
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wenjie Cheng | -- | 9772 | 2022-06-27 11:56:07 | | | |
| 2 | Wenjie Cheng | Meta information modification | 9772 | 2022-06-28 10:35:01 | | | | |
| 3 | Conner Chen | -4021 word(s) | 5751 | 2022-07-08 12:51:57 | | |
Video Upload Options
In children, vasovagal syncope and postural tachycardia syndrome constitute the major types of orthostatic intolerance. The clinical characteristics of postural tachycardia syndrome and vasovagal syncope are similar but their treatments differ. Therefore, their differential diagnosis is important to guide the correct treatment. Children suffering from vasovagal syncope or postural tachycardia syndrome might be treated using water, β-blockers, salt, or midodrine. However, the effificacy of the drugs varies. Biomarkers or certain hemodynamic parameters that can predict the treatment effects of individualized treatment for POTS or VVS have been used.
1. Introduction
2. Differential Diagnosis of POTS and VVS
| Cut-Off | Sensitivity | Specificity | Year | |
|---|---|---|---|---|
| Plasma H2S level | 98 μmol/L | 90% | 80% | 2012 |
| Serum iron level | 11.8 μmol/L, | 92.50% | 64.70% | 2013 |
| AI and 30/15 | AI: 28.180; 30/15: 1.025 |
95.80% | 80.80% | 2018 |
| dULF | 36.2 ms2 | 71.40% | 75.00% | 2019 |
2.1. The Plasma Hydrogen Sulfide (H2S) Level
2.2. The Serum Iron Level
2.3. Immediate Heart Rate Alteration Index AI and 30/15
2.4. Frequency Domain Indices of Heart Rate Variability (dULF)
3. Individualized Therapy
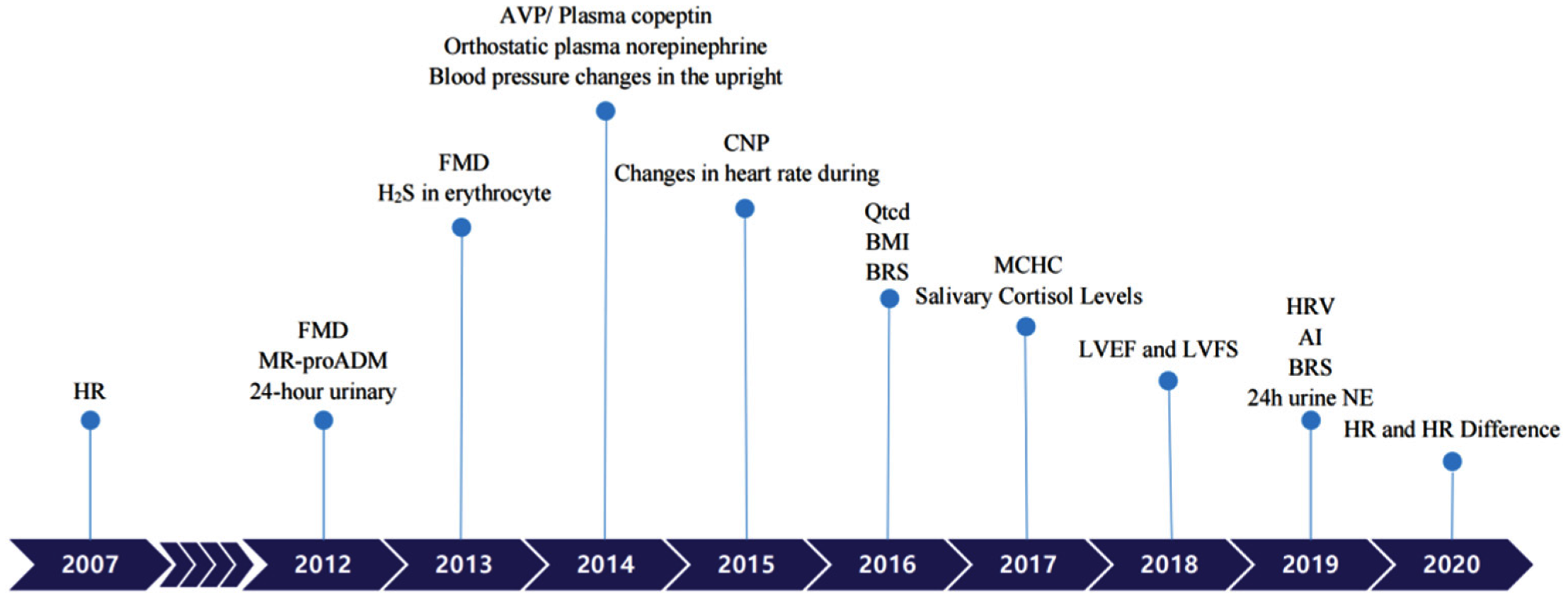 Figure 1. Biomarkers to predict individualized treatment for VVS and POTS in chronological order. POTS: Postural Tachycardia Syndrome; VVS: Vasovagal Syncope; HR: heart rate; FMD: Flow-mediated vasodilation response; MR-proADM: Pro-adrenomedullin; AVP: Arginine vasopressin; CNP: C-type natriuretic peptide; BMI: Body mass index; BRS: Baroreflex sensitivity; MCHC: Mean corpuscular hemoglobin concentration; LVEF: Left ventricular ejection fraction; LVFS: Left ventricular fractional shortening; AI: Acceleration index; HRV: Heart rate variability; 24 h urine NE: 24-h urine norepinephrine.
Figure 1. Biomarkers to predict individualized treatment for VVS and POTS in chronological order. POTS: Postural Tachycardia Syndrome; VVS: Vasovagal Syncope; HR: heart rate; FMD: Flow-mediated vasodilation response; MR-proADM: Pro-adrenomedullin; AVP: Arginine vasopressin; CNP: C-type natriuretic peptide; BMI: Body mass index; BRS: Baroreflex sensitivity; MCHC: Mean corpuscular hemoglobin concentration; LVEF: Left ventricular ejection fraction; LVFS: Left ventricular fractional shortening; AI: Acceleration index; HRV: Heart rate variability; 24 h urine NE: 24-h urine norepinephrine.|
Diagnosis |
Treatment |
Biological Markers or Predictors |
Cut-off |
Sensitivity |
Specificity |
Year |
|
|
POTS |
non-pharmacotherapy |
Qtcd [27] |
43.0 msec |
90% |
60% |
2016 |
|
|
|
|
Salivary cortisol levels [28] |
4.1 ng/mL |
83.30% |
68.70% |
2017 |
|
|
|
ORS |
24-h urinary sodium [29] |
124 mmol/24 h |
76.90% |
93% |
2012 |
|
|
|
|
Changes in heart rate during HUTT [30] |
pre-treatment increase in HR = 41 beats/min maximum upright HR in 10 min = 123 beats/min |
84% |
56% |
2015 |
|
|
|
|
BMI [31] |
18.02 |
92% |
82.80% |
2016 |
|
|
|
|
BRS [32] |
17.01 ms/mmHg |
85.70% |
87.50% |
2016 |
|
|
|
|
MCHC [33] |
347.5 g/L |
68.80% |
63.20% |
2017 |
|
|
|
midodrine hydrochloride |
MR-proADM [34] |
61.5 pg/mL |
100% |
71.60% |
2012 |
|
|
|
|
FMD [35] |
9.85% |
1-month |
76.9% |
93% |
2013 |
|
3-month |
71.6% |
80% |
|||||
|
|
|
H2S in erythrocyte [36] |
27.1 nmol/min/108 |
78.90% |
77.80% |
2013 |
|
|
|
|
Blood pressure changes in the upright position [37] |
SBP ≤ 0 mmHg; DBP ≤ 6.5 mmHg |
72% |
88% |
2014 |
|
|
|
|
AVP/Plasma copeptin [38] |
10.482 pmol/L |
81.30% |
76.50% |
2014 |
|
|
|
metoprolol |
Orthostatic plasma norepinephrine [39] |
3.59 pg/mL |
76.90% |
91.70% |
2014 |
|
|
|
|
AVP/Plasma copeptin [40] |
10.225 pmol/L |
90.50% |
78.60% |
2014 |
|
|
|
|
CNP [41] |
32.55 pg/mL |
95.80% |
70% |
2015 |
|
|
|
|
HRV [42] |
TR index ≤ 33.7; SDNN index ≤ 79.0ms |
85.3%, |
81.80% |
2019 |
|
|
|
|
HR and HR Difference [43] |
HR 5 ≥ 110 beats/min |
82.50% |
69.23% |
2020 |
|
|
|
|
HR 10 ≥ 112 beats/min |
84.62% |
69.70% |
|||
|
|
|
HR difference 5 ≥ 34 beats/min |
85.29% |
89.47% |
|||
|
|
|
HR difference 10 ≥ 37 beats/min |
97.56% |
64.86% |
|||
|
VVS |
orthostatic training |
AI [44] |
26.77 |
85.00% |
69.20% |
2019 |
|
|
|
midodrine hydrochloride |
FMD [45] |
8.85% |
90% |
80% |
2012 |
|
|
|
metoprolol |
HR [46] |
increase of 30 beats/min |
81% |
80% |
2007 |
|
|
|
|
LVEF and LVFS [47] |
two month |
LVEF > 70.5% |
80.00% |
100.00% |
2018 |
|
|
|
|
LVFS > 38.5% |
90.00% |
90.00% |
||
|
|
|
six month |
LVEF > 70.5% |
81.30% |
88.90% |
||
|
|
|
|
LVFS > 37.5% |
93.80% |
66.70% |
||
|
|
|
BRS [48] |
10 ms/mmHg |
82% |
83% |
2019 |
|
|
|
|
24 h urine NE [49] |
34.84 μg/24h |
70% |
100% |
2019 |
|
3.1. Individualized Therapy for POTS
3.1.1. Physiological Indicators Predicting the Efficacy of Non-Pharmacotherapy Treatment in Children with POTS
-
HR-corrected QT interval dispersion (QTcd)
-
Salivary cortisol levels
3.1.2. Physiological Predictors for the Oral Rehydration Salts Efficacy in Pediatric POTS
-
24 h urinary sodium
-
Changes in heart rate during head-up tilt test (HUTT).
-
Body mass index (BMI)
-
Baroreflex sensitivity (BRS)
-
Mean corpuscular hemoglobin concentration (MCHC)
3.1.3. Physiological Predictors for the Midodrine Hydrochloride Efficacy among POTS Children
-
Pro-adrenomedullin (MR-proADM)
-
Flow-mediated vasodilation response (FMD)
-
Hydrogen sulfide in erythrocyte (H2S)
-
Blood pressure changes in the upright position
-
Arginine vasopressin (AVP)/Plasma copeptin
3.1.4. Physiological Predictors for the Efficacy of Metoprolol in Pediatric POTS
-
Orthostatic plasma norepinephrine
-
AVP/Plasma copeptin
-
Plasma C-type natriuretic peptide (CNP)
-
Heart rate variability (HRV)
-
Heart Rate (HR) and Heart Rate Difference
3.2. VVS Individualized Therapy
3.2.1. Physiological Predictors for the Orthostatic Exercise Efficacy in Pediatric VVS
-
The Acceleration index (AI)
3.2.2. Physiological Indicators to Predict the Efficacy of a1-Adrenergic Receptor Agonists in Pediatric VVS
-
Flow-mediated vasodilation (FMD)
3.2.3. Physiological Indicators to Predict β-Blocker Efficacy in Pediatric VVS
-
Heart rate (HR)
-
LVFS (left ventricular fractional shortening) and LVEF (left ventricular ejection fraction)
-
Baroreflex sensitivity (BRS)
-
24 h urine norepinephrine (24 h urine NE)
References
- Du, J.; Zhang, Q. Research progress in orthostatic intolerance in children. Zhongguo Shi Yong Er Ke Za Zhi 2010, 25, 241–244.
- Duan, H.; Zhou, K.; Wang, C.; Hua, Y. Analysis of clinical features and related factor of syncope and head-up tilt test results in children. Zhonghua Er Ke Za Zhi 2016, 54, 269–272.
- Vernino, S.; Stiles, L.E. Autoimmunity in postural orthostatic tachycardia syndrome: Current understanding. Auton. Neurosci. 2018, 215, 78–82.
- Freeman, R.; Wieling, W.; Axelrod, F.B.; Benditt, D.G.; Benarroch, E.; Biaggioni, I.; Cheshire, W.; Chelimsky, T.C.; Cortelli, P.; Gibbons, C.H.; et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res. 2011, 21, 69–72.
- Benarroch, E.E. Postural tachycardia syndrome: A heterogeneous and multifactorial disorder. Mayo Clin. Proc. 2012, 87, 1214–1225.
- Stewart, J.M.; Medow, M.S.; Sutton, R.; Visintainer, P.; Jardine, D.L.; Wieling, W. Mechanisms of Vasovagal Syncope in the Young: Reduced Systemic Vascular Resistance Versus Reduced Cardiac Output. J. Am. Heart Assoc. 2017, 6, e004417.
- Song, J.; Jin, H.; Du, J. Diagnosis and treatment process in vasovagal syncope in children. Zhongguo Shi Yong Er Ke Za Zhi 2017, 32, 384–388.
- Tao, C.; Du, J. Research progress in the individualized treatment for postural tachycardia syndrome in children. Zhongguo Shi Yong Er Ke Za Zhi 2018, 33, 909–919.
- Chu, W.; Wang, C.; Lin, P.; Li, F.; Wu, L.; Xie, Z. Transient aphasia: A rare complication of head-up tilt test. Neurol. Sci. 2014, 35, 1127–1132.
- Wang, R. Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002, 16, 1792–1798.
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322, 587–590.
- Zhang, F.; Li, X.; Stella, C.; Chen, L.; Liao, Y.; Tang, C.; Jin, H.; Du, J. Plasma hydrogen sulfide in differential diagnosis between vasovagal syncope and postural orthostatic tachycardia syndrome in children. J. Pediatr. 2012, 160, 227–231.
- Verdon, F.; Burnand, B.; Stubi, C.-L.F.; Bonard, C.; Graff, M.; Michaud, A.; Bischoff, T.; De Vevey, M.; Studer, J.-P.; Herzig, L.; et al. Iron supplementation for unexplained fatigue in non-anaemic women: Double blind randomised placebo controlled trial. BMJ 2003, 326, 1124.
- Jarjour, I.T.; Jarjour, L.K. Low iron storage and mild anemia in postural tachycardia syndrome in adolescents. Clin. Auton. Res. 2013, 23, 175–179.
- Hoeldtke, R.D.; Streeten, D.H. Treatment of orthostatic hypotension with erythropoietin. N. Engl. J. Med. 1993, 329, 611–615.
- Stewart, J.M. Reduced iron stores and its effect on vasovagal syncope (simple faint). J. Pediatr. 2008, 153, 9–11.
- Li, J.; Zhang, Q.; Gao, J.; Jin, H.; Du, J. Significance of serum iron in the differential diagnosis between vasovagal syncope and postural orthostatic tachycardia syndrome in children. Beijing Da Xue Xue Bao Yi Xue Ban 2013, 45, 923–927.
- Sundkvist, G.; Lilja, B. Effect of the degree and speed of tilt on the immediate heart rate reaction. Clin. Physiol. 1983, 3, 381–386.
- Ewing, D.J.; Campbell, I.W.; Murray, A.; Neilson, J.M.; Clarke, B.F. Immediate heart-rate response to standing: Simple test for autonomic neuropathy in diabetes. Br. Med. J. 1978, 1, 145–147.
- Tao, C.; Chen, S.; Li, H.; Wang, Y.; Wang, Y.; Liu, P.; Liao, Y.; Zhang, C.; Tang, C.; Jin, H.; et al. Value of Immediate Heart Rate Alteration From Supine to Upright in Differential Diagnosis Between Vasovagal Syncope and Postural Tachycardia Syndrome in Children. Front. Pediatr. 2018, 6, 343.
- Wang, Y.; Zhang, C.; Chen, S.; Li, X.; Jin, H.; Du, J. Frequency Domain Indices of Heart Rate Variability are Useful for Differentiating Vasovagal Syncope and Postural Tachycardia Syndrome in Children. J. Pediatr. 2019, 207, 59–63.
- Qingyou, Z.; Junbao, D.; Chaoshu, T. The efficacy of midodrine hydrochloride in the treatment of children with vasovagal syncope. J. Pediatr. 2006, 149, 777–780.
- Fu, Q.; Vangundy, T.B.; Shibata, S.; Auchus, R.J.; Williams, G.H.; Levine, B.D. Exercise training versus propranolol in the treatment of the postural orthostatic tachycardia syndrome. Hypertension 2011, 58, 167–175.
- Chen, L.; Wang, L.; Sun, J.; Qin, J.; Tang, C.; Jin, H.; Du, J. Midodrine hydrochloride is effective in the treatment of children with postural orthostatic tachycardia syndrome. Circ. J. 2011, 75, 927–931.
- Fu, Q.; Levine, B.D. Exercise and non-pharmacological treatment of POTS. Auton. Neurosci. 2018, 215, 20–27.
- Sheldon, R.; Faris, P.; Tang, A.; Ayala-Paredes, F.; Guzman, J.; Marquez, M.; Morillo, C.A.; Krahn, A.D.; Kus, T.; Ritchie, M.D.; et al. Midodrine for the Prevention of Vasovagal Syncope: A Randomized Clinical Trial. Ann. Intern. Med. 2021, 174, 1349–1356.
- George, S.A.; Bivens, T.B.; Howden, E.J.; Saleem, Y.; Galbreath, M.M.; Hendrickson, D.; Fu, Q.; Levine, B.D. The international POTS registry: Evaluating the efficacy of an exercise training intervention in a community setting. Heart Rhythm 2016, 13, 943–950.
- van Lieshout, J.J.; ten Harkel, A.D.; Wieling, W. Physical manoeuvres for combating orthostatic dizziness in autonomic failure. Lancet 1992, 339, 897–898.
- Krediet, C.T.; van Lieshout, J.J.; Bogert, L.W.; Immink, R.V.; Kim, Y.S.; Wieling, W. Leg crossing improves orthostatic tolerance in healthy subjects: A placebo-controlled crossover study. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1768–H1772.
- Stewart, J.M.; Medow, M.S.; Montgomery, L.D.; McLeod, K. Decreased skeletal muscle pump activity in patients with postural tachycardia syndrome and low peripheral blood flow. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1216–H1222.
- Sato, Y.; Ichihashi, K.; Kikuchi, Y.; Shiraishi, H.; Momoi, M.Y. Autonomic function in adolescents with orthostatic dysregulation measured by heart rate variability. Hypertens. Res. 2007, 30, 601–605.
- Shenthar, J.; Gangwar, R.S.; Banavalikar, B.; Benditt, D.G.; Lakkireddy, D.; Padmanabhan, D. A randomized study of yoga therapy for the prevention of recurrent reflex vasovagal syncope. Europace 2021, 23, 1479–1486.
- Lu, W.; Yan, H.; Wu, S.; Chen, S.; Xu, W.; Jin, H.; Du, J. Electrocardiography-Derived Predictors for Therapeutic Response to Treatment in Children with Postural Tachycardia Syndrome. J. Pediatr. 2016, 176, 128–133.
- Miglis, M.G.; Muppidi, S.; Feakins, C.; Fong, L.; Prieto, T.; Jaradeh, S. Sleep disorders in patients with postural tachycardia syndrome. Clin. Auton. Res. 2016, 26, 67–73.
- Lin, J.; Han, Z.; Li, X.; Ochs, T.; Zhao, J.; Zhang, X.; Yang, J.; Liu, P.; Xiong, Z.; Gai, Y.; et al. Risk factors for postural tachycardia syndrome in children and adolescents. PLoS ONE 2014, 9, e113625.
- Lin, J.; Zhao, H.; Shen, J.; Jiao, F. Salivary Cortisol Levels Predict Therapeutic Response to a Sleep-Promoting Method in Children with Postural Tachycardia Syndrome. J. Pediatr. 2017, 191, 91–95.
- Fu, Q.; Shibata, S.; Hastings, J.L.; Prasad, A.; Palmer, M.D.; Levine, B.D. Evidence for unloading arterial baroreceptors during low levels of lower body negative pressure in humans. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H480–H488.
- Fu, Q.; VanGundy, T.B.; Galbreath, M.M.; Shibata, S.; Jain, M.; Hastings, J.L.; Bhella, P.S.; Levine, B.D. Cardiac origins of the postural orthostatic tachycardia syndrome. J. Am. Coll. Cardiol. 2010, 55, 2858–2868.
- Stewart, J.M.; Taneja, I.; Medow, M.S. Reduced central blood volume and cardiac output and increased vascular resistance during static handgrip exercise in postural tachycardia syndrome. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1908–H1917.
- Lu, C.-C.; Diedrich, A.; Tung, C.-S.; Paranjape, S.; Harris, P.A.; Byrne, D.W.; Jordan, J.; Robertson, D. Water ingestion as prophylaxis against syncope. Circulation 2003, 108, 2660–2665.
- Mathias, C.J.; Young, T.M. Water drinking in the management of orthostatic intolerance due to orthostatic hypotension, vasovagal syncope and the postural tachycardia syndrome. Eur. J. Neurol. 2004, 11, 613–619.
- Zhang, Q.; Liao, Y.; Tang, C.; Du, J.; Jin, H. Twenty-four-hour urinary sodium excretion and postural orthostatic tachycardia syndrome. J. Pediatr. 2012, 161, 281–284.
- Chen, G.; Du, J.; Jin, H.; Huang, Y. Postural Tachycardia Syndrome in Children and Adolescents: Pathophysiology and Clinical Management. Front. Pediatr. 2020, 8, 474.
- Stewart, J.M.; Boris, J.R.; Chelimsky, G.; Fischer, P.R.; Fortunato, J.E.; Grubb, B.P.; Heyer, G.L.; Jarjour, I.T.; Medow, M.S.; Numan, M.T.; et al. Pediatric Disorders of Orthostatic Intolerance. Pediatrics 2018, 141, e20171673.
- Stewart, J.M. A new guideline for diagnosis and treatment of syncope in children and adolescents that stimulates further thought and discussion. Sci. Bull. 2018, 63, 1527–1528.
- Lin, J.; Liu, P.; Wang, Y.; Li, H.; Li, X.; Zhao, J.; Tang, C.; Du, J.; Jin, H. Evaluation of the changes in heart rate during head-up test predicting the efficacy of oral rehydration salts on postural tachycardia syndrome in children. Zhonghua Er Ke Za Zhi 2015, 53, 25–29.
- Antonini-Canterin, F.; Di Nora, C.; Poli, S.; Sparacino, L.; Cosei, I.; Ravasel, A.; Popescu, A.C.; Popescu, B.A. Obesity, Cardiac Remodeling, and Metabolic Profile: Validation of a New Simple Index beyond Body Mass Index. J. Cardiovasc. Echogr. 2018, 28, 18–25.
- Bunsawat, K.; Lefferts, E.C.; Grigoriadis, G.; Wee, S.O.; Kilianek, M.M.; Fadel, P.J.; Clifford, P.S.; Fernhall, B.; Baynard, T. Central and Peripheral Postexercise Blood Pressure and Vascular Responses in Young Adults with Obesity. Med. Sci. Sports Exerc. 2021, 53, 994–1002.
- Stewart, J.M.; Taneja, I.; Medow, M.S. Reduced body mass index is associated with increased angiotensin II in young women with postural tachycardia syndrome. Clin. Sci. 2007, 113, 449–457.
- Li, H.; Wang, Y.; Liu, P.; Chen, Y.; Feng, X.; Tang, C.; Du, J.; Jin, H. Body Mass Index (BMI) is Associated with the Therapeutic Response to Oral Rehydration Solution in Children with Postural Tachycardia Syndrome. Pediatr. Cardiol. 2016, 37, 1313–1318.
- Mustafa, H.I.; Raj, S.R.; Diedrich, A.; Black, B.K.; Paranjape, S.Y.; Dupont, W.D.; Williams, G.H.; Biaggioni, I.; Robertson, D. Altered systemic hemodynamic and baroreflex response to angiotensin II in postural tachycardia syndrome. Circ. Arrhythm. Electrophysiol. 2012, 5, 173–180.
- Raj, S.R. Postural tachycardia syndrome (POTS). Circulation 2013, 127, 2336–2342.
- Harrington, F.; Murray, A.; Ford, G.A. Relationship of baroreflex sensitivity and blood pressure in an older population. J. Hypertens. 2000, 18, 1629–1633.
- Li, H.; Liao, Y.; Wang, Y.; Liu, P.; Sun, C.; Chen, Y.; Tang, C.; Jin, H.; Du, J. Baroreflex Sensitivity Predicts Short-Term Outcome of Postural Tachycardia Syndrome in Children. PLoS ONE 2016, 11, e0167525.
- Lin, C.J.; Chu, Y.K.; Chern, C.M. RBC volume deficiency in patients with excessive orthostatic decrease in cerebral blood flow velocity. J. Chin. Med. Assoc. 2014, 77, 174–178.
- Lu, W.; Yan, H.; Wu, S.; Xu, W.; Jin, H.; Du, J. Hemocytometric Measures Predict the Efficacy of Oral Rehydration for Children with Postural Tachycardia Syndrome. J. Pediatr. 2017, 187, 220–224.
- Zhang, F.W.; Liao, Y.; Li, X.Y.; Chen, L.; Jin, H.F.; DU, J. Therapies for postural tachycardia syndrome in children. Zhonghua Er Ke Za Zhi 2011, 49, 428–432.
- Zhang, F.; Li, X.; Ochs, T.; Chen, L.; Liao, Y.; Tang, C.; Jin, H.; Du, J. Midregional pro-adrenomedullin as a predictor for therapeutic response to midodrine hydrochloride in children with postural orthostatic tachycardia syndrome. J. Am. Coll. Cardiol. 2012, 60, 315–320.
- Stewart, J.M.; Montgomery, L.D. Regional blood volume and peripheral blood flow in postural tachycardia syndrome. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1319–H1327.
- Strieper, M.J.; Campbell, R.M. Efficacy of alpha-adrenergic agonist therapy for prevention of pediatric neurocardiogenic syncope. J. Am. Coll. Cardiol. 1993, 22, 594–597.
- Liao, Y.; Yang, J.; Zhang, F.; Chen, S.; Liu, X.; Zhang, Q.; Ai, Y.; Wang, Y.; Tang, C.; Du, J.; et al. Flow-mediated vasodilation as a predictor of therapeutic response to midodrine hydrochloride in children with postural orthostatic tachycardia syndrome. Am. J. Cardiol. 2013, 112, 816–820.
- Yang, J.; Zhao, J.; Du, S.; Liu, D.; Fu, C.; Li, X.; Chen, S.; Tang, C.; Du, J.; Jin, H. Postural orthostatic tachycardia syndrome with increased erythrocytic hydrogen sulfide and response to midodrine hydrochloride. J. Pediatr. 2013, 163, 1169–1173.
- Franklin, L.; Bauce, L.; Pittman, Q.J. Depletion of central catecholamines reduces pressor responses to arginine vasopressin. Brain Res. 1988, 438, 295–298.
- Deng, W.; Liu, Y.; Liu, A.D.; Holmberg, L.; Ochs, T.; Li, X.; Yang, J.; Tang, C.; Du, J.; Jin, H. Difference between supine and upright blood pressure associates to the efficacy of midodrine on postural orthostatic tachycardia syndrome (POTS) in children. Pediatr. Cardiol. 2014, 35, 719–725.
- Lamarre-Cliche, M.; du Souich, P.; de Champlain, J.; Larochelle, P. Pharmacokinetic and pharmacodynamic effects of midodrine on blood pressure, the autonomic nervous system, and plasma natriuretic peptides: A prospective, randomized, single-blind, two-period, crossover, placebo-controlled study. Clin. Ther. 2008, 30, 1629–1638.
- Preibisz, J.J.; Sealey, J.E.; Laragh, J.H.; Cody, R.J.; Weksler, B.B. Plasma and platelet vasopressin in essential hypertension and congestive heart failure. Hypertension 1983, 5, I129–I138.
- Barat, C.; Simpson, L.; Breslow, E. Properties of human vasopressin precursor constructs: Inefficient monomer folding in the absence of copeptin as a potential contributor to diabetes insipidus. Biochemistry 2004, 43, 8191–8203.
- Zhao, J.; Tang, C.; Jin, H.; Du, J. Plasma copeptin and therapeutic effectiveness of midodrine hydrochloride on postural tachycardia syndrome in children. J. Pediatr. 2014, 165, 290–294.
- Wyller, V.B.; Thaulow, E.; Amlie, J.P. Treatment of chronic fatigue and orthostatic intolerance with propranolol. J. Pediatr. 2007, 150, 654–655.
- Lai, C.C.; Fischer, P.R.; Brands, C.K.; Fisher, J.L.; Porter, C.-B.J.; Driscoll, S.W.; Graner, K.K. Outcomes in adolescents with postural orthostatic tachycardia syndrome treated with midodrine and beta-blockers. Pacing Clin. Electrophysiol. 2009, 32, 234–238.
- Ladage, D.; Schwinger, R.H.; Brixius, K. Cardio-selective beta-blocker: Pharmacological evidence and their influence on exercise capacity. Cardiovasc. Ther. 2013, 31, 76–83.
- Kimpinski, K.; Figueroa, J.J.; Singer, W.; Sletten, D.M.; Iodice, V.; Sandroni, P.; Fischer, P.R.; Opfer-Gehrking, T.L.; Gehrking, J.A.; Low, P.A. A prospective, 1-year follow-up study of postural tachycardia syndrome. Mayo Clin. Proc. 2012, 87, 746–752.
- Zhang, Q.; Chen, X.; Li, J.; Du, J. Orthostatic plasma norepinephrine level as a predictor for therapeutic response to metoprolol in children with postural tachycardia syndrome. J. Transl. Med. 2014, 12, 249.
- Szinnai, G.; Morgenthaler, N.G.; Berneis, K.; Struck, J.; Müller, B.; Keller, U.; Christ-Crain, M. Changes in plasma copeptin, the c-terminal portion of arginine vasopressin during water deprivation and excess in healthy subjects. J. Clin. Endocrinol. Metab. 2007, 92, 3973–3978.
- Zhao, J.; Du, S.; Yang, J.; Lin, J.; Tang, C.; Du, J.; Jin, H. Usefulness of plasma copeptin as a biomarker to predict the therapeutic effectiveness of metoprolol for postural tachycardia syndrome in children. Am. J. Cardiol. 2014, 114, 601–605.
- Lin, J.; Han, Z.; Li, H.; Chen, S.; Li, X.; Liu, P.; Wang, Y.; Tang, C.; Du, J.; Jin, H. Plasma C-type natriuretic peptide as a predictor for therapeutic response to metoprolol in children with postural tachycardia syndrome. PLoS ONE 2015, 10, e0121913.
- Wang, Y.; Zhang, C.; Chen, S.; Liu, P.; Wang, Y.; Tang, C.; Jin, H.; Du, J. Heart Rate Variability Predicts Therapeutic Response to Metoprolol in Children With Postural Tachycardia Syndrome. Front. Neurosci. 2019, 13, 1214.
- Bateman, R.J.; Boychuk, C.R.; Philbin, K.E.; Mendelowitz, D. β adrenergic receptor modulation of neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Neuroscience 2012, 210, 58–66.
- Deng, X.; Zhang, Y.; Liao, Y.; Du, J. Efficacy of β-Blockers on Postural Tachycardia Syndrome in Children and Adolescents: A Systematic Review and Meta-Analysis. Front. Pediatr. 2019, 7, 460.
- Wang, S.; Zou, R.; Cai, H.; Wang, Y.; Ding, Y.; Tan, C.; Yang, M.; Li, F.; Wang, C. Heart Rate and Heart Rate Difference Predicted the Efficacy of Metoprolol on Postural Tachycardia Syndrome in Children and Adolescents. J. Pediatr. 2020, 224, 110–114.
- Nair, N.; Padder, F.A.; Kantharia, B.K. Pathophysiology and management of neurocardiogenic syncope. Am. J. Manag. Care 2003, 9, 327–336.
- Santini, L.; Capria, A.; Brusca, V.; Violo, A.; Smurra, F.; Scarfò, I.; Forleo, G.B.; Papavasileiou, L.P.; Borzi, M.; Romeo, F. An increased endothelial-independent vasodilation is the hallmark of the neurally mediated syncope. Clin. Cardiol. 2012, 35, 107–110.
- Di Girolamo, E.; Di Iorio, C.; Leonzio, L.; Sabatini, P.; Barsotti, A. Usefulness of a tilt training program for the prevention of refractory neurocardiogenic syncope in adolescents: A controlled study. Circulation 1999, 100, 1798–1801.
- Sundkvist, G.; Lilja, B.; Manhem, P.; Almér, L.O. Responses of plasma catecholamines to tilt in patients with diabetes mellitus. Acta Med. Scand. 1984, 216, 223–227.
- Tao, C.; Li, X.; Tang, C.; Jin, H.; Du, J. Acceleration Index Predicts Efficacy of Orthostatic Training on Vasovagal Syncope in Children. J. Pediatr. 2019, 207, 54–58.
- Stewart, J.M. Orthostatic intolerance in pediatrics. J. Pediatr. 2002, 140, 404–411.
- Low, P.A.; Gilden, J.L.; Freeman, R.; Sheng, K.N.; McElligott, M.A. Efficacy of midodrine vs. placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA 1997, 277, 1046–1051.
- Samniah, N.; Sakaguchi, S.; Lurie, K.G.; Iskos, D.; Benditt, D.G. Efficacy and safety of midodrine hydrochloride in patients with refractory vasovagal syncope. Am. J. Cardiol. 2001, 88, 80–83.
- Kaufmann, H.; Saadia, D.; Voustianiouk, A. Midodrine in neurally mediated syncope: A double-blind, randomized, crossover study. Ann. Neurol. 2002, 52, 342–345.
- Galetta, F.; Franzoni, F.; Plantinga, Y.; Ghiadoni, L.; Merico, G.; Tocchini, L.; Braccini, L.; Rossi, M.; Carpi, A.; Taddei, S.; et al. Endothelial function in young subjects with vaso-vagal syncope. Biomed. Pharmacother. 2006, 60, 448–452.
- Zhang, F.; Liao, Y.; Li, X.; Chen, L.; Jin, H.; Du, J. The predictive value of flow-mediated vasodilation on therapeutic efficacy of midodrine hydrochloride for vasovagal syncope in children. Zhongguo Shi Yong Er Ke Za Zhi 2012, 27, 102–105.
- Chen, L.Y.; Shen, W.K. Neurocardiogenic syncope: Latest pharmacological therapies. Expert Opin. Pharmacother. 2006, 7, 1151–1162.
- Brignole, M.; Menozzi, C.; Gianfranchi, L.; Lolli, G.; Bottoni, N.; Oddone, D. A controlled trial of acute and long-term medical therapy in tilt-induced neurally mediated syncope. Am. J. Cardiol. 1992, 70, 339–342.
- Sheldon, R.; Rose, S.; Flanagan, P.; Koshman, M.L.; Killam, S. Effect of beta blockers on the time to first syncope recurrence in patients after a positive isoproterenol tilt table test. Am. J. Cardiol. 1996, 78, 536–539.
- Di Girolamo, E.; Di Iorio, C.; Sabatini, P.; Leonzio, L.; Barsotti, A. Evaluation of the effects of diverse therapeutic treatments versus no treatment of patients with neurocardiogenic syncope. Cardiologia 1998, 43, 833–837.
- Madrid, A.H.; Ortega, J.; Rebollo, J.G.; Manzano, J.G.; Segovia, J.G.; Sánchez, A.; Peña, G.; Moro, C. Lack of efficacy of atenolol for the prevention of neurally mediated syncope in a highly symptomatic population: A prospective, double-blind, randomized and placebo-controlled study. J. Am. Coll. Cardiol. 2001, 37, 554–559.
- Ventura, R.; Maas, R.; Zeidler, D.; Schoder, V.; Nienaber, C.A.; Schuchert, A.; Meinertz, T. A randomized and controlled pilot trial of beta-blockers for the treatment of recurrent syncope in patients with a positive or negative response to head-up tilt test. Pacing Clin. Electrophysiol. 2002, 25, 816–821.
- Flevari, P.; Livanis, E.G.; Theodorakis, G.N.; Zarvalis, E.; Mesiskli, T.; Kremastinos, D.T. Vasovagal syncope: A prospective, randomized, crossover evaluation of the effect of propranolol, nadolol and placebo on syncope recurrence and patients’ well-being. J. Am. Coll. Cardiol. 2002, 40, 499–504.
- Sheldon, R. The Prevention of Syncope Trial (POST) Results. In Proceedings of the 25th Annual Scientific Sessions, San Francisco, CA, USA, 19–22 May 2004.
- Chen, L.; Du, J.-B.; Zhang, Q.-Y.; Wang, C.; Du, Z.-D.; Wang, H.-W.; Tian, H.; Chen, J.-J.; Wang, Y.-L.; Hu, X.-F.; et al. A multicenter study on treatment of autonomous nerve-mediated syncope in children with beta-receptor blocker. Zhonghua Er Ke Za Zhi 2007, 45, 885–888.
- Kuchinskaia, E.A.; Pevzner, A.V.; Vershuta, E.V.; Al’Bitskaia, K.V.; Kheĭmets, G.I.; Rogoza, A.; Golitsyn, S.P. Comparative efficacy and tolerance of atenolol and midodrine in patients with vasovagal syncopes. Ter. Arkh. 2006, 78, 64–68.
- Zhang, Q.; JB, D.; Zhen, J.; Li, W.; Wang, Y. Hemodynamic changes during head-up tilt test and predictive value thereof in predicting the efficacy of metoprolol therapy in children with vasovagal syncope. Zhonghua Yi Xue Za Zhi 2007, 87, 1260–1262.
- Mitro, P.; Rybárová, E.; Zemberová, E.; Tkác, I. Enhanced plasma catecholamine and cAMP response during the head-up tilt test in patients with vasovagal syncope. Wien. Klin. Wochenschr. 2005, 117, 353–358.
- Edner, M.; Brodin, L.; Al-Khalili, F.; Svane, B.; Moor, E.; Ståhle, A.; Nordlander, R. Changes in Systolic and Diastolic Function Indexes Throughout Dobutamine Stress Echocardiography in Healthy Volunteers and Patients with Ischemic Heart Disease. Echocardiography 1998, 15, 625–634.
- Song, J.; Li, H.; Wang, Y.; Liu, P.; Li, X.; Tang, C.; Jin, H.; Du, J. Left Ventricular Ejection Fraction and Fractional Shortening are Useful for the Prediction of the Therapeutic Response to Metoprolol in Children with Vasovagal Syncope. Pediatr. Cardiol. 2018, 39, 1366–1372.
- Yao, R.; Huang, J. Pathophysiology and treatment of vasovagal syncope. Adv. Cardiovasc. Dis. 2013, 34, 221–224.
- Zhang, Q.; Jin, H.; Wang, L.; Chen, J.; Tang, C.; Du, J. Rand.domized comparison of metoprolol versus conventional treatment in preventing recurrence of vasovagal syncope in children and adolescents. Med. Sci. Monit. 2008, 14, CR199–CR203.
- Wang, Y.; Wang, Y.; Li, X.; Du, J.; Zhang, H.; Jin, H.; Liao, Y. Efficacy of Increased Salt and Water Intake on Pediatric Vasovagal Syncope: A Meta-Analysis Based on Global Published Data. Front. Pediatr. 2021, 9, 663016.
- Tao, C.; Li, X.; Tang, C.; Jin, H.; Du, J. Baroreflex Sensitivity Predicts Response to Metoprolol in Children With Vasovagal Syncope: A Pilot Study. Front. Neurosci. 2019, 13, 1329.
- White, C.M.; Tsikouris, J.P. A review of pathophysiology and therapy of patients with vasovagal syncope. Pharmacotherapy 2000, 20, 158–165.
- Li, H.; Liao, Y.; Han, Z.; Wang, Y.; Liu, P.; Zhang, C.; Tang, C.; Du, J.; Jin, H. Head-up tilt test provokes dynamic alterations in total peripheral resistance and cardiac output in children with vasovagal syncope. Acta Paediatr. 2018, 107, 1786–1791.
- Vigh, A.G.; DeAntonio, H.J. Acute and long-term beta-adrenergic blockade for patients with neurocardiogenic syncope. J. Am. Coll. Cardiol. 1996, 28, 798.
- Sheldon, R.; Connolly, S.; Rose, S.; Klingenheben, T.; Krahn, A.; Morillo, C.; Talajic, M.; Ku, T.; Fouad-Tarazi, F.; Ritchie, D.; et al. Prevention of Syncope Trial (POST): A randomized, placebo-controlled study of metoprolol in the prevention of vasovagal syncope. Circulation 2006, 113, 1164–1170.
- Kong, Q.; Yang, X.; Cai, Z.; Pan, Y.; Wang, M.; Liu, M.; Zhao, C. Twenty-four-hour urine NE level as a predictor of the therapeutic response to metoprolol in children with recurrent vasovagal syncope. Ir. J. Med. Sci. 2019, 188, 1279–1287.




