significantly among different milk yields by RNA-seq [67]. Since the expression of ABC is also different between different breeds of cattle, the difference in immunity may also be related to ABC. And Lopez et al. further verified this conclusion, ABC is indeed related to the immune system of cattle [68]. Although this conclusion was obtained by RNA-seq, it was speculated that this is caused by the different content of VLCFAs in vivo and the autophagy function of peroxisome; the latest research also suggests that ABC is involved in intracellular cholesterol-mediated autophagy [69]. In addition, the role of ABC was also found in the reproductive system of sheep [70], revealing that ABC may be related to the degeneration of germ cells. Although researchers have paid more attention to the transport function of ABCD, it would like to be emphasized that the X-linked adrenoleukodystrophy (X-ALD) remains a matter of concern because it lacks peroxisomal β-oxidation caused by ABCD deletion. At present, the only effective treatment is hematopoietic stem cell transplantation (HSCT), but the risk of death remains high. Some studies have pointed out that the β-oxidation defects can be restored by overexpressing ABCD1/2 in cells, whereas the understanding of its mechanism is still incomplete [71][72][73]. As mentioned above, a comprehensive understanding of peroxisomal β-oxidation is urgently needed.
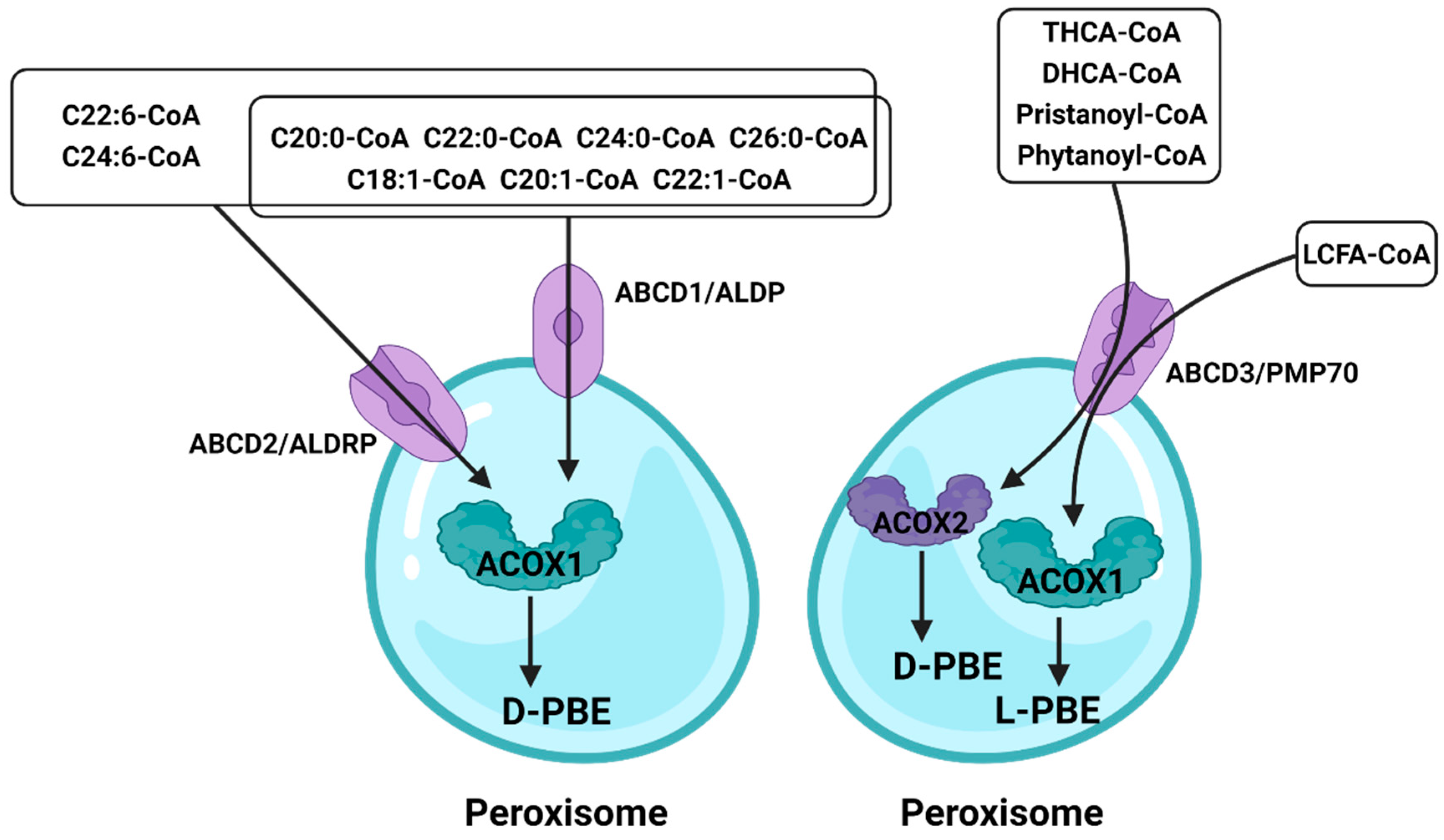
Figure 3. The role of the ATP-binding cassette in peroxisomal β-oxidation.
Different fatty acids enter peroxisome through different ATP-binding cassettes, and ABCD2 has a broader role. Fatty acids that enter peroxisomes through ABCD3 are processed by different enzymes to achieve oxidation results.
5. Others
New regulators involved in the regulation of peroxisomal β-oxidation have constantly been identified. AMPK, for example, promotes FAO by activating the expression of PPARα and ACOX1 [74][75], whereas mTOR promotes the accumulation of FAs by shutting down β-oxidation [76][77]. Hence, these are considered the AMPK-mTOR pathway for regulating β-oxidation [78]. The NAD-dependent protein lysine deacylases of the sirtuin family regulate various physiological functions, from energy metabolism to stress responses [79]. Numerous studies have found that Sirtuin has a variety of catalytic activities [80]. These pleiotropic enzymatic activities give sirtuins their far-reaching functions in maintaining genome integrity, regulating metabolism homeostasis, and promoting organismal longevity. And a recent study found that sirtuin 5 (SIRT5) functions similarly to mTOR, which shuts down peroxisomal β-oxidation by inhibiting the activity of ACOX1 [81]. Other reports have studied CoA in the process of β-oxidation and found that Nudt7 and Nudt19 in the Nudt superfamily can exert positive effects on CoA substrates in certain metabolic processes to promote the metabolic process [82][83]. With the upsurge of research on noncoding RNAs (ncRNAs), some play positive or inhibitory roles in peroxisomal β-oxidation, such as miR-222, miR-25-3p, circ_0005379, and others [84][85][86][87][88][89], which mainly target the rate-limiting enzyme ACOX1. Recently, Li et al. analyzed the ACOX1 transcript and found that miR-532-3P could regulate the expression of ACOX1 by targeting the complementary sequence in the 3’-UTR, thereby participating in lipid metabolism [84].
Moreover, other enzymes involved in peroxisomal β-oxidation were also regulated. Several studies have demonstrated that phosphatidylserine (PS) can bind to D-bifunctional protein (D-BP) and localize to peroxisomes, implying that PS can also affect the β-oxidation process [90]. It is worth noting that some studies have pointed out that acetylation is important for the normal functioning of D-BP [91][92], but related studies are not common; thus, it is unknown which factors affect its acetylation process. Another key regulatory enzyme of peroxisomal β-oxidation, Acetyl-CoA acyltransferase 1 (ACAA1), has been extensively studied. In the breeding of livestock and poultry, researchers often explore its effects on adipocytes; for example, in goats and sheep, ACAA1 is involved in regulating adipogenesis. Studies have shown that ACAA1 deficiency increased lipid accumulation and the triglyceride content and promoted sheep preadipocyte differentiation [93]. At the same time, it was also proved that a regulatory relationship between ACAA1 and PPARγ. Although it does not point out the relationship between such a phenomenon and peroxisomal β-oxidation, it is not difficult to speculate that peroxisomal β-oxidation plays an important role. And in humans or model animals, researchers have focused on the diseases caused by it [94]. At present, it is generally believed that the expression of PPAR affects the expression of ACCA1, which in turn affects the process of β-oxidation.
References
- Wagner, N.; Wagner, K.D. The Role of PPARs in Disease. Cells 2020, 9, 2367.
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metab. Clin. Exp. 2021, 114, 154338.
- Cai, D.; Li, Y.; Zhang, K.; Zhou, B.; Guo, F.; Holm, L.; Liu, H.Y. Co-option of PPARα in the regulation of lipogenesis and fatty acid oxidation in CLA-induced hepatic steatosis. J. Cell. Physiol. 2021, 236, 4387–4402.
- Jia, Y.; Liu, N.; Viswakarma, N.; Sun, R.; Schipma, M.J.; Shang, M.; Thorp, E.B.; Kanwar, Y.S.; Thimmapaya, B.; Reddy, J.K. PIMT/NCOA6IP Deletion in the Mouse Heart Causes Delayed Cardiomyopathy Attributable to Perturbation in Energy Metabolism. Int. J. Mol. Sci. 2018, 19, 1485.
- Liu, H.Y.; Gu, H.; Li, Y.; Hu, P.; Yang, Y.; Li, K.; Li, H.; Zhang, K.; Zhou, B.; Wu, H.; et al. Dietary Conjugated Linoleic Acid Modulates the Hepatic Circadian Clock Program via PPARα/REV-ERBα-Mediated Chromatin Modification in Mice. Front. Nutr. 2021, 8, 711398.
- Angeli, E.; Trionfini, V.; Gareis, N.C.; Matiller, V.; Huber, E.; Rey, F.; Salvetti, N.R.; Ortega, H.H.; Hein, G.J. Protein and gene expression of relevant enzymes and nuclear receptor of hepatic lipid metabolism in grazing dairy cattle during the transition period. Res. Vet. Sci. 2019, 123, 223–231.
- Zhang, X.; Zhang, S.; Ma, L.; Jiang, E.; Xu, H.; Chen, R.; Yang, Q.; Chen, H.; Li, Z.; Lan, X. Reduced representation bisulfite sequencing (RRBS) of dairy goat mammary glands reveals DNA methylation profiles of integrated genome-wide and critical milk-related genes. Oncotarget 2017, 8, 115326–115344.
- Chen, Z.; Luo, J.; Sun, S.; Cao, D.; Shi, H.; Loor, J.J. miR-148a and miR-17-5p synergistically regulate milk TAG synthesis via PPARGC1A and PPARA in goat mammary epithelial cells. RNA Biol. 2017, 14, 326–338.
- Li, X.; Li, Y.; Ding, H.; Dong, J.; Zhang, R.; Huang, D.; Lei, L.; Wang, Z.; Liu, G.; Li, X. Insulin suppresses the AMPK signaling pathway to regulate lipid metabolism in primary cultured hepatocytes of dairy cows. J. Dairy Res. 2018, 85, 157–162.
- Zou, D.; Liu, R.; Shi, S.; Du, J.; Tian, M.; Wang, X.; Hou, M.; Duan, Z.; Ma, Y. BHBA regulates the expressions of lipid synthesis and oxidation genes in sheep hepatocytes through the AMPK pathway. Res. Vet. Sci. 2021, 140, 153–163.
- Socha, B.M.; Łada, P.; Szczepańska, A.A.; Łupicka, M.; Korzekwa, A.J. The influence of experimentally induced endometritis on the PPAR expression profile in the bovine endometrium. Theriogenology 2018, 122, 74–83.
- Cañón-Beltrán, K.; Cajas, Y.N.; Peréz-Cerezales, S.; Leal, C.L.V.; Agirregoitia, E.; Gutierrez-Adán, A.; González, E.M.; Rizos, D. Nobiletin enhances the development and quality of bovine embryos in vitro during two key periods of embryonic genome activation. Sci. Rep. 2021, 11, 11796.
- Cai, D.; Li, H.; Zhou, B.; Han, L.; Zhang, X.; Yang, G.; Yang, G. Conjugated linoleic acid supplementation caused reduction of perilipin1 and aberrant lipolysis in epididymal adipose tissue. Biochem. Biophys. Res. Commun. 2012, 422, 621–626.
- El Ouarrat, D.; Isaac, R.; Lee, Y.S.; Oh, D.Y.; Wollam, J.; Lackey, D.; Riopel, M.; Bandyopadhyay, G.; Seo, J.B.; Sampath-Kumar, R.; et al. TAZ Is a Negative Regulator of PPARγ Activity in Adipocytes and TAZ Deletion Improves Insulin Sensitivity and Glucose Tolerance. Cell Metab. 2020, 31, 162–173.e165.
- Cai, D.; Liu, H.; Zhao, R. Nuclear Receptors in Hepatic Glucose and Lipid Metabolism During Neonatal and Adult Life. Curr. Protein Pept. Sci. 2017, 18, 548–561.
- Dean, J.M.; He, A.; Tan, M.; Wang, J.; Lu, D.; Razani, B.; Lodhi, I.J. MED19 Regulates Adipogenesis and Maintenance of White Adipose Tissue Mass by Mediating PPARγ-Dependent Gene Expression. Cell Rep. 2020, 33, 108228.
- Hassan, F.U.; Nadeem, A.; Li, Z.; Javed, M.; Liu, Q.; Azhar, J.; Rehman, M.S.; Cui, K.; Rehman, S.U. Role of Peroxisome Proliferator-Activated Receptors (PPARs) in Energy Homeostasis of Dairy Animals: Exploiting Their Modulation through Nutrigenomic Interventions. Int. J. Mol. Sci. 2021, 22, 2463.
- Gao, S.T.; Girma, D.D.; Bionaz, M.; Ma, L.; Bu, D.P. Hepatic transcriptomic adaptation from prepartum to postpartum in dairy cows. J. Dairy Sci. 2021, 104, 1053–1072.
- Schmitt, E.; Ballou, M.A.; Correa, M.N.; DePeters, E.J.; Drackley, J.K.; Loor, J.J. Dietary lipid during the transition period to manipulate subcutaneous adipose tissue peroxisome proliferator-activated receptor-γ co-regulator and target gene expression. J. Dairy Sci. 2011, 94, 5913–5925.
- Fan, Y.; Han, Z.; Lu, X.; Zhang, H.; Arbab, A.A.I.; Loor, J.J.; Yang, Y.; Yang, Z. Identification of Milk Fat Metabolism-Related Pathways of the Bovine Mammary Gland during Mid and Late Lactation and Functional Verification of the ACSL4 Gene. Genes 2020, 11, 1357.
- Sandri, E.C.; Camêra, M.; Sandri, E.M.; Harvatine, K.J.; De Oliveira, D.E. Peroxisome proliferator-activated receptor gamma (PPARγ) agonist fails to overcome trans-10, cis-12 conjugated linoleic acid (CLA) inhibition of milk fat in dairy sheep. Anim. Int. J. Anim. Biosci. 2018, 12, 1405–1412.
- Zhang, Y.; Wang, Y.; Wang, X.; Ji, Y.; Cheng, S.; Wang, M.; Zhang, C.; Yu, X.; Zhao, R.; Zhang, W.; et al. Acetyl-coenzyme A acyltransferase 2 promote the differentiation of sheep precursor adipocytes into adipocytes. J. Cell. Biochem. 2019, 20, 8021–8031.
- Xu, X.; Zhao, R.; Ma, W.; Zhao, Q.; Zhang, G. Comparison of lipid deposition of intramuscular preadipocytes in Tan sheep co-cultured with satellite cells or alone. J. Anim. Physiol. Anim. Nutr. 2021, 10, 1111.
- Wallace, J.M.; Milne, J.S.; Aitken, B.W.; Aitken, R.P.; Adam, C.L. Ovine prenatal growth-restriction and sex influence fetal adipose tissue phenotype and impact postnatal lipid metabolism and adiposity in vivo from birth until adulthood. PLoS ONE 2020, 15, e0228732.
- Fan, Y.; Ren, C.; Meng, F.; Deng, K.; Zhang, G.; Wang, F. Effects of algae supplementation in high-energy dietary on fatty acid composition and the expression of genes involved in lipid metabolism in Hu sheep managed under intensive finishing system. Meat Sci. 2019, 157, 107872.
- Vargas-Bello-Pérez, E.; Zhao, W.; Bionaz, M.; Luo, J.; Loor, J.J. Nutrigenomic Effect of Saturated and Unsaturated Long Chain Fatty Acids on Lipid-Related Genes in Goat Mammary Epithelial Cells: What Is the Role of PPARγ? Vet. Sci. 2019, 6, 54.
- Fensterseifer, S.R.; Austin, K.J.; Ford, S.P.; Alexander, B.M. Effects of maternal obesity on maternal and fetal plasma concentrations of adiponectin and expression of adiponectin and its receptor genes in cotyledonary and adipose tissues at mid- and late-gestation in sheep. Anim. Reprod. Sci. 2018, 197, 231–239.
- Yao, Y.C.; Song, X.T.; Zhai, Y.F.; Liu, S.; Lu, J.; Xu, X.; Qi, M.Y.; Zhang, J.N.; Huang, H.; Liu, Y.F.; et al. Transcriptome analysis of sheep follicular development during prerecruitment, dominant, and mature stages after FSH superstimulation. Domest. Anim. Endocrinol. 2021, 74, 106563.
- Hassanpour, H.; Khalaji-Pirbalouty, V.; Adibi, M.; Nazari, H. Involvement of peroxisome proliferator-activated receptors in the estradiol production of ovine Sertoli cells. Vet. Res. Forum Int. Q. J. 2017, 8, 251–257.
- Penna-de-Carvalho, A.; Graus-Nunes, F.; Rabelo-Andrade, J.; Mandarim-de-Lacerda, C.A.; Souza-Mello, V. Enhanced pan-peroxisome proliferator-activated receptor gene and protein expression in adipose tissue of diet-induced obese mice treated with telmisartan. Exp. Physiol. 2014, 99, 1663–1678.
- Tong, L.; Wang, L.; Yao, S.; Jin, L.; Yang, J.; Zhang, Y.; Ning, G.; Zhang, Z. PPARδ attenuates hepatic steatosis through autophagy-mediated fatty acid oxidation. Cell Death Dis. 2019, 10, 197.
- Kim, N.H.; Kim, S.G. Fibrates Revisited: Potential Role in Cardiovascular Risk Reduction. Diabetes Metab. J. 2020, 44, 213–221.
- Yamashita, S.; Masuda, D.; Matsuzawa, Y. Pemafibrate, a New Selective PPARα Modulator: Drug Concept and Its Clinical Applications for Dyslipidemia and Metabolic Diseases. Curr. Atheroscler. Rep. 2020, 22, 5.
- De Vries, E.; Bolier, R.; Goet, J.; Parés, A.; Verbeek, J.; de Vree, M.; Drenth, J.; van Erpecum, K.; van Nieuwkerk, K.; van der Heide, F.; et al. Fibrates for Itch (FITCH) in Fibrosing Cholangiopathies: A Double-Blind, Randomized, Placebo-Controlled Trial. Gastroenterology 2021, 160, 734–743.e736.
- Rubio, B.; Mora, C.; Pintado, C.; Mazuecos, L.; Fernández, A.; López, V.; Andrés, A.; Gallardo, N. The nutrient sensing pathways FoxO1/3 and mTOR in the heart are coordinately regulated by central leptin through PPARβ/δ. Implications in cardiac remodeling. Metab. Clin. Exp. 2021, 115, 154453.
- Park, H.; He, A.; Tan, M.; Johnson, J.M.; Dean, J.M.; Pietka, T.A.; Chen, Y.; Zhang, X.; Hsu, F.F.; Razani, B.; et al. Peroxisome-derived lipids regulate adipose thermogenesis by mediating cold-induced mitochondrial fission. J. Clin. Investig. 2019, 129, 694–711.
- Moreira-Pais, A.; Ferreira, R.; Neves, J.S.; Vitorino, R.; Moreira-Gonçalves, D.; Nogueira-Ferreira, R. Sex differences on adipose tissue remodeling: From molecular mechanisms to therapeutic interventions. J. Mol. Med. 2020, 98, 483–493.
- Park, H.J.; Choi, J.M. Sex-specific regulation of immune responses by PPARs. Exp. Mol. Med. 2017, 49, e364.
- Hara, S.; Furukawa, F.; Mukai, K.; Yazawa, T.; Kitano, T. Peroxisome proliferator-activated receptor alpha is involved in the temperature-induced sex differentiation of a vertebrate. Sci. Rep. 2020, 10, 11672.
- Pierrot, N.; Ris, L.; Stancu, I.C.; Doshina, A.; Ribeiro, F.; Tyteca, D.; Baugé, E.; Lalloyer, F.; Malong, L.; Schakman, O.; et al. Sex-regulated gene dosage effect of PPARα on synaptic plasticity. Life Sci. Alliance 2019, 2, e201800262.
- De Felice, M.; Melis, M.; Aroni, S.; Muntoni, A.L.; Fanni, S.; Frau, R.; Devoto, P.; Pistis, M. The PPARα agonist fenofibrate attenuates disruption of dopamine function in a maternal immune activation rat model of schizophrenia. CNS Neurosci. Ther. 2019, 25, 549–561.
- Luft, C.; Levices, I.P.; Pedrazza, L.; de Oliveira, J.R.; Donadio, M.V.F. Sex-dependent metabolic effects of pregestational exercise on prenatally stressed mice. J. Dev. Orig. Health Dis. 2021, 12, 271–279.
- Bapat, S.P.; Whitty, C.; Mowery, C.T.; Liang, Y.; Yoo, A.; Jiang, Z.; Peters, M.C.; Zhang, L.J.; Vogel, I.; Zhou, C.; et al. Obesity alters pathology and treatment response in inflammatory disease. Nature 2022, 604, 337–342.
- Xin, G.; Chen, Y.; Topchyan, P.; Kasmani, M.Y.; Burns, R.; Volberding, P.J.; Wu, X.; Cohn, A.; Chen, Y.; Lin, C.W.; et al. Targeting PIM1-Mediated Metabolism in Myeloid Suppressor Cells to Treat Cancer. Cancer Immunol. Res. 2021, 9, 454–469.
- Li, C.; Wang, G.; Sivasami, P.; Ramirez, R.N.; Zhang, Y.; Benoist, C.; Mathis, D. Interferon-α-producing plasmacytoid dendritic cells drive the loss of adipose tissue regulatory T cells during obesity. Cell Metab. 2021, 33, 1610–1623.e1615.
- Kim, S.; Lee, N.; Park, E.S.; Yun, H.; Ha, T.U.; Jeon, H.; Yu, J.; Choi, S.; Shin, B.; Yu, J.; et al. T-Cell Death Associated Gene 51 Is a Novel Negative Regulator of PPARγ That Inhibits PPARγ-RXRα Heterodimer Formation in Adipogenesis. Mol. Cells 2021, 44, 1–12.
- Van Herck, M.A.; Vonghia, L.; Kwanten, W.J.; Vanwolleghem, T.; Ebo, D.G.; Michielsen, P.P.; De Man, J.G.; Gama, L.; De Winter, B.Y.; Francque, S.M. Adoptive Cell Transfer of Regulatory T Cells Exacerbates Hepatic Steatosis in High-Fat High-Fructose Diet-Fed Mice. Front. Immunol. 2020, 11, 1711.
- Fontecha-Barriuso, M.; Martin-Sanchez, D.; Martinez-Moreno, J.M.; Monsalve, M.; Ramos, A.M.; Sanchez-Niño, M.D.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A.B. The Role of PGC-1α and Mitochondrial Biogenesis in Kidney Diseases. Biomolecules 2020, 10, 347.
- Fontecha-Barriuso, M.; Martín-Sánchez, D.; Martinez-Moreno, J.M.; Carrasco, S.; Ruiz-Andrés, O.; Monsalve, M.; Sanchez-Ramos, C.; Gómez, M.J.; Ruiz-Ortega, M.; Sánchez-Niño, M.D.; et al. PGC-1α deficiency causes spontaneous kidney inflammation and increases the severity of nephrotoxic AKI. J. Pathol. 2019, 249, 65–78.
- Bagattin, A.; Hugendubler, L.; Mueller, E. Transcriptional coactivator PGC-1alpha promotes peroxisomal remodeling and biogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 20376–20381.
- Huang, T.Y.; Zheng, D.; Houmard, J.A.; Brault, J.J.; Hickner, R.C.; Cortright, R.N. Overexpression of PGC-1α increases peroxisomal activity and mitochondrial fatty acid oxidation in human primary myotubes. Am. J. Physiol. Endocrinol. Metab. 2017, 312, e253–e263.
- Huang, T.Y.; Linden, M.A.; Fuller, S.E.; Goldsmith, F.R.; Simon, J.; Batdorf, H.M.; Scott, M.C.; Essajee, N.M.; Brown, J.M.; Noland, R.C. Combined effects of a ketogenic diet and exercise training alter mitochondrial and peroxisomal substrate oxidative capacity in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2021, 320, e1053–e1067.
- Summermatter, S.; Baum, O.; Santos, G.; Hoppeler, H.; Handschin, C. Peroxisome proliferator-activated receptor coactivator 1 (PGC-1) promotes skeletal muscle lipid refueling in vivo by activating de novo lipogenesis and the pentose phosphate pathway. J. Biol. Chem. 2010, 285, 32793–32800.
- Held-Hoelker, E.; Klein, S.L.; Rings, F.; Salilew-Wondim, D.; Saeed-Zidane, M.; Neuhoff, C.; Tesfaye, D.; Schellander, K.; Hoelker, M. Cryosurvival of in vitro produced bovine embryos supplemented with l-Carnitine and concurrent reduction of fatty acids. Theriogenology 2017, 96, 145–152.
- Tan, X.; Zhu, T.; Zhang, L.; Fu, L.; Hu, Y.; Li, H.; Li, C.; Zhang, J.; Liang, B.; Liu, J. miR-669a-5p promotes adipogenic differentiation and induces browning in preadipocytes. Adipocyte 2022, 11, 120–132.
- Zhou, X.; Yan, Q.; Yang, H.; Ren, A.; He, Z.; Tan, Z. Maternal intake restriction programs the energy metabolism, clock circadian regulator and mTOR signals in the skeletal muscles of goat offspring probably via the protein kinase A-cAMP-responsive element-binding proteins pathway. Anim. Nutr. 2021, 7, 1303–1314.
- Distel, B.; Erdmann, R.; Gould, S.J.; Blobel, G.; Crane, D.I.; Cregg, J.M.; Dodt, G.; Fujiki, Y.; Goodman, J.M.; Just, W.W.; et al. A unified nomenclature for peroxisome biogenesis factors. J. Cell Biol. 1996, 135, 1–3.
- Barros-Barbosa, A.; Rodrigues, T.A.; Ferreira, M.J.; Pedrosa, A.G.; Teixeira, N.R.; Francisco, T.; Azevedo, J.E. The intrinsically disordered nature of the peroxisomal protein translocation machinery. FEBS J. 2019, 286, 24–38.
- Wang, W.; Xia, Z.J.; Farré, J.C.; Subramani, S. TRIM37, a novel E3 ligase for PEX5-mediated peroxisomal matrix protein import. J. Cell Biol. 2017, 216, 2843–2858.
- Klouwer, F.C.C.; Falkenberg, K.D.; Ofman, R.; Koster, J.; van Gent, D.; Ferdinandusse, S.; Wanders, R.J.A.; Waterham, H.R. Autophagy Inhibitors Do Not Restore Peroxisomal Functions in Cells with the Most Common Peroxisome Biogenesis Defect. Front. Cell Dev. Biol. 2021, 9, 661298.
- Lotz-Havla, A.S.; Woidy, M.; Guder, P.; Friedel, C.C.; Klingbeil, J.M.; Bulau, A.M.; Schultze, A.; Dahmen, I.; Noll-Puchta, H.; Kemp, S.; et al. iBRET Screen of the ABCD1 Peroxisomal Network and Mutation-Induced Network Perturbations. J. Proteome Res. 2021, 20, 4366–4380.
- Coppa, A.; Guha, S.; Fourcade, S.; Parameswaran, J.; Ruiz, M.; Moser, A.B.; Schlüter, A.; Murphy, M.P.; Lizcano, J.M.; Miranda-Vizuete, A.; et al. The peroxisomal fatty acid transporter ABCD1/PMP-4 is required in the C. elegans hypodermis for axonal maintenance: A worm model for adrenoleukodystrophy. Free Radic. Biol. Med. 2020, 152, 797–809.
- Zierfuss, B.; Weinhofer, I.; Kühl, J.S.; Köhler, W.; Bley, A.; Zauner, K.; Binder, J.; Martinović, K.; Seiser, C.; Hertzberg, C.; et al. Vorinostat in the acute neuroinflammatory form of X-linked adrenoleukodystrophy. Ann. Clin. Transl. Neurol. 2020, 7, 639–652.
- Violante, S.; Achetib, N.; van Roermund, C.W.T.; Hagen, J.; Dodatko, T.; Vaz, F.M.; Waterham, H.R.; Chen, H.; Baes, M.; Yu, C.; et al. Peroxisomes can oxidize medium- and long-chain fatty acids through a pathway involving ABCD3 and HSD17B4. FASEB J. 2019, 33, 4355–4364.
- Wang, J.; Ma, H.; Zhao, S.; Huang, J.; Yang, Y.; Tabashnik, B.E.; Wu, Y. Functional redundancy of two ABC transporter proteins in mediating toxicity of Bacillus thuringiensis to cotton bollworm. PLoS Pathog. 2020, 16, e1008427.
- Zhao, S.; Jiang, D.; Wang, F.; Yang, Y.; Tabashnik, B.E.; Wu, Y. Independent and Synergistic Effects of Knocking out Two ABC Transporter Genes on Resistance to Bacillus thuringiensis Toxins Cry1Ac and Cry1Fa in Diamondback Moth. Toxins 2020, 13, 9.
- Ahmad, S.M.; Bhat, B.; Bhat, S.A.; Yaseen, M.; Mir, S.; Raza, M.; Iquebal, M.A.; Shah, R.A.; Ganai, N.A. SNPs in Mammary Gland Epithelial Cells Unraveling Potential Difference in Milk Production Between Jersey and Kashmiri Cattle Using RNA Sequencing. Front. Genet. 2021, 12, 666015.
- Lopez, B.I.; Santiago, K.G.; Lee, D.; Ha, S.; Seo, K. RNA Sequencing (RNA-Seq) Based Transcriptome Analysis in Immune Response of Holstein Cattle to Killed Vaccine against Bovine Viral Diarrhea Virus Type I. Animals 2020, 10, 344.
- Xu, J.; Zhou, Y.; Yang, Y.; Lv, C.; Liu, X.; Wang, Y. Involvement of ABC-transporters and acyltransferase 1 in intracellular cholesterol-mediated autophagy in bovine alveolar macrophages in response to the Bacillus Calmette-Guerin (BCG) infection. BMC Immunol. 2020, 21, 26.
- Sales, A.D.; Duarte, A.B.G.; Rocha, R.M.P.; Brito, I.R.; Locatelli, Y.; Alves, B.G.; Alves, K.A.; Figueiredo, J.R.; Rodrigues, A.P.R. Transcriptional downregulation of ABC transporters is related to follicular degeneration after vitrification and in vitro culture of ovine ovarian tissue. Theriogenology 2022, 177, 127–132.
- Xu, Q.; Fan, Y.; Loor, J.J.; Liang, Y.; Sun, X.; Jia, H.; Zhao, C.; Xu, C. Adenosine 5’-monophosphate-activated protein kinase ameliorates bovine adipocyte oxidative stress by inducing antioxidant responses and autophagy. J. Dairy Sci. 2021, 104, 4516–4528.
- Xu, T.; Lu, X.; Arbab, A.A.I.; Wu, X.; Mao, Y.; Loor, J.J.; Yang, Z. Metformin acts to suppress β-hydroxybutyric acid-mediated inflammatory responses through activation of AMPK signaling in bovine hepatocytes. J. Anim. Sci. 2021, 99, skab153.
- Zhang, Y.; Xu, Y.; Chen, B.; Zhao, B.; Gao, X.J. Selenium Deficiency Promotes Oxidative Stress-Induced Mastitis via Activating the NF-κB and MAPK Pathways in Dairy Cow. Biol. Trace Elem. Res. 2022, 200, 2716–2726.
- Zhou, F.; Ding, M.; Gu, Y.; Fan, G.; Liu, C.; Li, Y.; Sun, R.; Wu, J.; Li, J.; Xue, X.; et al. Aurantio-Obtusin Attenuates Non-Alcoholic Fatty Liver Disease Through AMPK-Mediated Autophagy and Fatty Acid Oxidation Pathways. Front. Pharmacol. 2021, 12, 826628.
- Xie, Y.H.; Xiao, Y.; Huang, Q.; Hu, X.F.; Gong, Z.C.; Du, J. Role of the CTRP6/AMPK pathway in kidney fibrosis through the promotion of fatty acid oxidation. Eur. J. Pharmacol. 2021, 892, 173755.
- Zhang, X.; Li, L.; Li, Y.; Li, Z.; Zhai, W.; Sun, Q.; Yang, X.; Roth, M.; Lu, S. mTOR regulates PRMT1 expression and mitochondrial mass through STAT1 phosphorylation in hepatic cell. Biochim. Biophys. Acta. Mol. Cell Res. 2021, 1868, 119017.
- Liu, Z.; Liao, W.; Yin, X.; Zheng, X.; Li, Q.; Zhang, H.; Zheng, L.; Feng, X. Resveratrol-induced brown fat-like phenotype in 3T3-L1 adipocytes partly via mTOR pathway. Food Nutr. Res. 2020, 64, 3656.
- Wang, R.; Hu, W. Asprosin promotes β-cell apoptosis by inhibiting the autophagy of β-cell via AMPK-mTOR pathway. J. Cell. Physiol. 2021, 236, 215–221.
- Dai, H.; Sinclair, D.A.; Ellis, J.L.; Steegborn, C. Sirtuin activators and inhibitors: Promises, achievements, and challenges. Pharmacol. Ther. 2018, 188, 140–154.
- Curry, A.M.; White, D.S.; Donu, D.; Cen, Y. Human Sirtuin Regulators: The “Success” Stories. Front. Physiol. 2021, 12, 752117.
- Chen, X.F.; Tian, M.X.; Sun, R.Q.; Zhang, M.L.; Zhou, L.S.; Jin, L.; Chen, L.L.; Zhou, W.J.; Duan, K.L.; Chen, Y.J.; et al. SIRT5 inhibits peroxisomal ACOX1 to prevent oxidative damage and is downregulated in liver cancer. EMBO Rep. 2018, 19, e45124.
- Shumar, S.A.; Kerr, E.W.; Geldenhuys, W.J.; Montgomery, G.E.; Fagone, P.; Thirawatananond, P.; Saavedra, H.; Gabelli, S.B.; Leonardi, R. Nudt19 is a renal CoA diphosphohydrolase with biochemical and regulatory properties that are distinct from the hepatic Nudt7 isoform. J. Biol. Chem. 2018, 293, 4134–4148.
- Shumar, S.A.; Kerr, E.W.; Fagone, P.; Infante, A.M.; Leonardi, R. Overexpression of Nudt7 decreases bile acid levels and peroxisomal fatty acid oxidation in the liver. J. Lipid Res. 2019, 60, 1005–1019.
- Li, C.; Jiang, L.; Jin, Y.; Zhang, D.; Chen, J.; Qi, Y.; Fan, R.; Luo, J.; Xu, L.; Ma, W.; et al. Lipid metabolism disorders effects of 6:2 chlorinated polyfluorinated ether sulfonate through Hsa-miRNA-532-3p/Acyl-CoA oxidase 1(ACOX1) pathway. Ecotoxicol. Environ. Saf. 2021, 228, 113011.
- Zhang, F.; Xiong, Q.; Tao, H.; Liu, Y.; Zhang, N.; Li, X.F.; Suo, X.J.; Yang, Q.P.; Chen, M.X. ACOX1, regulated by C/EBPα and miR-25-3p, promotes bovine preadipocyte adipogenesis. J. Mol. Endocrinol. 2021, 66, 195–205.
- Wang, J.J.; Zhang, Y.T.; Tseng, Y.J.; Zhang, J. miR-222 targets ACOX1, promotes triglyceride accumulation in hepatocytes. Hepatobiliary Pancreat. Dis. Int. HBPD INT 2019, 18, 360–365.
- Lai, Y.H.; Liu, H.; Chiang, W.F.; Chen, T.W.; Chu, L.J.; Yu, J.S.; Chen, S.J.; Chen, H.C.; Tan, B.C. MiR-31-5p-ACOX1 Axis Enhances Tumorigenic Fitness in Oral Squamous Cell Carcinoma Via the Promigratory Prostaglandin E2. Theranostics 2018, 8, 486–504.
- Li, G.; Fu, S.; Chen, Y.; Jin, W.; Zhai, B.; Li, Y.; Sun, G.; Han, R.; Wang, Y.; Tian, Y.; et al. MicroRNA-15a Regulates the Differentiation of Intramuscular Preadipocytes by Targeting ACAA1, ACOX1 and SCP2 in Chickens. Int. J. Mol. Sci. 2019, 20, 63.
- Zhou, H.X.; Wang, L.Y.; Chen, S.; Wang, D.D.; Fang, Z. circ_0005379 inhibits the progression of oral squamous cell carcinoma by regulating the miR-17-5p/acyl-CoA oxidase 1 axis. Hua Xi Kou Qiang Yi Xue Za Zhi = Huaxi Kouqiang Yixue Zazhi = West China J. Stomatol. 2021, 39, 425–433.
- Lee, S.A.; Lee, J.; Kim, K.; Moon, H.; Min, C.; Moon, B.; Kim, D.; Yang, S.; Park, H.; Lee, G.; et al. The Peroxisomal Localization of Hsd17b4 Is Regulated by Its Interaction with Phosphatidylserine. Mol. Cells 2021, 44, 214–222.
- Zhang, Y.; Xu, Y.Y.; Yao, C.B.; Li, J.T.; Zhao, X.N.; Yang, H.B.; Zhang, M.; Yin, M.; Chen, J.; Lei, Q.Y. Acetylation targets HSD17B4 for degradation via the CMA pathway in response to estrone. Autophagy 2017, 13, 538–553.
- Huang, H.; Liu, R.; Huang, Y.; Feng, Y.; Fu, Y.; Chen, L.; Chen, Z.; Cai, Y.; Zhang, Y.; Chen, Y. Acetylation-mediated degradation of HSD17B4 regulates the progression of prostate cancer. Aging 2020, 12, 14699–14717.
- Wang, Y.; Li, X.; Cao, Y.; Xiao, C.; Liu, Y.; Jin, H.; Cao, Y. Effect of the ACAA1 Gene on Preadipocyte Differentiation in Sheep. Front. Genet. 2021, 12, 649140.
- Ranea-Robles, P.; Violante, S.; Argmann, C.; Dodatko, T.; Bhattacharya, D.; Chen, H.; Yu, C.; Friedman, S.L.; Puchowicz, M.; Houten, S.M. Murine deficiency of peroxisomal L-bifunctional protein (EHHADH) causes medium-chain 3-hydroxydicarboxylic aciduria and perturbs hepatic cholesterol homeostasis. Cell. Mol. Life Sci. CMLS 2021, 78, 5631–5646.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.9K
Revisions:
2 times
(View History)
Update Date:
07 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




