Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xinyu Song | + 2801 word(s) | 2801 | 2022-06-21 08:50:17 | | | |
| 2 | Conner Chen | -25 word(s) | 2776 | 2022-06-21 09:43:52 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Song, X.; Liu, P.; Yu, L.; Zille, A. Antimicrobial Masks as Personal protective equipment. Encyclopedia. Available online: https://encyclopedia.pub/entry/24252 (accessed on 08 February 2026).
Song X, Liu P, Yu L, Zille A. Antimicrobial Masks as Personal protective equipment. Encyclopedia. Available at: https://encyclopedia.pub/entry/24252. Accessed February 08, 2026.
Song, Xinyu, Pengyan Liu, Liangmin Yu, Andrea Zille. "Antimicrobial Masks as Personal protective equipment" Encyclopedia, https://encyclopedia.pub/entry/24252 (accessed February 08, 2026).
Song, X., Liu, P., Yu, L., & Zille, A. (2022, June 21). Antimicrobial Masks as Personal protective equipment. In Encyclopedia. https://encyclopedia.pub/entry/24252
Song, Xinyu, et al. "Antimicrobial Masks as Personal protective equipment." Encyclopedia. Web. 21 June, 2022.
Copy Citation
Shortage of personal protective equipment (PPE) is often projected in response to public health emergencies such as infection outbreaks and pandemics. Respiratory protective devices (RPDs), namely medical face masks and respirators, are considered the last defence for the front-line healthcare workers. To contribute to the mitigation of RPDs shortage, new technology such as antimicrobial treated PPE that can reduce the risks of fomite during the donning and doffing process with an extended lifespan gets increasingly prevalent.
respiratory protective devices
filtering facepiece respirators
medical face masks
antimicrobial coating
photocatalytic and photothermal
nanoparticles
1. Introduction
Personal protective equipment, commonly referred to as PPE, is defined by the Occupational Safety and Health Administration (OSHA) as equipment worn to minimize exposure to a variety of hazards [1]. Many guidelines have been issued to advise the practical use of PPE in various work professionals [2][3][4]. In the Hierarchy of Hazard Controls, PPE is considered an option of the last resort, providing a barrier to prevent work injuries from potentially hazardous work environments [5]. PPE type varies and includes head and scalp protection, respiratory protection, eye protection, hearing protection, hand and arm protection, foot and leg protection, body protection, and height and access protection [6][7]. PPE has been commonly used in healthcare settings, such as at surgical sites and in infection outbreaks [8][9]. Lately, the growing pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has placed healthcare workers (HCWs) at great risk of coronavirus disease 2019 (COVID-19) infection [10]. The use of PPE in healthcare and community settings plays a crucial role in preventing the transmission of SARS-CoV-2 during COVID-19 patient care [11]. World Health Organisation (WHO) has recommended the use of PPE, including gloves, medical masks, goggles or a face shield and gowns, as well as for specific procedures, respirators (i.e., N95 or FFP2 standard or equivalent) and aprons in different activities involving contact with COIVD-19 patients [11].
In the context of PPE for infection control, respiratory protective devices (RPDs) undertake as part of the package for personal protection [12]. It is also considered the last line of non-invasive defence for HCWs against respiratory infection transmission [13][14]. RPDs can be divided into medical masks and respirators according to their qualifications and functions. Medical masks are also known as surgical masks or face masks termed by U.S. Food and Drug Administration (FDA), or medical face masks termed by European Committee for Standardization (CEN) Technical Committee (TC) 205. In general, three nonwoven layers compose the most commonly used medical face masks. The middle layer is usually fabricated by the nonwoven meltblown process featuring electrostatic charging, and the two outer layers are usually fabricated by the nonwoven spunbond process [15]. Medical masks were originally designed to be used in the operation room, avoiding contaminants generated by the wearer on the wound and meanwhile preventing blood or other potentially infectious agents from reaching the wearer’s skin, mouth or mucous membranes (by splashes) [16].
2. Antimicrobial Masks
In the view of PPE shortage, several studies have demonstrated strategies of incorporating functional agents (antimicrobial agents, superhydrophobic materials, electrical chargers, etc.) on masks and respirators in vitro that can reduce the risks of self-inoculation during doffing of the equipment [17][18][19][20]. To summarise, the mode of action of antimicrobial masks can be categorized into the followings: (1) superhydrophobic surface treatment avoiding pathogens’ adhesion and allowing mask self-cleaning; (2) incorporating antimicrobial agents achieving self-disinfectant features (chemical, photodynamic or photothermal antimicrobial actions); (3) extending the electrostatic charges of masks for sustained and improved pathogens filtration efficacy [21]. Among all, the second type of mode of action is the most employed one in the current research.
Antimicrobial agents such as quaternary ammonium compounds (QACs), metal ions (in the form of nanoparticles or nanowire, or nanorods) and natural plant extracts have been frequently utilized for the development of antimicrobial masks, exerting antibacterial or antiviral actions [22][23]. QACs are a common type of antimicrobial agent with broad-spectrum applications, which have been commonly applied in textile materials, including polyethylene terephthalate (PET), cellulose, polyamide, polypropylene (PP), etc. [24]. However, their low stability, non-adhesion or weak attachment on the substrate surface and potential toxicity result in decreased antimicrobial performance and wide application in practice [25]. In the study of Tuñón-Molina et al., a type of PET transparent mask coated with benzalkonium chloride (BAK) was prepared, which enabled to inactivate enveloped viruses (e.g., the phage phi 6 and severe acute respiratory syndrome coronavirus 2) in less than a minute of contact time [26]. The potent antimicrobial activity of the PET sample against viruses and bacteria was attributed to the positively charged nitrogen atoms of BAK, which can destroy the phospholipid bilayer, glycoprotein envelope and spike glycoprotein of the virus or destroy the bacterial membranes. The PET with BAK coating simple also showed antibacterial ability against MRSA and MRSE with inhibition zones of 0.61 ± 0.03 and 0.57 ± 0.05, respectively. It is unfortunate that the study did not investigate the safety issue regarding the use of these BAK-coated masks in practice, such as the potential risks of respiratory exposure to this toxic compound. Similarly, Martí et al. developed the BAK bio-functional coating on a nonwoven mask filter by the dip-coating method, which exhibited >99% of SARS-CoV-2 particles reduction in just one-minute treatment [27]. In the study of Kumaran et al., terpyridine methylammonium chloride (TMAC) and adenine hexyl ammonium chloride (AHAC) were conjugated with lignin respectively to synthesize lignin 2,2′,4′-terpyridine methylammonium chloride (LTMAC) and lignin adenine hexyl ammonium chloride (LAHAC) and cross-linked to form a permanent antimicrobial coating on the surface of the face masks in the form of spray or infiltration (Figure 1(Ai)) [28]. In the evaluation of the antiviral ability of LTMAC and LAHAC-coated PP face mask, human coronaviruses (alpha coronavirus: HCoV-229E and beta coronavirus: HCoV-OC43) were inactivated in 5 min and achieved 3–6 log reduction in 30 min (Figure 1(Aii–Av)). In addition, the LTMAC- and LAHAC-coated face masks significantly killed K. pneumoniae both in the medium composition of distilled water and artificial saliva and in the transmission modes of droplets and aerosols, which also showed a consistently time-dependent bacterial inactivation. However, the stability of the polymer coating on the mask surface needs to be verified.
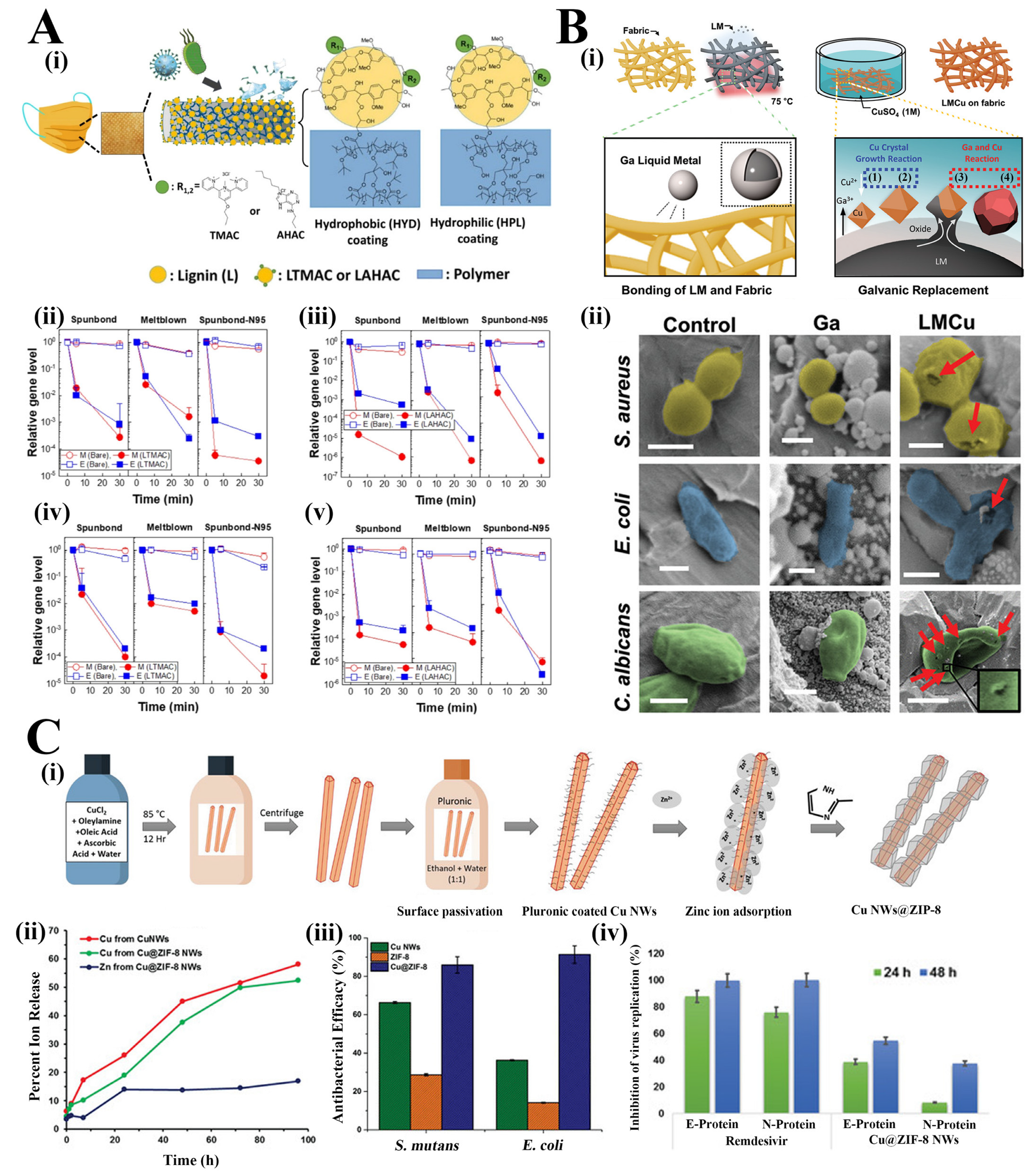
Figure 1. (Ai). Schematic illustration of the UV cross-linked coating on the surface of the face mask. Gene expression level change of HCoV-229E (Aii,Aiii) and HCoV-OC43 (Aiv,Av) on lignin-based, LTMAC- (Aii,Aiv) and LAHAC- (Aiii,Av) coated face mask fabrics (spunbond: outer layer of SM, meltblown: middle layer of SM and spunbond-N95: outer layer of N95) (n = 3, mean ± SD) [28]. (Bi). Schematic illustration of the gallium-copper (LMCu) coatings on fabric by galvanic replacement. (Bii). False coloured SEM images of S. aureus, E. coli and C. albicans cells on the control, Ga coated, and LMCu coated fabric (scale bars: 500 nm for S. aureus and E. coli and 2 µm for C. albicans): red arrows indicated the physically damaged and perforated cells [20]. (Ci). Schematic illustration of the synthesis of core-shell Cu@ZIF-8 NWs. (Cii). Release profile of Cu2+ and Zn2+ from the Cu NWs and Cu@ZIF-8 NWs in cell media (n = 3. p < 0.05). (Ciii). Antimicrobial efficacy (percent reduction at OD 600 nm after 26 h) of Cu NWs, ZIF-8 and Cu@ZIF-8 NWs against S. mutans and E. coli at concentrations of 375 µg·mL−1. (Civ). Antiviral effects (percentage reduction) of Cu@ZIF-8 NWs and Remdesivir at 24 h and 48 h post infection [29].
Metal ions in the form of nanoparticles or nanowires or nanorods have been frequently utilized for the development of antimicrobial masks against various bacteria, fungi and viruses [22][30]. However, the weak coating adhesion between the metal particles (e.g., CuNPs, AgNPs, ZnNPs) and the textile substrate is the key issue hindering their further application in antimicrobial masks. In the study of Kwon et al., gallium liquid metal (LM) particles were used to increase the adhesion between liquid metal copper alloy (LMCu) particles and the fabric, which meanwhile achieved the reduction of Cu ions into metallic Cu by galvanic replacement (Figure 1(Bi)) [20]. After 20 min interaction between bacteria/fungi and LMCu coated fabric, significant cell death was observed (S. aureus (96.8 ± 4%), E. coli (99.7 ± 1%), and C. albicans (97.6 ± 4%)) (Figure 1(Bii)). Additionally, the LMCu coated fabric rapidly killed S. aureus in 10 s or even less. Moreover, LMCu coated fabric exhibited over 90% viral titer reduction in the antiviral test against prototype human coronavirus (HCoV 229E). Assisted with gallium LM particles, LMCu coating has a great potential for antimicrobial masks in the critical pandemic period. However, the toxicity assessment shall be performed to evaluate the potential risk of wearing these LMCu coated masks before their scaled application. In addition to the form of NPs, Cu has been produced in the shape of nanowires for a high surface-to-volume ratio. For example, in the study of Kumar et al., the surface of the blown polypropylene filtration media was dip coated with copper@ZIF-8 core-shell nanowires (Cu@ZIF-8 NWs), in which the Cu NWs were firstly passivated with the pluronic F-127 block copolymer for the following growth of ZIF-8 and meanwhile to prevent Cu NW degradation (Figure 1(Ci)) [29]. The Cu@ZIF-8 NWs exhibited lower cytotoxicity with three tested cells (A549 adenocarcinomic human alveolar basal epithelial cells, human gingival epithelial-like and primary gingival fibroblasts) in comparison to the bare CuNWs owning to the sustained and controlled release of copper ions (Figure 1(Cii)). In addition, Cu@ZIF-8 NWs showed more effective bactericidal activity against Streptococcus mutans (S. mutans) (86% inhibition) and E. coli (91% inhibition) than bare Cu NWs or ZIF-8, attributing to the synergistic antimicrobial effects between the Cu nucleus and the ZIF-8 shell (Figure 1(Ciii)). Additionally, Cu@ZIF-8 NWs demonstrated stronger antiviral activity in comparison with the positive control Remdesivir in the in vitro investigation (Figure 1(Civ)). However, the antiviral activity of the Cu@ZIF-8 NWs treated masks upon the exposure to virus-laden aerosols needs to be further explored as well as the general properties of masks in terms of the mechanical strength and filter efficiency.
Various natural plants extract exhibits strong antibacterial and antiviral properties, such as fructus arctii, sage, lycoris radiata, cinnamon and licorice, among others [31]. Son et al. prepared the AC + CO + PU antimicrobial nanofiber mat by polyurethane (PU) mixing solution activated carbon (AC) and cinnamon essential oil (CO) as the antibacterial agents via the electrospinning method, which exhibited a good inactivation effect of S. aureus and E. coli [32]. Researchers have found various compounds in licorice with antiviral and antimicrobial properties, especially 18-β glycyrrhetinic acid (GA) and glycyrrhizin (GL). In the study of Chowdhury et al., the licorice root extract that contains the antiviral substance glycyrrhetinic acid (GLR) was added to polyvinyl alcohol (PVA) solution to fabricate the bio-based filtration mask with a random porosity and orientation by electrospinning (nanofibers diameter ranging from 15 μm to 30 μm) (Figure 2(Ai)) [33]. The airflow rate of 85 L min−1 (to maintain good breathability) can be reached with a pore size of 75 nm, which is smaller than the size of COVID-19 (Figure 2(Aii)). Additionally, the filtering efficacy was not affected when increasing the airflow rate in comparison with N95. However, the antivirus activity and the potential cytotoxicity of the licorice root-based masks need to be further evaluated. Moreover, the fluid-resistant and particulate filtration capacities need to be investigated for its final use as an antimicrobial face mask in practice.
Considering sustained and controlled antimicrobial action on face masks, researchers have proposed light-induced inactivation of pathogens upon near-infrared (NIR) light, Ultraviolet (UV) light or visible (Vis) light irradiation [34]. Photothermal and photodynamic modes of action act as a green and effective way to eliminate biological threats and generate heat or ROS to kill pathogens. In the study of Wu et al., the photodynamic BC-BPTCD-RF nanofibers (BBR-NFs) were produced by an esterification reaction between the carboxyl group of Benzophenone tetracarboxylic dianhydride (BPTCD) and the hydroxyl groups on bacterial cellulose nanofibers (BC-NFs), followed by grafting with Riboflavin (RF), which was further loaded through the high-pressure airflow onto the surface of the nonwoven fibers (Figure 2(Bi)) [35]. The BBR-NFs showed excellent antibacterial effects, achieving 99.999% and 99.9% contact-killing against E. coli and S. aureus by the sustained release of ROS owing to the forming of the intramolecular energy transfer channels and hydrogen transport after effective absorption of visible light (Figure 2(Bii)). In addition, the BBR-NFs exhibited excellent antiviral effects against a simulated virus T7 bacteriophage, achieving 5 log plaque-forming units (PFU) reduction both in 1 h under light conditions and in 90 min under dark conditions (Figure 2(Biii)). Similarly, Monmaturapoj et al. modified hydroxyapatite with anatase TiO2 composite (HA/TiO2) (HA50:Ti50) by solid-state reaction method, which showed an excellent antimicrobial effect [36]. HA/TiO2 composite at 0.5 mg·mL−1 dose exhibited antiviral activity against the H1N1 influenza A virus, achieving more than 2 log/hour reduction of virus titer upon UV irradiation for 60 min because of the production of ROS (especially hydroxyl free radicals and peroxide) by TiO2 particles upon UV light exposure, while for final application in practice, the persistence of the antiviral effects against the SARS-CoV-2 virus as well as the filtration efficacy shall be further verified. Furthermore, researchers have also attempted to combine several antimicrobial modes of action together for enhanced/synergistic antimicrobial activity. In the study of Kumar et al., shellac/copper nanoparticles (CuNPs) were coated on the PP nonwoven surgical masks by dual-channel spray method, where the bio-adhesive shellac bonded tightly to the antimicrobial CuNPs, increasing the hydrophobicity and photoactivity of the surface for self-cleaning property (Figure 2(Ci)) [37]. The nanocoated photoactive antiviral masks (PAM) exhibited substantial E. coli MG1655 reduction (∼4 log) under the sunlight for 5 min, attributing to the generation of free radicals by the rapid rising of the mask surface temperature over 70 °C (Figure 2(Cii,Ciii)). Additionally, the concentration of extracellular vesicles as the model of COVID-19-virus-like particles (VLPs) decreased by 2–3 log on the PAM under sunlight for 5 min owing to the self-cleaning ability of PAM. Overall, the PAM showed excellent photocatalytic and self-cleaning activity, providing a pragmatic solution in the critical pandemic situation.
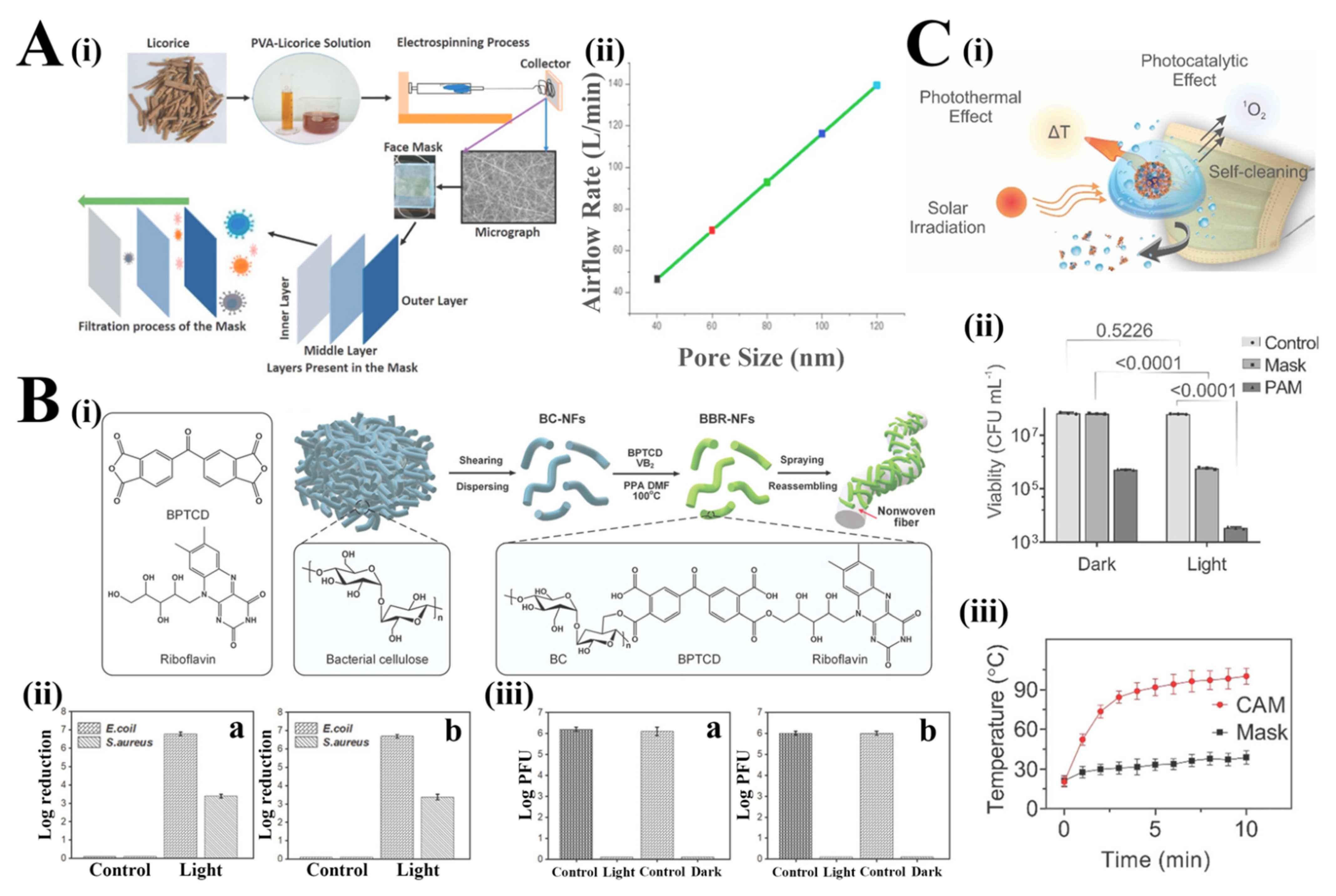
Figure 2. (Ai) Schematic illustration of the production of biobased antiviral face mask by electrospinning process. (Aii) Estimated airflow rate through the licorice membrane with a varying pore size [33]. Reprinted from Environ. Res., 192/110294, Chowdhury, M.A.; Shuvho, M.B.A.; Shahid, M.A.; Haque, A.K.M.M.; Kashem, M.A.; Lam, S.S.; Ong, H.C.; Uddin, M.A.; Mofijur, M., Prospect of biobased antiviral face mask to limit the coronavirus outbreak. (Bi) Schematic illustration of the fabrication of BBR-NFs and their encapsulation with the nonwoven fibers. (Bii) E Bactericidal activity of BBR@protective suit against E. coli and S. aureus under visible light irradiation (a) and dark conditions (b). (Biii) Viricidal assay against T7 phage for BBR@protective suit (a) and BBR@mask (b) under visible light irradiation and dark conditions [35]. (Ci). Schematic illustration of the virus inactivation in respiratory droplets through photothermal, photocatalytic and hydrophobic self-cleaning processes under solar irradiation. (Cii). Colony-forming unit (CFU) number of viable E. coli after solar illumination treatment on the surface of the control, PAM and raw surgical mask (data are presented as means ± SD and n = 3). (Ciii). Increase in the CAM and Mask surface temperature as a function of solar illumination time (data are presented as mean ± SD and n = 3) [37].
The development of antimicrobial masks can indeed extend the lifespan of medical masks. The up-to-date developed antimicrobial masks in literature are summarized in Table 2. In combination with decontamination of the used masks, these strategies can potentially solve the global shortage of masks during the pandemic. Nevertheless, in addition to possible protocols for reuse of the medical masks or respirators, good training and guidance for proper reuse of them are fundamental to ensure their efficiency in infection prevention and control (IPC) [38][39].
Table 2. Strategies for the fabrication of antimicrobial masks.
| Coating Method | Antimicrobial Agents | Mode of Action | Tested Microorganisms | Ref. |
|---|---|---|---|---|
| Polyethylene terephthalate transparent masks dip-coated with benzalkonium chloride | The positively charged nitrogen atoms of BAK | Chemically antimicrobial action | MRSA and MRSE | [26] |
| Cross-linked to form a permanent antimicrobial coating on the surface of the face masks | 2,2′,4′-terpyridine methylammonium chloride (LTMAC) and lignin adenine hexyl ammonium chloride (LAHAC) | Chemically antimicrobial action | Alpha coronavirus: HCoV-229E, beta coronavirus: HCoV-OC43 and K. pneumoniae | [28] |
| The deposition of liquid metal copper alloy (LMCu) particles on the fabric by spontaneous galvanic replacement reaction | The reduction of Cu ions into metallic Cu by galvanic replacement | Chemically antimicrobial action | S. aureus, E. coli, C. albicans and prototype human coronavirus (HCoV 229E) |
[20] |
| The surface of the blown polypropylene filtration media was dip-coated with copper@ZIF-8 core-shell nanowires | The synergistic antimicrobial effects between the Cu nucleus and the ZIF-8 shell | Chemically antimicrobial action | S. mutans and E. coli | [29] |
| Glycyrrhetinic acid was added to polyvinyl alcohol solution to fabricate the biobased filtration mask by electrospinning | Glycyrrhetinic acid | Chemically antimicrobial action | N.a. | [33] |
| BC-BPTCD-RF nanofiber was loaded through the high-pressure airflow onto the surface of the nonwoven fibers | BC-BPTCD-RF nanofibers | Photoactive antiviral (combined photocatalytic and photothermal properties) | E. coli, S. aureus and simulated virus T7 bacteriophage |
[35] |
| Shellac/copper nanoparticles (CuNPs) were coated on the polypropylene masks by a dual-channel spray method | The generation of free radicals by the rapid rising of the mask surface temperature over 70 °C under the sunlight | Light-induced inactivation upon irradiation with near-UV and visible light | E. coli and extracellular vesicles | [37] |
References
- OSHA. Personal Protective Equipment; US Department of Labor, Ed.; Occupational Safety and Health Administration: Washington, DC, USA, 2004.
- University of Washington. Guidelines For Personal Protective Equipment (PPE); EH&S Occupational Safety and Health Office: Washington, DC, USA, 2017; p. 35.
- HSE. The Health and Safety Toolbox: How to Control Risks at Work; Health and Safety Executive: Merseyside, UK. Available online: https://www.hse.gov.uk/toolbox/index.htm (accessed on 2 June 2022).
- OSHC. Guidelines for the Use of Personal Protective Equipment; National Council for Occupational Safety and Health, Ed.; Occupational Safety & Health Council: Hongkong, China, 2001; p. 17.
- NIOSH. Workplace Safety & Health Topics; The National Institute for Occupational Safety and Health: Washington, DC, USA, 2005.
- The European Parliament; The Council of the European Union. REGULATION (EU) 2016/425 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL on personal protective equipment and repealing Council Directive 89/686/EEC. Off. J. Eur. Union 2016, OJ L 81, 51–98.
- Taylor, N.A.S.; Lewis, M.C.; Notley, S.R.; Peoples, G.E. A fractionation of the physiological burden of the personal protective equipment worn by firefighters. Eur. J. Appl. Physiol. 2011, 112, 2913–2921.
- Akduman, D.; Kim, L.E.; Parks, R.L.; L’Ecuyer, P.B.; Mutha, S.; Jeffe, D.B.; Fraser, V.J. Use of Personal Protective Equipment and Operating Room Behaviors in Four Surgical Subspecialties: Personal Protective Equipment and Behaviors in Surgery; The Official Journal of the Society of Hospital Epidemiologists of America, Washington University School of Medicine: Washington, DC, USA, 1999; pp. 110–114.
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L. Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am. J. Infect. Control. 2007, 35, S65–S164.
- Newman, M. COVID-19: Doctors’ leaders warn that staff could quit and may die over lack of protective equipment. BMJ 2020, 368, m1257.
- World Health Organization. Rational Use of Personal Protective Equipment (PPE) for Coronavirus Disease (COVID-19): Interim Guidance, 19 March 2020; World Health Organization: Geneva, Switzerland, 2020.
- bin-Reza, F.; Lopez Chavarrias, V.; Nicoll, A.; Chamberland, M.E. The use of masks and respirators to prevent transmission of influenza: A systematic review of the scientific evidence. Influenza Other Respir. Viruses 2012, 6, 257–267.
- Seale, H.; Dwyer, D.E.; Cowling, B.J.; Wang, Q.; Yang, P.; MacIntyre, C.R. A review of medical masks and respirators for use during an influenza pandemic. Influenza Other Respir. Viruses 2009, 3, 205–206.
- Klompas, M.; Morris, C.A.; Sinclair, J.; Pearson, M.; Shenoy, E.S. Universal Masking in Hospitals in the COVID-19 Era. N. Engl. J. Med. 2020, 382, e63.
- Drabek, J.; Zatloukal, M. Meltblown technology for production of polymeric microfibers/nanofibers: A review. Phys. Fluids 2019, 31, 091301.
- Institute of Medicine. Reusability of Facemasks During an Influenza Pandemic; The National Academies Press: Washington, DC, USA, 2006.
- Tavis, J.E.; Borkow, G.; Zhou, S.S.; Page, T.; Gabbay, J. A Novel Anti-Influenza Copper Oxide Containing Respiratory Face Mask. PLoS ONE 2010, 5, e11295.
- Rengasamy, S.; Fisher, E.; Shaffer, R.E. Evaluation of the survivability of MS2 viral aerosols deposited on filtering face piece respirator samples incorporating antimicrobial technologies. Am. J. Infect. Control. 2010, 38, 9–17.
- Li, Y.; Leung, P.; Yao, L.; Song, Q.W.; Newton, E. Antimicrobial effect of surgical masks coated with nanoparticles. J. Hosp. Infect. 2006, 62, 58–63.
- Kwon, K.Y.; Cheeseman, S.; Frias-De-Diego, A.; Hong, H.; Yang, J.; Jung, W.; Yin, H.; Murdoch, B.J.; Scholle, F.; Crook, N.; et al. A Liquid Metal Mediated Metallic Coating for Antimicrobial and Antiviral Fabrics. Adv. Mater. 2021, 33, e2104298.
- Seidi, F.; Deng, C.; Zhong, Y.; Liu, Y.; Huang, Y.; Li, C.; Xiao, H. Functionalized Masks: Powerful Materials against COVID-19 and Future Pandemics. Small 2021, 17, e2102453.
- Blosi, M.; Costa, A.L.; Ortelli, S.; Belosi, F.; Ravegnani, F.; Varesano, A.; Tonetti, C.; Zanoni, I.; Vineis, C. Polyvinyl alcohol/silver electrospun nanofibers: Biocidal filter media capturing virus-size particles. J. Appl. Polym. Sci. 2021, 138, 51380.
- Shanmugam, V.; Babu, K.; Garrison, T.F.; Capezza, A.J.; Olsson, R.T.; Ramakrishna, S.; Hedenqvist, M.S.; Singha, S.; Bartoli, M.; Giorcelli, M.; et al. Potential natural polymer-based nanofibres for the development of facemasks in countering viral outbreaks. J. Appl. Polym. Sci. 2021, 138, 50658.
- Elena, P.; Miri, K. Formation of contact active antimicrobial surfaces by covalent grafting of quaternary ammonium compounds. Colloids Surf. B Biointerfaces 2018, 169, 195–205.
- Bureš, F. Quaternary Ammonium Compounds: Simple in Structure, Complex in Application. Top. Curr. Chem. 2019, 377, 14.
- Tuñón-Molina, A.; Martí, M.; Muramoto, Y.; Noda, T.; Takayama, K.; Serrano-Aroca, Á. Antimicrobial Face Shield: Next Generation of Facial Protective Equipment against SARS-CoV-2 and Multidrug-Resistant Bacteria. Int. J. Mol. Sci. 2021, 22, 9518.
- Martí, M.; Tuñón-Molina, A.; Aachmann, F.; Muramoto, Y.; Noda, T.; Takayama, K.; Serrano-Aroca, Á. Protective Face Mask Filter Capable of Inactivating SARS-CoV-2, and Methicillin-Resistant Staphylococcus aureus and Staphylococcus epidermidis. Polymers 2021, 13, 207.
- Kumaran, S.; Oh, E.; Han, S.; Choi, H.-J. Photopolymerizable, Universal Antimicrobial Coating to Produce High-Performing, Multifunctional Face Masks. Nano Lett. 2021, 21, 5422–5429.
- Kumar, A.; Sharma, A.; Chen, Y.; Jones, M.M.; Vanyo, S.T.; Li, C.; Visser, M.B.; Mahajan, S.D.; Sharma, R.K.; Swihart, M.T. Core-Shell Nanowires for Reusable Antimicrobial Face Masks. Adv. Funct. Mater. 2020, 31, 2008054.
- Pollard, Z.A.; Karod, M.; Goldfarb, J.L. Metal leaching from antimicrobial cloth face masks intended to slow the spread of COVID-19. Sci. Rep. 2021, 11, 19216.
- Duong-Quy, S.; Ngo-Minh, X.; Tang-Le-Quynh, T.; Tang-Thi-Thao, T.; Nguyen-Quoc, B.; Le-Quang, K.; Tran-Thanh, D.; Doan-Thi-Quynh, N.; Canty, E.; Do, T.; et al. The use of exhaled nitric oxide and peak expiratory flow to demonstrate improved breathability and antimicrobial properties of novel face mask made with sustainable filter paper and Folium Plectranthii amboinicii oil: Additional option for mask shortage during COVID-19 pandemic. Multidiscip. Respir. Med. 2020, 15, 664.
- Son, B.C.; Park, C.H.; Kim, C.S. Fabrication of Antimicrobial Nanofiber Air Filter Using Activated Carbon and Cinnamon Essential Oil. J. Nanosci. Nanotechnol. 2020, 20, 4376–4380.
- Chowdhury, M.A.; Shuvho, M.B.A.; Shahid, M.A.; Haque, A.K.M.M.; Kashem, M.A.; Lam, S.S.; Ong, H.C.; Uddin, M.A.; Mofijur, M. Prospect of biobased antiviral face mask to limit the coronavirus outbreak. Environ. Res. 2021, 192, 110294.
- Margarucci, L.M.; Gianfranceschi, G.; Romano Spica, V.; D’Ermo, G.; Refi, C.; Podico, M.; Vitali, M.; Romano, F.; Valeriani, F. Photocatalytic Treatments for Personal Protective Equipment: Experimental Microbiological Investigations and Perspectives for the Enhancement of Antimicrobial Activity by Micrometric TiO2. Int. J. Environ. Res. Public Health 2021, 18, 8662.
- Wu, F.; He, P.; Chang, X.; Jiao, W.; Liu, L.; Si, Y.; Yu, J.; Ding, B. Visible-Light-Driven and Self-Hydrogen-Donated Nanofibers Enable Rapid-Deployable Antimicrobial Bioprotection. Small 2021, 17, e2100139.
- Monmaturapoj, N.; Sri-on, A.; Klinsukhon, W.; Boonnak, K.; Prahsarn, C. Antiviral activity of multifunctional composite based on TiO2-modified hydroxyapatite. Mater. Sci. Eng. C 2018, 92, 96–102.
- Kumar, S.; Karmacharya, M.; Joshi, S.R.; Gulenko, O.; Park, J.; Kim, G.-H.; Cho, Y.-K. Photoactive Antiviral Face Mask with Self-Sterilization and Reusability. Nano Lett. 2020, 21, 337–343.
- European Centre for Disease Prevention and Control. Guidance for Wearing and Removing Personal Protective Equipment in Healthcare Settings for the Care of Patients with Suspected or Confirmed COVID-19; European Centre for Disease Prevention and Control: Solna, Sweden, 2020; p. 13.
- Ganczak, M.; Szych, Z. Surgical nurses and compliance with personal protective equipment. J. Hosp. Infect. 2007, 66, 346–351.
More
Information
Subjects:
Materials Science, Textiles
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
COVID-19
Revisions:
2 times
(View History)
Update Date:
21 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




