Skin aging is a multi-factorial process that affects nearly every aspect of skin biology and function. With age, an impairment of structures, quality characteristics, and functions of the dermal extracellular matrix (ECM) occurs in the skin, which leads to disrupted functioning of dermal fibroblasts (DFs), the main cells supporting morphofunctional organization of the skin. The DF functioning directly depends on the state of the surrounding collagen matrix (CM). The intact collagen matrix ensures proper adhesion and mechanical tension in DFs, which allows these cells to maintain collagen homeostasis while ECM correctly regulates cellular processes. When the integrity of CM is destroyed, mechanotransduction is disrupted, which is accompanied by impairment of DF functioning and destruction of collagen homeostasis, thereby contributing to the progression of aging processes in skin tissues.
- skin
- extracellular matrix
- collagen
- aging
- fibroblasts
1. Aging of Human Skin
2. Aging of the Epidermis and the Dermo-Epidermal Junction
| Chronological Aging | Photoaging | |
|---|---|---|
| HA in theepidermis | ↓ amount | ↓ amount |
| HA in the derma | amount not changed ↓ bioavailability |
↑ amount ↓ length |
| Total content of sulphated GAGs | ↓ amount | ↑ amount |
| Versican | ↓ mRNA expression ↑ amount in males |
↓ mRNA expression ↑ amount in the solar elastosis area |
| Decorin | ↓ mRNA expression ↓ amount ↓ size |
↓ amount in the solar elastosis area |
| Biglycan | ↓ mRNA expression ↓ amount |
↓ amount |
3. Aging of the Dermis
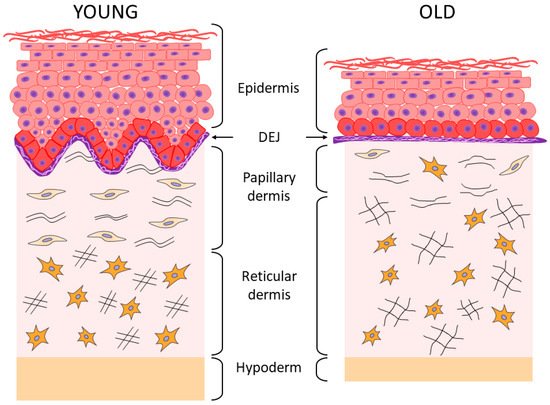
According to the results of histological and ultrastructural studies, the dermis is the skin layer focusing the most significant changes associated with skin aging [13][44][13,44]. This occurs primarily due to structural changes in dermal ECM, its reduction, and degradation [2]. The fibers (collagen and elastin) that constitute the skin framework and are the components of “the ground substance”, as well as proteoglycans (PG) and GAGs which play a key role in maintaining firmness and hydration of the skin, undergo particularly prominent degenerative changes [4][10][4,10]. As a result of these processes, firmness and elasticity of the skin is lost, its thickness decreases, and wrinkles form [18][34][40][41][18,34,40,41]. The changes affect both layers of the dermis; the papillary is the upper and thinner layer, while the reticular is the next more pronounced layer [4][10][42][45][4,10,42,45]. In the papillary layer that is adjacent to BM, a decrease in the content of perlecan (the main PG of BM) and GAG hyaluronic acid is detected (echographically visualized as a subepidermal anechogenic zone [46]), as well as a decrease in the density and spatial orientation of collagen fibers [47][48][49][47,48,49]. In the elastin network of the papillary layer of the dermis, the progressive atrophy of oxytalan fibers is observed, up to their complete disappearance [10][50][10,50]. In the reticular layer, a decrease in the density of collagen fibers is accompanied by a decrease in the thickness of their bundles and an increase in the space between them [51], while the thickening of fibers (elaunin and elastin) and a decrease in the number of functional fibers is observed in the elastic network [10][50][10,50]. Under the chronic UVA exposure, solar elastosis affects the elastin network of both dermal layers, i.e., there is the deposition and accumulation of elastin masses possessing the incomplete molecular organization and, therefore, the incomplete function. This phenomenon is explained by the stimulating effect of UV on the expression of the gene responsible for the synthesis of elastin. Solar elastosis zones are also characterized by the accumulation of PGs [5][10][5,10]. It should be emphasized that changes in the organization and structure of the collagen matrix are characteristic of both chrono- and photoaging of human skin [2]. The results of the study of skin biopsy samples of elderly people have shown that the accumulation of degraded/fragmented fibers and a decrease in de novo collagen synthesis correlate both with age and with the degree of photo-damage severity [51][52][53][54][51,52,53,54]. Along with degradation of the collagen–elastin matrix, changes also occur in “the ground substance” of the dermis, which is associated with the quantitative and qualitative transformation of GAGs and PGs (Table 1) responsible for hydration and elasticity of the skin. In particular, the bioavailability of HA decreases significantly with age (although its amount remains unchanged [34]), as well as biglycan [36]. Proteoglycan decorin, that is, the smallest in size and the most important regulator of the assembly of collagen fibers, also undergoes quantitative and qualitative changes, while a decrease in the molecular weight of its polysaccharide chains has a significant negative effect on skin elasticity since decorin is involved in fibrillogenesis and determines the diameter of fibrils [36][55][36,55]. It has been shown that during photoaging, the abnormal accumulation of HA, versican, and chondroitin sulfate is observed in the solar elastosis zones, while decorin is completely lost; all these phenomena are caused by chronic damage of the skin under UV exposure [36]. Histological examination of skin biopsy samples of young and elderly people confirms the changes described above. Thus, the histological picture of skin sections [4] showed that the “young” and photoprotected skin is characterized by the pronounced epidermal ridges and DEJs, as well as the highly organized network of collagen fibers and a cascade of elastic network fibers connecting BM with the papillary and reticular layers of the dermis; while the chronologically aged skin is characterized by the reduced collagen fibers and elastic network fibers (especially oxytalan fibers) and the reduced content of GAGs, whereas the photoaged skin is characterized by the reduction in collagen fibers, including type VII collagen in the area of DEJs, and by solar elastosis, that is, the accumulation of disorganized proteins of elastin fibers throughout the dermis and also the accumulation of GAGs. In the case of chronological aging of the photoprotected skin, the flattening of DEJs is observed, as well as the disorganization of the elastic network (mostly in the papillary layer), which is accompanied by the accumulation of amorphous elastin and a reduction in the number of collagen fibers. During photoaging, the skin of both young and elderly patients is characterized by the pronounced destruction of epidermal ridges and DEJs, degradation of the elastic network, and accumulation of amorphous elastin in both layers of the dermis. Aging and UV exposure cause the disorders observed in the integrative buffer system of the dermis, a decrease in functioning of the highly organized network of elastic fibers connecting all layers of the skin through cascading, and structural deformations of collagen fibers including their progressive fragmentation. All these events change the essential functional properties of the skin by reducing the skin hydration, elasticity, firmness, and strength [2][4][46][54][2,4,46,54]. As regards the collagen fragmentation, it is important to note the degradation of type I collagen fibers, the most common structural fiber-forming protein of the skin, which comprises 80–90% of the total collagen amount, while the other two fiber-forming collagens type III collagen and type V collagen are 8 to 12% and up to 5%, respectively [44]. With age, the level of total collagen in the skin decreases (by approximately 1% throughout the entire adult person’s life [56]), and the level of main collagen types I and III also decreases, especially at the age of over 60 [57]. According to other data, the increase in collagen type III/I ratio occurs with age due to the increased degradation of type I collagen [58].4. The Relationship between the State of Collagen Matrix and Functioning of Dermal Fibroblasts
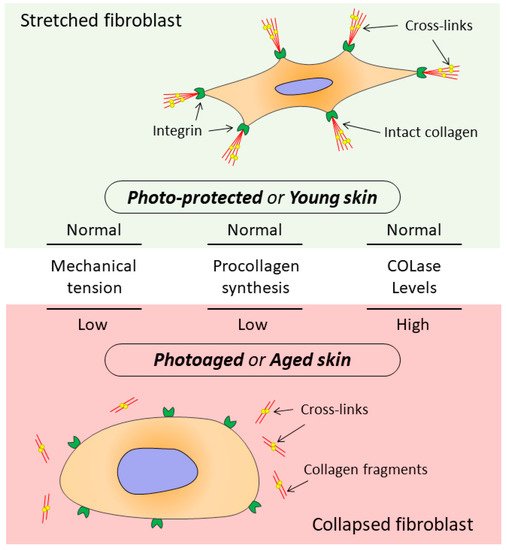
It has been shown that signals entering the dermis from outside are perceived by the ECM and transmitted to DFs, which, receiving these signals, provide ECM homeostasis [13][51][59][13,51,81]. At the same time, the DF functioning directly depends on the state of the surrounding collagen matrix (CM). The intact CM ensures proper adhesion and mechanical tension in DFs, which allows these cells to provide collagen homeostasis while the ECM fully regulates cellular processes including cell migration, proliferation, differentiation, and apoptosis [60][82]. When the CM integrity is damaged, which occurs both during chronological aging and photoaging, changes are observed in mechanotransduction (transmission of mechanical signals from ECM to cells), promoting development of the mechanism that disrupts DF functions (Figure 2) [13][53][61][62][13,53,83,84].