
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pavel Kopnin | -- | 2723 | 2022-06-20 11:40:58 | | | |
| 2 | Vivi Li | Meta information modification | 2723 | 2022-06-21 03:29:16 | | |
Video Upload Options
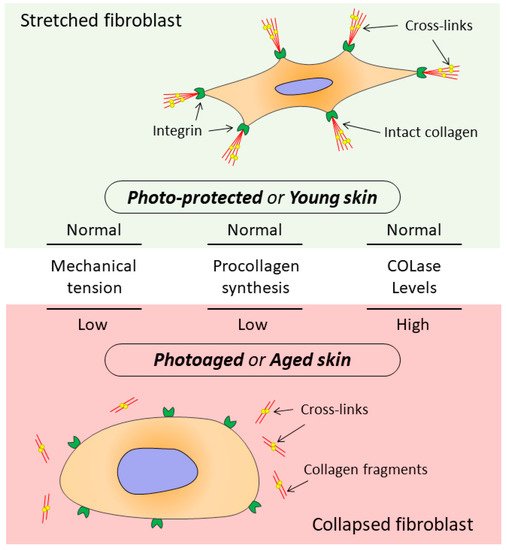
Skin aging is a multi-factorial process that affects nearly every aspect of skin biology and function. With age, an impairment of structures, quality characteristics, and functions of the dermal extracellular matrix (ECM) occurs in the skin, which leads to disrupted functioning of dermal fibroblasts (DFs), the main cells supporting morphofunctional organization of the skin. The DF functioning directly depends on the state of the surrounding collagen matrix (CM). The intact collagen matrix ensures proper adhesion and mechanical tension in DFs, which allows these cells to maintain collagen homeostasis while ECM correctly regulates cellular processes. When the integrity of CM is destroyed, mechanotransduction is disrupted, which is accompanied by impairment of DF functioning and destruction of collagen homeostasis, thereby contributing to the progression of aging processes in skin tissues.
1. Aging of Human Skin
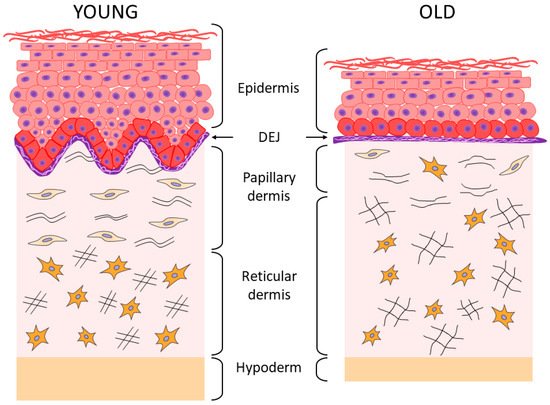
2. Aging of the Epidermis and the Dermo-Epidermal Junction
| Chronological Aging | Photoaging | |
|---|---|---|
| HA in theepidermis | ↓ amount | ↓ amount |
| HA in the derma | amount not changed ↓ bioavailability |
↑ amount ↓ length |
| Total content of sulphated GAGs | ↓ amount | ↑ amount |
| Versican | ↓ mRNA expression ↑ amount in males |
↓ mRNA expression ↑ amount in the solar elastosis area |
| Decorin | ↓ mRNA expression ↓ amount ↓ size |
↓ amount in the solar elastosis area |
| Biglycan | ↓ mRNA expression ↓ amount |
↓ amount |
3. Aging of the Dermis
4. The Relationship between the State of Collagen Matrix and Functioning of Dermal Fibroblasts
References
- Capri, M.; Salvioli, S. The genetics of human longevity. Ann. N. Y. Acad. Sci. 2006, 1067, 252–263.
- Rittie, L.; Fisher, G.J. Natural and Sun-Induced Aging of Human Skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370.
- Wang, A.S.; Dreesen, O. Biomarkers of Cellular Senescence and Skin Aging. Front. Genet. 2018, 9, 247.
- Langton, A.K.; Graham, H.K. Ageing significantly impacts the biomechanical function and structural composition of skin. Exp. Dermatol. 2019, 28, 981–984.
- Pedić, L.; Pondeljak, N. Recent information on photoaging mechanisms and the preventive role of topical sunscreen products. Acta Derm. APA 2020, 29, 201–207.
- Zhang, W.; Qu, J. The ageing epigenome and its rejuvenation. Nat. Rev. Mol. Cell Biol. 2020, 21, 137–150.
- Tewari, A.; Grage, M.M.L. UVA1 is skin deep: Molecular and clinical implications. Photochem. Photobiol. Sci. 2013, 12, 95–103.
- Yaar, M.; Eller, M.S. Fifty years of skin aging. J. Investig. Dermatol. Symp. Proc. 2002, 7, 51–58.
- Zhong, J.; Hua, N. A novel promising therapy for skin aging: Dermal multipotent stem cells against photoaged skin by activation of TGF-b/Smad and p38 MAPK signaling pathway. Med. Hypotheses 2011, 76, 343–346.
- Naylor, E.; Watson, R. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256.
- Fisher, G.; Kang, S. Mechanism of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1467.
- Miyamura, Y.; Coelho, S. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment. Cell Res. 2007, 20, 2–13.
- Fisher, G.; Varani, J. Looking older: Fibroblast Collapse and Therapeutic Implications. Arch. Dermatol. 2008, 144, 666–672.
- Varani, J.; Schuger, L. Reduced fibroblast interaction with intact collagen as a mechanism for depressed collagen synthesis in photodamaged skin. J. Investig. Dermatol. 2004, 122, 1471–1479.
- Proshkina, E.N.; Solovev, I.A. Key Molecular Mechanisms of Aging, Biomarkers, and Potential Interventions. Mol. Biol. 2020, 54, 883–921.
- Freemont, A.; Hoyland, J. Morphology, mechanisms and pathology of musculoskeletal ageing. J. Pathol. 2007, 211, 252–259.
- Farage, M.; Miller, K. Characteristics of the aging skin. Adv. Wound Care 2013, 2, 5–10.
- Mizukoshi, K.; Yonekura, K. Changes in dermal papilla structures due to aging in the facial cheek region. Ski. Res. Technol. 2015, 21, 224–231.
- Lynch, M.D.; Watt, F.M. Fibroblast heterogeneity: Implications for human disease. J. Clin. Investig. 2018, 128, 26–35.
- Langton, A.K.; Halai, P. The impact of intrinsic ageing on the protein composition of the dermal-epidermal junction. Mech. Ageing Dev. 2016, 156, 14–16.
- Mine, S.; Fortunel, N. Aging Alters Functionally Human Dermal Papillary Fibroblasts but Not Reticular Fibroblasts: A New View of Skin Morphogenesis and Aging. PLoS ONE 2008, 3, e4066.
- Gritsenko, D.A.; Orlova, O.A. Transcription factor p53 and skin aging. Adv. Gerontol. 2017, 30, 10–16. (In Russian)
- Alonso, L.; Fuchs, E. Stem cells of the skin epithelium. Proc. Natl. Acad. Sci. USA 2003, 100, 11830–11835.
- Dreesen, M.A.O.; Chojnowsk, A. Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J. Cell Biol. 2013, 200, 605–617.
- López-Otín, C.; Blasco, M.A. The hallmarks of aging. Cell 2013, 153, 1194–1217.
- Katoh, N.; Tennstedt, D. Gerontodermatology: The fragility of the epidermis in older adults. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1–20.
- Blume-Peytavi, U.; Kottner, J. Age-associated skin conditions and diseases: Current perspectives and future options. Gerontologist 2016, 56, 230–242.
- Barrandon, Y.; Green, H. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl. Acad. Sci. USA 1987, 84, 2302–2306.
- Liu, N.; Matsumura, H. Stem cell competition orchestrates skin homeostasis and ageing. Nature 2019, 568, 344–350.
- Watanabe, M.; Natsuga, K. Type XVII collagen coordinates proliferation in the interfollicular epidermis. Elife 2017, 6, e26635.
- Farage, M.A.; Miller, K.W. Structural characteristics of the aging skin: A review. Cutan. Ocul. Toxicol. 2007, 26, 343–357.
- Hendi, A. Melanocytes in nonlesional sun-exposed skin: A multicenter comparative study. J. Am. Acad. Dermatol. 2011, 65, 1186–1193.
- Chang, A. Geriatric Dermatology Review: Major Changes in Skin Function in Older Patients and Their Contribution to Common Clinical Challenges. J. Am. Med. Dir. Assoc. 2013, 14, 724–730.
- Zouboulis, C.; Ganceviciene, R. Aesthetic aspects of skin aging, prevention, and local treatment. Clin. Dermatol. 2019, 37, 365–372.
- Luebberding, S.; Krueger, N. Age-related changes in skin barrier function—Quantitative evaluation of 150 female subjects. Int. J. Cosmet. Sci. 2013, 35, 183–190.
- Meyer, L.J.; Stern, R. Age-dependent changes of hyaluronan in human skin. J. Investig. Dermatol. 1994, 102, 385–389.
- Oh, J.H.; Kim, Y.K. Intrinsic aging and photoagingdependent level changes of glycosaminoglycans and their correlation with water content in human skin. J. Dermatol. Sci. 2011, 62, 192–201.
- Lee, D.H.; Oh, J.-H. Glycosaminoglycan and proteoglycan in skin aging. J. Dermatol. Sci. 2016, 83, 174–181.
- Makrantonaki, E.; Zouboulis, C.C. Characteristics and pathomechanisms of endogenously aged skin. Dermatology 2007, 214, 352–360.
- Vazquez, F.; Palacios, S. Changes of the basement membrane and type IV collagen in human skin during aging. Maturitas 1996, 25, 209–215.
- Lavker, R.M.; Zheng, P.S. Morphology of aged skin. Clin. Geriatr. Med. 1989, 5, 53–67.
- Výbohová, D.; Mellová, Y. Qualitative changes of the capillary bed in aging human skin. Histol. Histopathol. 2012, 27, 961–967.
- El-Domyati, M.; Attia, S. Intrinsic aging vs. photoaging: A comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 2002, 11, 398–405.
- Shin, J.-W.; Kwon, S.-H. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126.
- Marcos-Garces, V.; Aguilar, P.M. Age-related dermal collagen changes during development, maturation and ageing—A morphometric and comparative study. J. Anat. 2014, 225, 98–108.
- Haydont, V.; Bernard, B. Age-related evolutions of the dermis: Clinical signs, fibroblast and extracellular matrix dynamics. Mech. Ageing Dev. 2019, 177, 150–156.
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251.
- Oh, J.H.; Kim, Y.K. Changes in glycosaminoglycans and related proteoglycans in intrinsically aged human skin in vivo. Exp. Dermatol. 2011, 5, 454–456.
- Ahmed, T.; Nash, A. Combining nano-physical and computational investigations to understand the nature of aging in dermal collagen. Int. J. Nanomed. 2017, 12, 3303–3314.
- Cole, M.A.; Quan, T. Extracellular matrix regulation of fibroblast function: Redefining our pe rspective on skin aging. J. Cell Commun. Signal. 2018, 12, 35–43.
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720.
- Varani, J.; Spearman, D. Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. Am. J. Pathol. 2001, 158, 931–942.
- Varani, J.; Dame, M.K. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868.
- Varani, J.; Warner, R.L. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J. Investig. Dermatol. 2000, 114, 480–486.
- Carrino, D.; Sorrell, J. Age-reiated changes in the proteogiycans of human skin. Arch. Biochem. Biophys. 2002, 373, 91–101.
- Shuster, S.; Black, M.M. The influence of age and sex on skin thickness, skin collagen and density. Br. J. Dermatol. 1975, 93, 639–643.
- Baroni, E.; Biondo-Simões, M. Influence of aging on the quality of the skin of white women: The role of collagen. Acta. Cir. Bras. 2012, 27, 736–740.
- Lovel, C.R.; Smolenski, K.A. Type I and III collagen content and fibre distribution in normal human skin during ageing. Br. J. Dermatol. 1987, 117, 419–428.
- Burridge, K.; Chrzanowska-Wodnicka, M. Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 1996, 12, 463–518.
- Kim, S.H.; Turnbull, J. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011, 209, 139–151.
- Silver, F.; DeVore, D. Role of mechanophysiology in aging of ECM: Effects in mechanochemical transduction. J. Appl. Physiol. 2003, 95, 2134–2141.
- Grinnell, F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003, 13, 264–269.
- Xu, Y.; Gurusrddappa, S. Multiple binding sites in collagen type I for the integrins a1b1 and a2b1. J. Biol. Chem. 2000, 275, 38981–38989.
- Stephens, P.; Genever, P. Non-epithelial oral mucosal progenitor cell populations. Oral Dis. 2007, 13, 1–10.
- Quan, T.; Little, E. Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: Impact of altered extracellular matrix microenvironment on dermal fibroblast function. J. Investig. Dermatol. 2013, 133, 1362–1366.
- Orringer, J.S.; Hammerberg, C. Molecular Effects of Photodynamic Therapy for Photoaging. Arch. Dermatol. 2008, 144, 1296–1302.
- Rinnerthaler, M.; Bischof, J. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589.





