In November of 2021, a recently evolved variant of SARS-CoV-2, omicron, was discovered. In just one month, omicron has spread to more than 89 countries resulting in a rapid rise in cases and a new wave of infections. With over 46 mutations, omicron brings concern to the public health and may be able to infect at a greater capacity than previous strains. Although able to infect double vaccinated and previously infected individuals, the booster vaccine may prove promising. However, more research is needed to fully elucidate the key function of each mutation and to better develop effective drugs. Marine resources may be a promising drug discovery avenue to investigate. Through viral entry blockade and preventing viral replication and protein synthesis, metabolites produced from marine organisms may be promising against the evolving SARS-CoV-2.
1. Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) first emerged almost two years ago and has been ongoing since
[1]. It is without a doubt that the SARS-CoV-2 pandemic has led to frustration and prevalent negative effects on the mental wellbeing of individuals as well as the economy
[2][3][4]. Vaccinations were effective at first, however, the solution was temporary, and the single-stranded SARS-CoV-2 virus is continuously evolving through mutations in key domains
[5][6][7][8]. Several SARS-CoV-2 variants including the alpha, beta and delta strains were associated with new waves of COVID-19 infection
[9][10]. The delta variant was shown to have a higher transmissible and infectious risk than other strains with reports of having a higher viral load and mutations granting a better immune response escape
[11][12][13].
On 24 November 2021, a new SARS-CoV-2 variant, known as omicron (B.1.1.529) was reported to World Health Organization (WHO)
[14]. The omicron variant was first reported on 11 November 2021, in Botswana and just a few days later, was identified in South Africa
[15][16]. To date (January 2022), omicron has been reported in more than 57 countries, with South Korea reporting its highest number of COVID-19 cases in December 2021, since the start of the pandemic
[17]. It has not been elucidated whether the transmissibility and severity status of the omicron variant is greater than the previous strains
[18][19]. Over 45 mutations and several gene deletions have been identified, some in the same genes as the previous variants and others being novel (
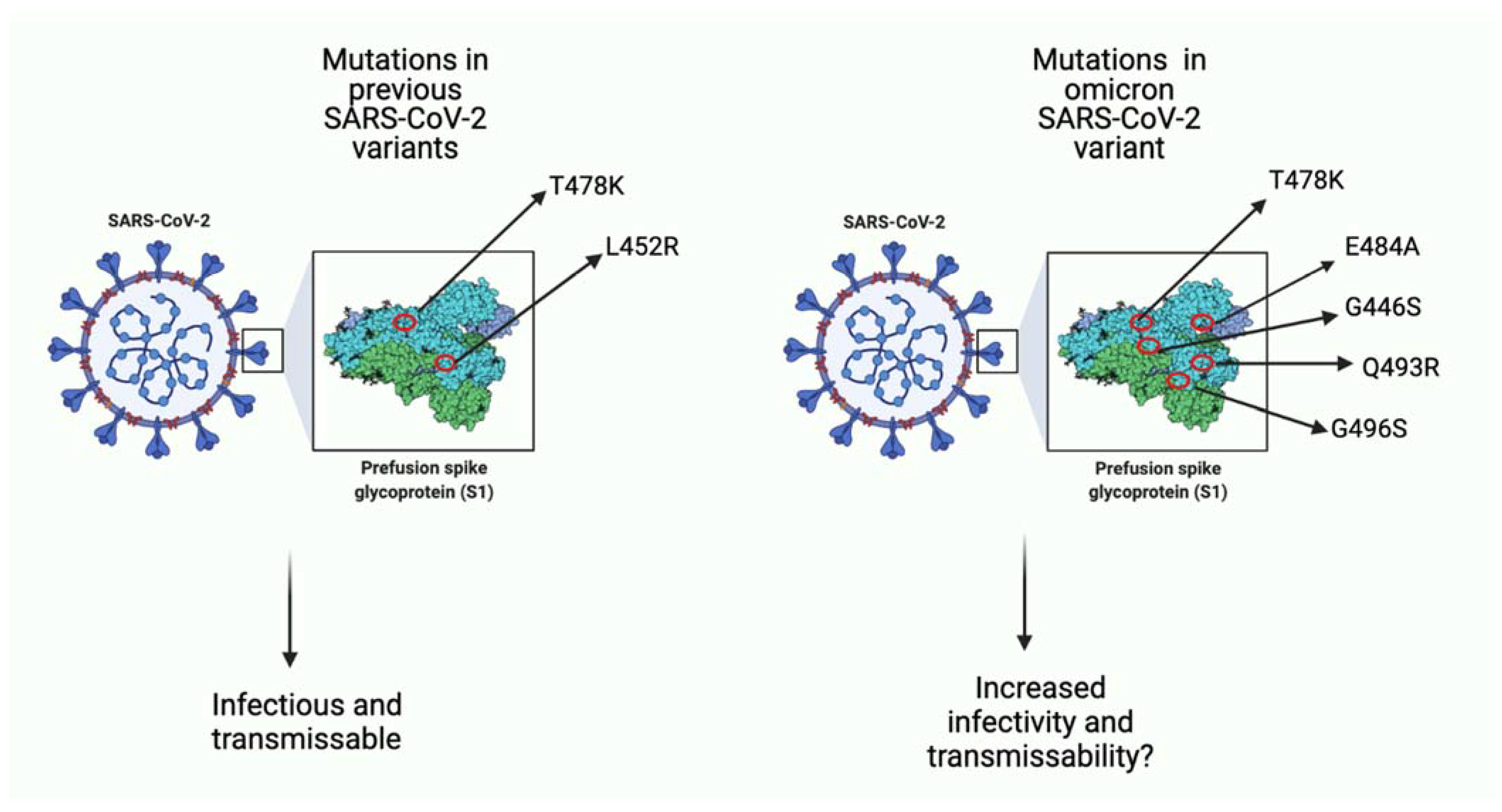
Figure 1)
[20]. Together, these mutations in key regions give the omicron strain the ability to escape the host immune system, rendering vaccinations less effective
[20][21]. Furthermore, it is not clear yet what the effects of several mutations are, and this serves as a barrier for developing new vaccinations or drugs for prevention and treatment of omicron viral infection
[22][23].
Figure 1. A comparison of few key missense mutations in the spike protein of previous SARS-CoV-2 variants and the omicron variant. As seen in the image on the left, previous variants (i.e., delta variant) had few mutations that favored viral infection. Seen on the right, omicron has more mutations, many of which function to increase binding affinity to host ACE-2 receptor and potentially increase infectivity ability as well as transmissibility of viral particles. Some mutations such as T478K overlap with previous variants, and some (i.e., E484A) are unique to omicron. (Created with
BioRender.com (accessed on 19 December 2021)).
Previous variants have shown to lower the efficacy of vaccinations. For example, the efficacy of the AstraZeneca ChAdOx1 vaccine decreased to 10% in South Africa because of the beta SARS-CoV-2 variant
[19]. The re-purposing of drugs used for anti-bacterial infections or other anti-viral infections may be a promising avenue to investigate. Marine resources have a variety of molecules that have shown to have anti-viral abilities and their repurposing for treatment against omicron may be promising
[24][25][26]. Specifically, inorganic polyphosphate has been shown to inhibit viral life cycle at several stages by blocking viral particle spike protein binding to host ACE-2 receptor and inhibiting viral protein synthesis once inside the cell
[26][27]. Similarly, lambda-carrageenan isolated from marine algae have also been shown to inhibit viral protein transcription and translation
[28]. Moreover, bromotyrosines have also been shown to have anti-bacterial and anti-viral activity, and have recently been isolated and purified for further research
[24][29]. Although some challenges are ahead such as large-scale production and more in vivo studies necessary for further development, more research is warranted to determine the full potential of marine metabolites against SARS-CoV-2 variants.
2. The Potential for Prevention/Treatment of SARS-CoV-2 Variants Using Marine Resources
Considering the rapid rise in cases as well as the continuously evolving SARS-CoV-2 virus, there needs to be more effective vaccinations and drugs to prevent and treat not only the omicron variant but other future variants as well. One such drug discovery avenue to investigate is marine resources
[30][31][32]. Marine resources harbor significant activity against cancer, viruses and bacteria, thus their ability to prevent or treat omicron viral infection should be investigated
[29][33][34][35][36]. In fact, marine resources, which can be isolated from invertebrates and cultivated under farming conditions (i.e., sponges)
[37][38], are highly advantageous over synthetic compounds as they lack religious constraints, have little to no toxins, are environmentally friendly and are metabolically compatible
[24]. Furthermore, marine compounds isolated from a single organism can often combat several stages of the viral life cycle, including both viral entry and replication
[39] (
Figure 2).
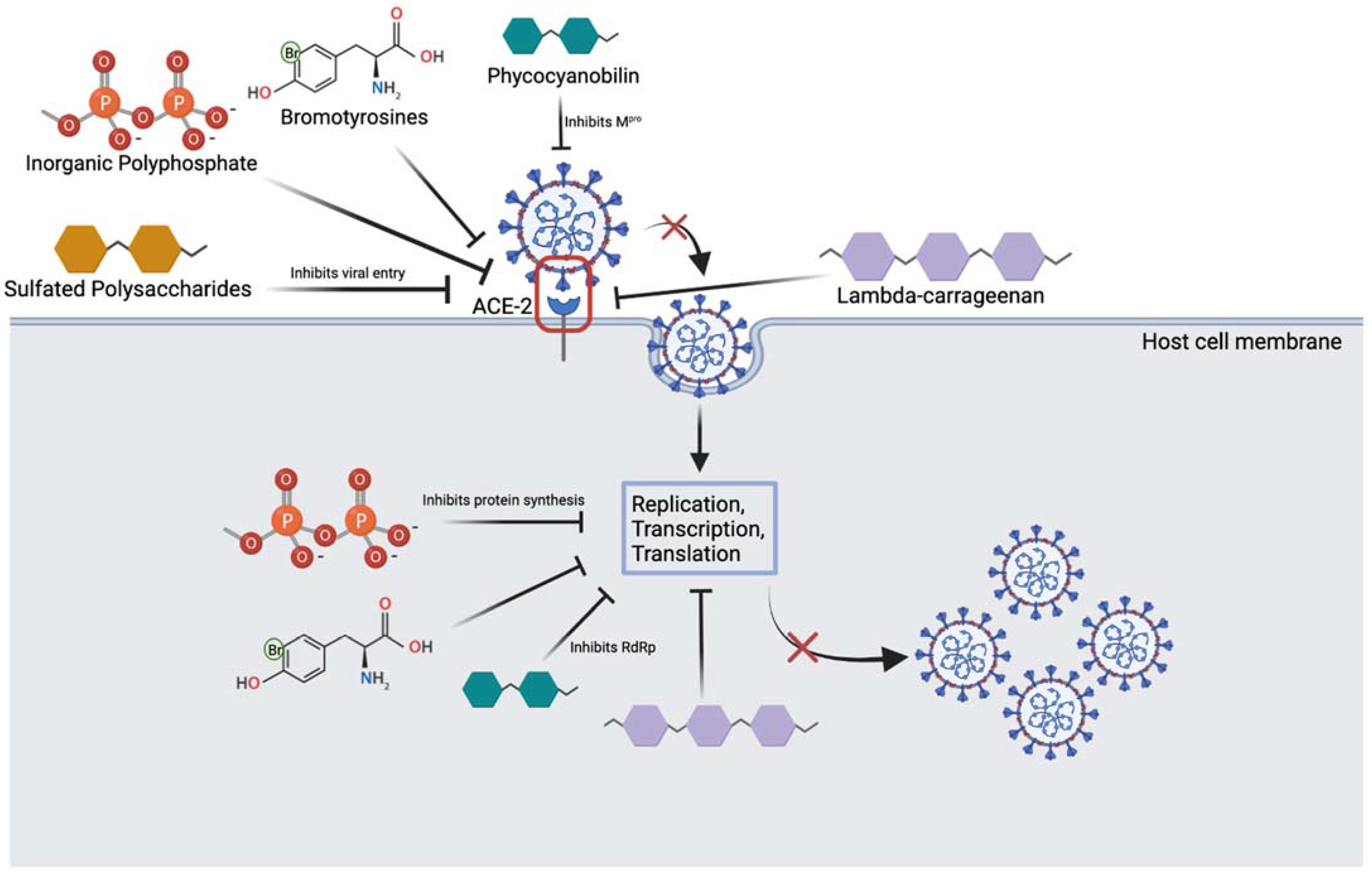
Figure 2. Overview of marine compounds interfering with the SARS-CoV-2 viral life cycle. Image depicts several marine substances that can inhibit various steps of the life cycle which may be relevant to treatment. (Created with
BioRender.com (accessed on 25 March 2022)).
Inorganic polyphosphate (polyP) are abundantly found in marine bacteria and sponges and have been shown to bind RBD of SARS-CoV-2 and prevent ACE-2 binding
[27]. Several phosphate units of polyP are thought to interact with the spike protein through basic residues like Arginine, Lysine and Histidine
[26][27]. Neuforth et al. found that polyP was able to inhibit the spike protein and ACE-2 interaction dose-dependently (up to 100 μg/mL)
[26]. This inhibition was found to be 70% effective and during the 24 h incubation period, 100 μg/mL of polyP had no toxic effect on the cells. In addition, polyP is versatile in that it has also been shown to stimulate the innate antiviral immune system, by upregulating the mucin gene, MUC1
[26][27][36]. By boosting the innate immune response, polyP may serve a protective role as immunity plays an important role in the onset of COVID-19 symptoms and infection in patients. To add, Ferrucci et al. found that a long chain polyP is able to impair replication and transcription of viral particles, and this prevents the synthesis of viral proteins
[26]. The study also found that polyP120 is able to bind to and downregulate the ACE-2 receptor by resulting in its degradation. It is clear that polyP may play a potential role in preventing SARS-CoV-2 infection, however more studies investigating whether polyP interacts with spike protein on conserved or mutated residues is warranted to elucidate its usefulness against the omicron variant. This is important as the omicron variant differs from previous SARS-CoV-2 strains due to mutations in the spike protein, half of which are in the RBD. Thus, targeting stages in the latter portion of the viral life cycle, boosting the innate immune system or targeting the ACE-2 receptor interaction may together serve as competent methods to inhibit viral infection.
Moreover, lambda-carrageenan is a polysaccharide isolated from red algae and has been shown to inhibit influenza virus and SARS-CoV-2 viral replication dose-dependently
[24][28]. Studies have found that like polyP, lambda-carrageenan is able to inhibit both viral entry and viral protein synthesis, by blocking transcription and translation of proteins. For example, in a study done by Jang et. al, SARS-CoV-2 viral protein and replication was suppressed dose-dependently in both Influenza A and SARS-CoV-2 viruses after lambda-carrageenan administration
[28]. These multi-mechanisms of action likely serve beneficial not only for omicron variant treatment and prevention, but future and more aggressive variants as well. Another molecule of interest that may be redirected for different strains of SARS-CoV-2 viruses includes the pigment compounds phycocyanobilins (PCBs)
[40]. In addition to their antioxidant properties, marine PCBs have been shown to inhibit a key conserved enzyme of the virus, main protease (M
pro)
[41]. PCBs can also interfere with RNA dependant RNA polymerase (RdRp) of SARS-CoV-2
[40]. These findings are important as they are conserved enzymes among the various viral strains, and the design of drugs that can directly act on these molecules may serve as promising treatments. Similar to the above compounds, sulfated polysaccharides isolated from cyanobacteria and marine algae have been shown to bind to the SARS-CoV-2 viral spike protein and inhibit entry into cells
[42]. One sulfated polysaccharide, iota-carrageenan, has been previously demonstrated to be effective against several respiratory virsuses, in vitro
[43]. A recent study by Morokutti-Kurz et al. found that this molecule was able to inhibit the entry of a SARS-CoV-2 psudeotyped lentivirus dose-dependently with an IC50 of 2.6 µg/mL
[44]. This is thought to be occurring through formation of a sulfated polysaccharide-ACE-2 complex which prevents the binding of the spike protein, and through the anionic sulfate groups interacting with the spike protein residues preventing ACE-2 interaction and thus, viral entry
[44].
To add, marine sponges have been shown to rapidly filter out viruses and have highly specialized defense mechanisms against viral particles. One promising compound, bromotyrosine, is produced by the marine demosponges of Verongiida order in specialized spherulocyte cells and has been shown to have promising anti-viral effects
[29][45][46][47]. These cells are sensitive to environmental stimuli and release bromotyrosine in response to damage. Muzychka et al. have recently isolated and purified a bromotyrosine derivative, 3,5-dibromoquinolacetic acid and found to have anti-bacterial capabilities
[29]. Bromotyrosines have been shown to also have antiviral activity against HIV-1 retrovirus by inhibiting its entry into host cells as well as its replication inside the cell. Bromotyrosines are promising compounds to investigate for SARS-CoV-2 infection blockade. More research is needed to understand if bromotyrosines can target conserved residues on the spike protein, and whether they can inhibit viral proliferation once inside the host cell. Both bacterial and COVID-like viruses contain a lipid wall and cell adhesive proteins on their surface. While current drug development aims at targeting their surface proteins, their constant mutation significantly limits drug efficacy. The lipid wall or capsule is conserved in structure and does not mutate as frequently; this makes it a promising drug target. Researchers suggest that bromotyrosines act through lipid surface of both bacterial and viruses protecting the sponges from such pathogens
[29]. If so, bromotyrosines should be effective against any kind of viruses, including omicron.
Promisingly, a recent study by Bharathi et al. identified natural bioactive compounds isolated from various marine seaweed species, as inhibitors of the omicron variant
[48]. Molecular docking analysis showed that two compounds, caffeic acid hexoside and phloretin isolated from
Sargassum wightii were able to inhibit important residues necessary for ACE-2 interaction (ASN417, SER496, TYR501, and HIS505)
[48]. This is significant as these 4 residues in the RBD of the omicron spike protein were found to most strongly bind ACE2 receptor. Phloretin has also been shown to activate transcription factors that can upregulate the expression of antioxidant enzymes like superoxide dismutase and glutathione peroxidase
[49]. These enzymes play an important role in reducing oxidative stress and inflammation in SARS-CoV-2 infected patients suggesting it has promising effects. Furthermore, several other compounds including cholestan-3-ol and 2-methylene from the
Corallina officinalis were able to inhibit the novel mutated residues, LEU452 and ALA484 on the RBD of the spike protein
[48]. This inhibition was found to be through hydrogen bonding and alkyl interactions. All together, it is clear that several seaweed compounds as well as compounds isolated from other marine organisms may be effective against omicron infection, however future experimental studies are warranted to support these findings (
Table 1).