Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Xinyu Song.
Shortage of personal protective equipment (PPE) is often projected in response to public health emergencies such as infection outbreaks and pandemics. Respiratory protective devices (RPDs), namely medical face masks and respirators, are considered the last defence for the front-line healthcare workers. To contribute to the mitigation of RPDs shortage, new technology such as antimicrobial treated PPE that can reduce the risks of fomite during the donning and doffing process with an extended lifespan gets increasingly prevalent.
- respiratory protective devices
- filtering facepiece respirators
- medical face masks
- antimicrobial coating
- photocatalytic and photothermal
- nanoparticles
1. Introduction
Personal protective equipment, commonly referred to as PPE, is defined by the Occupational Safety and Health Administration (OSHA) as equipment worn to minimize exposure to a variety of hazards [1]. Many guidelines have been issued to advise the practical use of PPE in various work professionals [2][3][4]. In the Hierarchy of Hazard Controls, PPE is considered an option of the last resort, providing a barrier to prevent work injuries from potentially hazardous work environments [5]. PPE type varies and includes head and scalp protection, respiratory protection, eye protection, hearing protection, hand and arm protection, foot and leg protection, body protection, and height and access protection [6][7]. PPE has been commonly used in healthcare settings, such as at surgical sites and in infection outbreaks [8][9]. Lately, the growing pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has placed healthcare workers (HCWs) at great risk of coronavirus disease 2019 (COVID-19) infection [10]. The use of PPE in healthcare and community settings plays a crucial role in preventing the transmission of SARS-CoV-2 during COVID-19 patient care [11]. World Health Organisation (WHO) has recommended the use of PPE, including gloves, medical masks, goggles or a face shield and gowns, as well as for specific procedures, respirators (i.e., N95 or FFP2 standard or equivalent) and aprons in different activities involving contact with COIVD-19 patients [11].
In the context of PPE for infection control, respiratory protective devices (RPDs) undertake as part of the package for personal protection [12]. It is also considered the last line of non-invasive defence for HCWs against respiratory infection transmission [13][14]. RPDs can be divided into medical masks and respirators according to their qualifications and functions. Medical masks are also known as surgical masks or face masks termed by U.S. Food and Drug Administration (FDA), or medical face masks termed by European Committee for Standardization (CEN) Technical Committee (TC) 205. In general, three nonwoven layers compose the most commonly used medical face masks. The middle layer is usually fabricated by the nonwoven meltblown process featuring electrostatic charging, and the two outer layers are usually fabricated by the nonwoven spunbond process [15]. Medical masks were originally designed to be used in the operation room, avoiding contaminants generated by the wearer on the wound and meanwhile preventing blood or other potentially infectious agents from reaching the wearer’s skin, mouth or mucous membranes (by splashes) [16].
2. Antimicrobial Masks
In the view of PPE shortage, several studies have demonstrated strategies of incorporating functional agents (antimicrobial agents, superhydrophobic materials, electrical chargers, etc.) on masks and respirators in vitro that can reduce the risks of self-inoculation during doffing of the equipment [17][18][19][20]. To summarise, the mode of action of antimicrobial masks can be categorized into the followings: (1) superhydrophobic surface treatment avoiding pathogens’ adhesion and allowing mask self-cleaning; (2) incorporating antimicrobial agents achieving self-disinfectant features (chemical, photodynamic or photothermal antimicrobial actions); (3) extending the electrostatic charges of masks for sustained and improved pathogens filtration efficacy [21]. Among all, the second type of mode of action is the most employed one in the current research. Antimicrobial agents such as quaternary ammonium compounds (QACs), metal ions (in the form of nanoparticles or nanowire, or nanorods) and natural plant extracts have been frequently utilized for the development of antimicrobial masks, exerting antibacterial or antiviral actions [22][23]. QACs are a common type of antimicrobial agent with broad-spectrum applications, which have been commonly applied in textile materials, including polyethylene terephthalate (PET), cellulose, polyamide, polypropylene (PP), etc. [24]. However, their low stability, non-adhesion or weak attachment on the substrate surface and potential toxicity result in decreased antimicrobial performance and wide application in practice [25]. In the study of Tuñón-Molina et al., a type of PET transparent mask coated with benzalkonium chloride (BAK) was prepared, which enabled to inactivate enveloped viruses (e.g., the phage phi 6 and severe acute respiratory syndrome coronavirus 2) in less than a minute of contact time [26]. The potent antimicrobial activity of the PET sample against viruses and bacteria was attributed to the positively charged nitrogen atoms of BAK, which can destroy the phospholipid bilayer, glycoprotein envelope and spike glycoprotein of the virus or destroy the bacterial membranes. The PET with BAK coating simple also showed antibacterial ability against MRSA and MRSE with inhibition zones of 0.61 ± 0.03 and 0.57 ± 0.05, respectively. It is unfortunate that the study did not investigate the safety issue regarding the use of these BAK-coated masks in practice, such as the potential risks of respiratory exposure to this toxic compound. Similarly, Martí et al. developed the BAK bio-functional coating on a nonwoven mask filter by the dip-coating method, which exhibited >99% of SARS-CoV-2 particles reduction in just one-minute treatment [27]. In the study of Kumaran et al., terpyridine methylammonium chloride (TMAC) and adenine hexyl ammonium chloride (AHAC) were conjugated with lignin respectively to synthesize lignin 2,2′,4′-terpyridine methylammonium chloride (LTMAC) and lignin adenine hexyl ammonium chloride (LAHAC) and cross-linked to form a permanent antimicrobial coating on the surface of the face masks in the form of spray or infiltration (Figure 1(Ai)) [28]. In the evaluation of the antiviral ability of LTMAC and LAHAC-coated PP face mask, human coronaviruses (alpha coronavirus: HCoV-229E and beta coronavirus: HCoV-OC43) were inactivated in 5 min and achieved 3–6 log reduction in 30 min (Figure 1(Aii–Av)). In addition, the LTMAC- and LAHAC-coated face masks significantly killed K. pneumoniae both in the medium composition of distilled water and artificial saliva and in the transmission modes of droplets and aerosols, which also showed a consistently time-dependent bacterial inactivation. However, the stability of the polymer coating on the mask surface needs to be verified.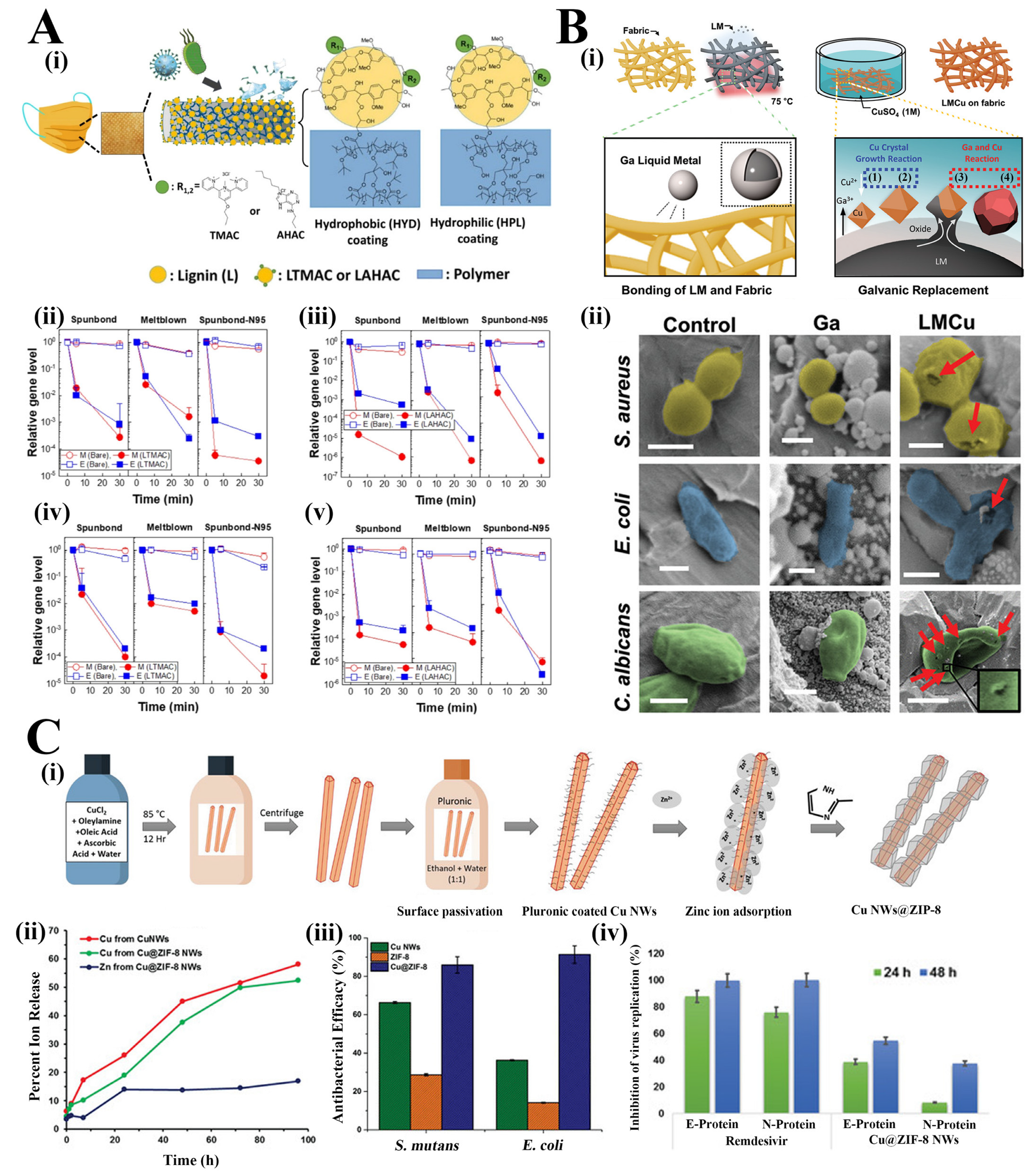
Figure 1. (Ai). Schematic illustration of the UV cross-linked coating on the surface of the face mask. Gene expression level change of HCoV-229E (Aii,Aiii) and HCoV-OC43 (Aiv,Av) on lignin-based, LTMAC- (Aii,Aiv) and LAHAC- (Aiii,Av) coated face mask fabrics (spunbond: outer layer of SM, meltblown: middle layer of SM and spunbond-N95: outer layer of N95) (n = 3, mean ± SD) [28] Reprinted (adapted) with permission from Reference [28]. Copyright 2022 American Chemical Society. (Bi). Schematic illustration of the gallium-copper (LMCu) coatings on fabric by galvanic replacement. (Bii). False coloured SEM images of S. aureus, E. coli and C. albicans cells on the control, Ga coated, and LMCu coated fabric (scale bars: 500 nm for S. aureus and E. coli and 2 µm for C. albicans): red arrows indicated the physically damaged and perforated cells [20]. (Ci). Schematic illustration of the synthesis of core-shell Cu@ZIF-8 NWs. (Cii). Release profile of Cu2+ and Zn2+ from the Cu NWs and Cu@ZIF-8 NWs in cell media (n = 3. p < 0.05). (Ciii). Antimicrobial efficacy (percent reduction at OD 600 nm after 26 h) of Cu NWs, ZIF-8 and Cu@ZIF-8 NWs against S. mutans and E. coli at concentrations of 375 µg·mL−1. (Civ). Antiviral effects (percentage reduction) of Cu@ZIF-8 NWs and Remdesivir at 24 h and 48 h post infection [29].
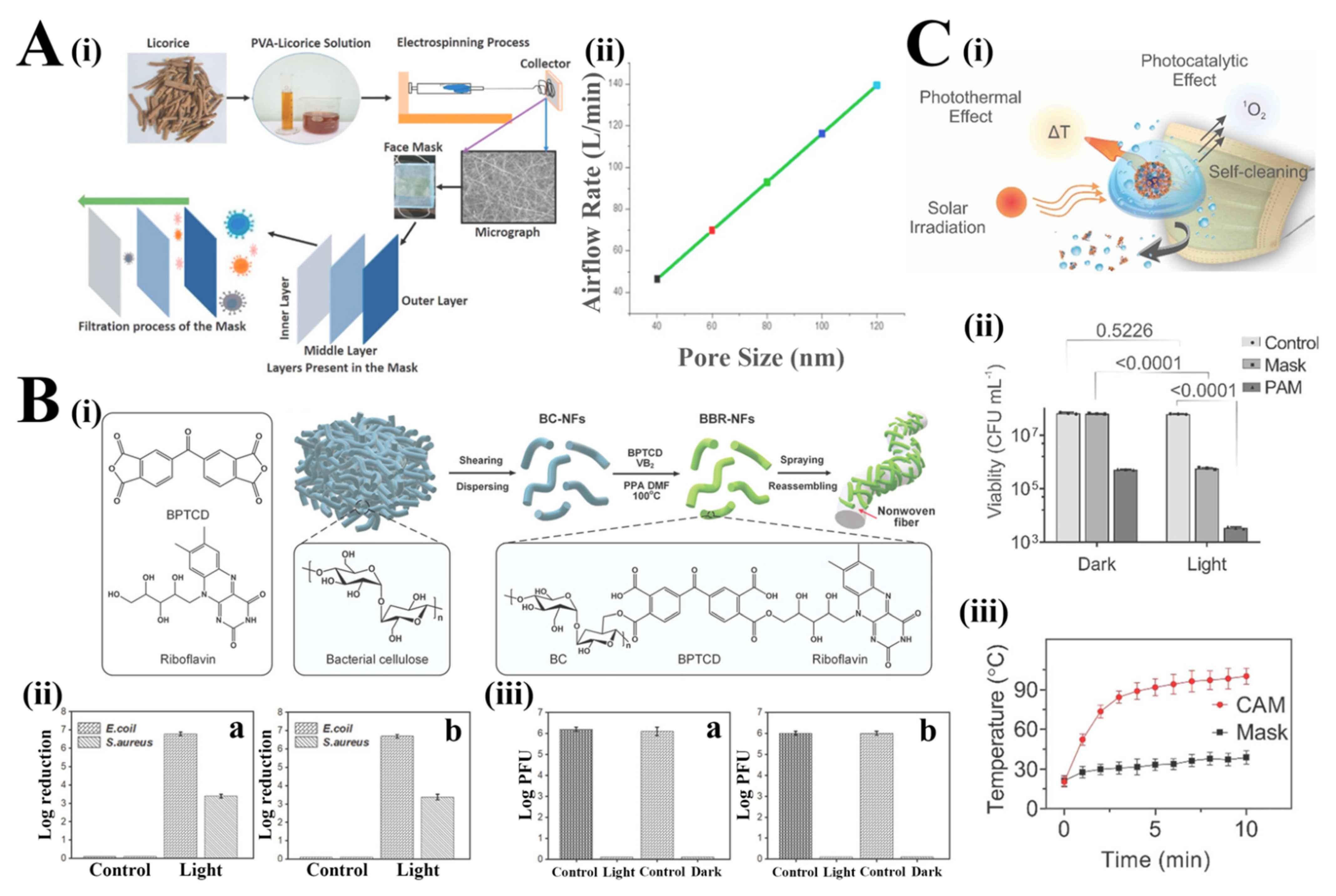
Figure 2. (Ai) Schematic illustration of the production of biobased antiviral face mask by electrospinning process. (Aii) Estimated airflow rate through the licorice membrane with a varying pore size [33]. Reprinted from Environ. Res., 192/110294, Chowdhury, M.A.; Shuvho, M.B.A.; Shahid, M.A.; Haque, A.K.M.M.; Kashem, M.A.; Lam, S.S.; Ong, H.C.; Uddin, M.A.; Mofijur, M., Prospect of biobased antiviral face mask to limit the coronavirus outbreak, Copyright (2022), with permission from Elsevier. (Bi) Schematic illustration of the fabrication of BBR-NFs and their encapsulation with the nonwoven fibers. (Bii) E Bactericidal activity of BBR@protective suit against E. coli and S. aureus under visible light irradiation (a) and dark conditions (b). (Biii) Viricidal assay against T7 phage for BBR@protective suit (a) and BBR@mask (b) under visible light irradiation and dark conditions [35]. (Ci). Schematic illustration of the virus inactivation in respiratory droplets through photothermal, photocatalytic and hydrophobic self-cleaning processes under solar irradiation. (Cii). Colony-forming unit (CFU) number of viable E. coli after solar illumination treatment on the surface of the control, PAM and raw surgical mask (data are presented as means ± SD and n = 3). (Ciii). Increase in the CAM and Mask surface temperature as a function of solar illumination time (data are presented as mean ± SD and n = 3) [37]. Reprinted (adapted) with permission from Reference [37]. Copyright 2022 American Chemical Society.
Table 2.
Strategies for the fabrication of antimicrobial masks.
| Coating Method | Antimicrobial Agents | Mode of Action | Tested Microorganisms | Ref. |
|---|---|---|---|---|
| Polyethylene terephthalate transparent masks dip-coated with benzalkonium chloride | The positively charged nitrogen atoms of BAK | Chemically antimicrobial action | MRSA and MRSE | [26] |
| Cross-linked to form a permanent antimicrobial coating on the surface of the face masks | 2,2′,4′-terpyridine methylammonium chloride (LTMAC) and lignin adenine hexyl ammonium chloride (LAHAC) | Chemically antimicrobial action | Alpha coronavirus: HCoV-229E, beta coronavirus: HCoV-OC43 and K. pneumoniae | [28] |
| The deposition of liquid metal copper alloy (LMCu) particles on the fabric by spontaneous galvanic replacement reaction | The reduction of Cu ions into metallic Cu by galvanic replacement | Chemically antimicrobial action | S. aureus, E. coli, C. albicans and prototype human coronavirus (HCoV 229E) |
[20] |
| The surface of the blown polypropylene filtration media was dip-coated with copper@ZIF-8 core-shell nanowires | The synergistic antimicrobial effects between the Cu nucleus and the ZIF-8 shell | Chemically antimicrobial action | S. mutans and E. coli | [29] |
| Glycyrrhetinic acid was added to polyvinyl alcohol solution to fabricate the biobased filtration mask by electrospinning | Glycyrrhetinic acid | Chemically antimicrobial action | N.a. | [33] |
| BC-BPTCD-RF nanofiber was loaded through the high-pressure airflow onto the surface of the nonwoven fibers | BC-BPTCD-RF nanofibers | Photoactive antiviral (combined photocatalytic and photothermal properties) | E. coli, S. aureus and simulated virus T7 bacteriophage |
[35] |
| Shellac/copper nanoparticles (CuNPs) were coated on the polypropylene masks by a dual-channel spray method | The generation of free radicals by the rapid rising of the mask surface temperature over 70 °C under the sunlight | Light-induced inactivation upon irradiation with near-UV and visible light | E. coli and extracellular vesicles | [37] |
References
- OSHA. Personal Protective Equipment; US Department of Labor, Ed.; Occupational Safety and Health Administration: Washington, DC, USA, 2004.
- University of Washington. Guidelines For Personal Protective Equipment (PPE); EH&S Occupational Safety and Health Office: Washington, DC, USA, 2017; p. 35.
- HSE. The Health and Safety Toolbox: How to Control Risks at Work; Health and Safety Executive: Merseyside, UK. Available online: https://www.hse.gov.uk/toolbox/index.htm (accessed on 2 June 2022).
- OSHC. Guidelines for the Use of Personal Protective Equipment; National Council for Occupational Safety and Health, Ed.; Occupational Safety & Health Council: Hongkong, China, 2001; p. 17.
- NIOSH. Workplace Safety & Health Topics; The National Institute for Occupational Safety and Health: Washington, DC, USA, 2005.
- The European Parliament; The Council of the European Union. REGULATION (EU) 2016/425 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL on personal protective equipment and repealing Council Directive 89/686/EEC. Off. J. Eur. Union 2016, OJ L 81, 51–98.
- Taylor, N.A.S.; Lewis, M.C.; Notley, S.R.; Peoples, G.E. A fractionation of the physiological burden of the personal protective equipment worn by firefighters. Eur. J. Appl. Physiol. 2011, 112, 2913–2921.
- Akduman, D.; Kim, L.E.; Parks, R.L.; L’Ecuyer, P.B.; Mutha, S.; Jeffe, D.B.; Fraser, V.J. Use of Personal Protective Equipment and Operating Room Behaviors in Four Surgical Subspecialties: Personal Protective Equipment and Behaviors in Surgery; The Official Journal of the Society of Hospital Epidemiologists of America, Washington University School of Medicine: Washington, DC, USA, 1999; pp. 110–114.
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L. Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am. J. Infect. Control. 2007, 35, S65–S164.
- Newman, M. COVID-19: Doctors’ leaders warn that staff could quit and may die over lack of protective equipment. BMJ 2020, 368, m1257.
- World Health Organization. Rational Use of Personal Protective Equipment (PPE) for Coronavirus Disease (COVID-19): Interim Guidance, 19 March 2020; World Health Organization: Geneva, Switzerland, 2020.
- bin-Reza, F.; Lopez Chavarrias, V.; Nicoll, A.; Chamberland, M.E. The use of masks and respirators to prevent transmission of influenza: A systematic review of the scientific evidence. Influenza Other Respir. Viruses 2012, 6, 257–267.
- Seale, H.; Dwyer, D.E.; Cowling, B.J.; Wang, Q.; Yang, P.; MacIntyre, C.R. A review of medical masks and respirators for use during an influenza pandemic. Influenza Other Respir. Viruses 2009, 3, 205–206.
- Klompas, M.; Morris, C.A.; Sinclair, J.; Pearson, M.; Shenoy, E.S. Universal Masking in Hospitals in the COVID-19 Era. N. Engl. J. Med. 2020, 382, e63.
- Drabek, J.; Zatloukal, M. Meltblown technology for production of polymeric microfibers/nanofibers: A review. Phys. Fluids 2019, 31, 091301.
- Institute of Medicine. Reusability of Facemasks During an Influenza Pandemic; The National Academies Press: Washington, DC, USA, 2006.
- Tavis, J.E.; Borkow, G.; Zhou, S.S.; Page, T.; Gabbay, J. A Novel Anti-Influenza Copper Oxide Containing Respiratory Face Mask. PLoS ONE 2010, 5, e11295.
- Rengasamy, S.; Fisher, E.; Shaffer, R.E. Evaluation of the survivability of MS2 viral aerosols deposited on filtering face piece respirator samples incorporating antimicrobial technologies. Am. J. Infect. Control. 2010, 38, 9–17.
- Li, Y.; Leung, P.; Yao, L.; Song, Q.W.; Newton, E. Antimicrobial effect of surgical masks coated with nanoparticles. J. Hosp. Infect. 2006, 62, 58–63.
- Kwon, K.Y.; Cheeseman, S.; Frias-De-Diego, A.; Hong, H.; Yang, J.; Jung, W.; Yin, H.; Murdoch, B.J.; Scholle, F.; Crook, N.; et al. A Liquid Metal Mediated Metallic Coating for Antimicrobial and Antiviral Fabrics. Adv. Mater. 2021, 33, e2104298.
- Seidi, F.; Deng, C.; Zhong, Y.; Liu, Y.; Huang, Y.; Li, C.; Xiao, H. Functionalized Masks: Powerful Materials against COVID-19 and Future Pandemics. Small 2021, 17, e2102453.
- Blosi, M.; Costa, A.L.; Ortelli, S.; Belosi, F.; Ravegnani, F.; Varesano, A.; Tonetti, C.; Zanoni, I.; Vineis, C. Polyvinyl alcohol/silver electrospun nanofibers: Biocidal filter media capturing virus-size particles. J. Appl. Polym. Sci. 2021, 138, 51380.
- Shanmugam, V.; Babu, K.; Garrison, T.F.; Capezza, A.J.; Olsson, R.T.; Ramakrishna, S.; Hedenqvist, M.S.; Singha, S.; Bartoli, M.; Giorcelli, M.; et al. Potential natural polymer-based nanofibres for the development of facemasks in countering viral outbreaks. J. Appl. Polym. Sci. 2021, 138, 50658.
- Elena, P.; Miri, K. Formation of contact active antimicrobial surfaces by covalent grafting of quaternary ammonium compounds. Colloids Surf. B Biointerfaces 2018, 169, 195–205.
- Bureš, F. Quaternary Ammonium Compounds: Simple in Structure, Complex in Application. Top. Curr. Chem. 2019, 377, 14.
- Tuñón-Molina, A.; Martí, M.; Muramoto, Y.; Noda, T.; Takayama, K.; Serrano-Aroca, Á. Antimicrobial Face Shield: Next Generation of Facial Protective Equipment against SARS-CoV-2 and Multidrug-Resistant Bacteria. Int. J. Mol. Sci. 2021, 22, 9518.
- Martí, M.; Tuñón-Molina, A.; Aachmann, F.; Muramoto, Y.; Noda, T.; Takayama, K.; Serrano-Aroca, Á. Protective Face Mask Filter Capable of Inactivating SARS-CoV-2, and Methicillin-Resistant Staphylococcus aureus and Staphylococcus epidermidis. Polymers 2021, 13, 207.
- Kumaran, S.; Oh, E.; Han, S.; Choi, H.-J. Photopolymerizable, Universal Antimicrobial Coating to Produce High-Performing, Multifunctional Face Masks. Nano Lett. 2021, 21, 5422–5429.
- Kumar, A.; Sharma, A.; Chen, Y.; Jones, M.M.; Vanyo, S.T.; Li, C.; Visser, M.B.; Mahajan, S.D.; Sharma, R.K.; Swihart, M.T. Core-Shell Nanowires for Reusable Antimicrobial Face Masks. Adv. Funct. Mater. 2020, 31, 2008054.
- Pollard, Z.A.; Karod, M.; Goldfarb, J.L. Metal leaching from antimicrobial cloth face masks intended to slow the spread of COVID-19. Sci. Rep. 2021, 11, 19216.
- Duong-Quy, S.; Ngo-Minh, X.; Tang-Le-Quynh, T.; Tang-Thi-Thao, T.; Nguyen-Quoc, B.; Le-Quang, K.; Tran-Thanh, D.; Doan-Thi-Quynh, N.; Canty, E.; Do, T.; et al. The use of exhaled nitric oxide and peak expiratory flow to demonstrate improved breathability and antimicrobial properties of novel face mask made with sustainable filter paper and Folium Plectranthii amboinicii oil: Additional option for mask shortage during COVID-19 pandemic. Multidiscip. Respir. Med. 2020, 15, 664.
- Son, B.C.; Park, C.H.; Kim, C.S. Fabrication of Antimicrobial Nanofiber Air Filter Using Activated Carbon and Cinnamon Essential Oil. J. Nanosci. Nanotechnol. 2020, 20, 4376–4380.
- Chowdhury, M.A.; Shuvho, M.B.A.; Shahid, M.A.; Haque, A.K.M.M.; Kashem, M.A.; Lam, S.S.; Ong, H.C.; Uddin, M.A.; Mofijur, M. Prospect of biobased antiviral face mask to limit the coronavirus outbreak. Environ. Res. 2021, 192, 110294.
- Margarucci, L.M.; Gianfranceschi, G.; Romano Spica, V.; D’Ermo, G.; Refi, C.; Podico, M.; Vitali, M.; Romano, F.; Valeriani, F. Photocatalytic Treatments for Personal Protective Equipment: Experimental Microbiological Investigations and Perspectives for the Enhancement of Antimicrobial Activity by Micrometric TiO2. Int. J. Environ. Res. Public Health 2021, 18, 8662.
- Wu, F.; He, P.; Chang, X.; Jiao, W.; Liu, L.; Si, Y.; Yu, J.; Ding, B. Visible-Light-Driven and Self-Hydrogen-Donated Nanofibers Enable Rapid-Deployable Antimicrobial Bioprotection. Small 2021, 17, e2100139.
- Monmaturapoj, N.; Sri-on, A.; Klinsukhon, W.; Boonnak, K.; Prahsarn, C. Antiviral activity of multifunctional composite based on TiO2-modified hydroxyapatite. Mater. Sci. Eng. C 2018, 92, 96–102.
- Kumar, S.; Karmacharya, M.; Joshi, S.R.; Gulenko, O.; Park, J.; Kim, G.-H.; Cho, Y.-K. Photoactive Antiviral Face Mask with Self-Sterilization and Reusability. Nano Lett. 2020, 21, 337–343.
- European Centre for Disease Prevention and Control. Guidance for Wearing and Removing Personal Protective Equipment in Healthcare Settings for the Care of Patients with Suspected or Confirmed COVID-19; European Centre for Disease Prevention and Control: Solna, Sweden, 2020; p. 13.
- Ganczak, M.; Szych, Z. Surgical nurses and compliance with personal protective equipment. J. Hosp. Infect. 2007, 66, 346–351.
More
