Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Luca Di Pietrantonio | -- | 1090 | 2022-06-13 12:59:18 | | | |
| 2 | Camila Xu | + 1 word(s) | 1091 | 2022-06-14 03:58:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Di Pietrantonio, L.; Verzella, M.; Affede, E.; Cozzolino, V.; Cicchitti, L. New Model Regarding the Characteristics of Somatic Dysfunction. Encyclopedia. Available online: https://encyclopedia.pub/entry/23974 (accessed on 07 February 2026).
Di Pietrantonio L, Verzella M, Affede E, Cozzolino V, Cicchitti L. New Model Regarding the Characteristics of Somatic Dysfunction. Encyclopedia. Available at: https://encyclopedia.pub/entry/23974. Accessed February 07, 2026.
Di Pietrantonio, Luca, Marco Verzella, Erika Affede, Vincenzo Cozzolino, Luca Cicchitti. "New Model Regarding the Characteristics of Somatic Dysfunction" Encyclopedia, https://encyclopedia.pub/entry/23974 (accessed February 07, 2026).
Di Pietrantonio, L., Verzella, M., Affede, E., Cozzolino, V., & Cicchitti, L. (2022, June 13). New Model Regarding the Characteristics of Somatic Dysfunction. In Encyclopedia. https://encyclopedia.pub/entry/23974
Di Pietrantonio, Luca, et al. "New Model Regarding the Characteristics of Somatic Dysfunction." Encyclopedia. Web. 13 June, 2022.
Copy Citation
Somatic dysfunction (SD) is classified by the ICD 11 as a “Biomechanical lesion, not elsewhere classified”; however, the definitions are not equally shared and codified by osteopathic professionals.
exclusion zone water
interstitial fluid pressure
water
somatic dysfunction
1. Introduction
The main means available to osteopathic medicine is to assess tissues by palpating, in particular tissues of the musculoskeletal system, with the aim of diagnosing a possible somatic dysfunction (SD).
By underscoring some contradictory aspects, several researchers have called into question SD, by defining it as a nosological entity detectable on palpation [1][2][3].
SD is classified by the ICD 11 [4] as a “Biomechanical lesion, not elsewhere classified”; however, the definitions are not equally shared and codified by osteopathic professionals [1][5][6][7].
SD presents the characteristics of impaired or altered function of components related to the somatic system, involving skeletal, arthrodial, and myofascial structures, and osteopathic manipulative treatment (OMT) is aimed at the treatment of SD [8][9][10].
The osteopathic literature describes the relationship between SD and OMT in many studies [10][11][12][13].
OMT is a drug-free manual medicine, a patient-centered, whole-body intervention. OMT has shown positive effects in different fields such as gynecology and obstetrics, neonatology, chronic inflammatory disease management, and musculoskeletal disorders [14][15][16][17][18][19].
There are many aspects to consider regarding the etiology and diagnosis of SD, and the osteopathic literature provides details on the signs that characterize it, including tissue texture changes [8][20][21][22].
Over the last few years, some researchers have proposed a variety of interpretation models in order to clarify the mechanisms of onset and the inherent characteristics of tissue alterations concerning SD. Among such models, there are also clinical reasoning and decision-making procedures suitable to establish a treatment routine [23][24][25][26][27].
Recent knowledge suggests that tissue, and, in particular, connective tissue, may react by modulating the inflammation degree. This issue should also be extended to any response to OMT, and several studies show the efficacy of OMT on inflammatory tissue levels [28][29][30][31][32][33][34][35].
LGI would act on the ECM, and alter its structure, such as in fibrosis, which is defined as a lesion of the connective component in an organ or tissue [36].
These alterations occur through mechanisms mediated by the environment in which the tissues are placed, namely water [37][38][39][40][41].
The water under consideration is water present in living matter. It has particular biophysical characteristics, which could exemplify the functioning of both healthy and injured tissues [39][42][43].
2. New Model Regarding the Characteristics of Somatic Dysfunction
With the acronym TART (tenderness, asymmetry, range of motion abnormality, and tissue texture changes), osteopathic literature accurately provides the characteristic elements of SD, at which OMT is aimed [7][8][21]. However, some researchers disagree on the relevance to be attributed to different clinical signs: some indicate the range of motion abnormality as fundamental for a diagnosis of SD, but there is no univocal evidence on the reproducibility in the evaluation [44]. Other researchers suggest the need for the presence of at least 2 of these 4 signs; still, others do not consider the sign of hypersensitivity or tenderness [1][5][6][7][45]. Regarding the asymmetries of the musculoskeletal structures, these can occur for a variety of causes, and are, therefore, difficult to attribute solely to SD [46][47][48].
In light of the results of this research, researchers believe that among the 4 clinical signs considered, tissue texture changes are the most significant to define an SD, thus proposing the hypothesis that SD can be compared to a condition of LGI.
The mechanisms underlying SD are still widely discussed in the literature, but it is reasonable to think that without first having tissue texture changes, caused by inflammatory phenomena, the presence of the other three clinical signs is not possible.
The researchers suggest that an inflammatory phenomenon could determine an alteration of the tissue as described in the chapters above, and only subsequently tenderness, altered movement, and asymmetry of the musculoskeletal structures can occur (Figure 1D).
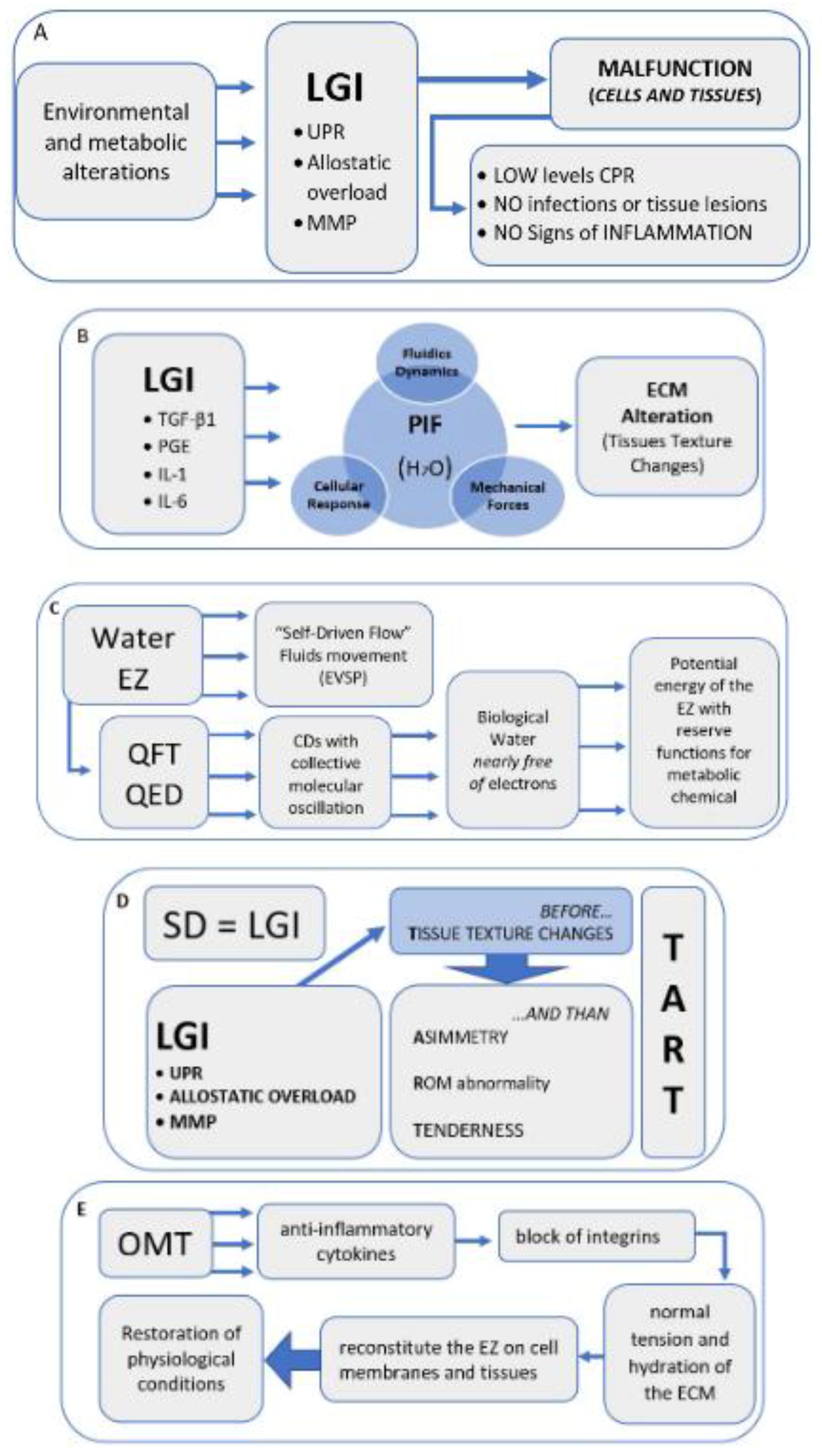 Figure 1. Narrative flow charts.
Figure 1. Narrative flow charts.The timing just described could be explained by one of the most accredited mechanisms of the onset and maintenance of SD: the neurogenic inflammation [1][49][50], in which the primary afferent nociceptors (PAN) determine the release in the periphery of neuropeptides, such as substance P and calcitonin gene-related peptide (CGRP). The neurotransmitters mentioned above are released into the peripheral peri-vascular and extracellular space through an antidromic signal, causing a local inflammatory response with alterations of the surrounding tissue. It should be noted that this area, by means of the axonal branch, can be very large [51].
These neuropeptides have vasoactive functions, recalling immune cells, activating mast cells, and releasing histamine, thus acting on the trophic state of the innervated organ [21][49][51][52]. Together, they contribute to the possible genesis of tissue alterations, also influencing the recovery of tissue lesions and their repair [53].
The nerve fibers involved would be predominantly the poorly myelinated C or A-delta, fibers of the interoceptive component which, therefore, represent the afferent portion of the sympathetic efference [54].
It has been demonstrated that sympathetic efference plays a decisive role in the onset of inflammatory phenomena [55]. These findings agree with what Denslow and Korr underscored regarding SD, as it pertains to expressiveness of phenomena related to neurogenic inflammation [56] and autonomic sympathetic innervation [57].
There may be mechanisms capable of leading to tissue alteration, which are associated with the dynamics of neurogenic inflammation. These dynamics are all probably linked to inflammatory phenomena, such as the unfolded protein response (UPR) [58][59][60][61], as well as the alteration of the functions of the MMP [62][63], which would determine changes to the functions of the ECM [64][65]. Last but not least, the allostatic overload would cause tissue alteration [66].
SD does not represent a real pathological condition [4]. In fact, as for LGI, it would not have a direct cause, such as trauma or tissue injury. Rather, SD appears as an alteration in tissue function, a sign of altered homeostasis, often lasting over time, and, like LGI, it can be placed between a homeostatic basal state and the actual inflammatory response [37][67].
There are studies on the efficacy of OMT in healthy people diagnosed with non-symptomatic SD [45][68][69][70]; the clinical conditions of these subjects could be associated with LGI, in which the blood inflammation markers are modest.
However, the signs of DS are not associated with the classic signs of inflammation. SD represents a sign of metabolic alteration that manifests itself with the alteration of the tissue texture, leading to tissue fibrosis and possible sclerosis and, therefore, is diagnosable through palpation [20][21][36].
The existence of a restriction barrier within the range of motion, a characteristic sign of SD [20][21][22], implies the alteration, both quantitative and qualitative, of a tissue or a joint region in a given district. This alteration is generated on an inflammatory substrate, without necessarily showing signs of classic inflammation [22].
References
- Fryer, G. Somatic dysfunction: An osteopathic conundrum. Int. J. Osteopath. Med. 2016, 22, 52–63.
- Chaitow, L. Somatic dysfunction and fascia’s gliding-potential. J. Bodyw. Mov. Ther. 2014, 18, 1–3.
- Moran, R. Somatic dysfunction—Conceptually fascinating, but does it help us address health needs? Int. J. Osteopath. Med. 2016, 22, 1–2.
- World Health Organization (WHO). International Statistical Classification of Diseases and Related Health Problems (ICD 11); WHO: Geneva, Switzerland, 2018.
- Ehrenfeuchter, W.C.; Kappler, R.E. Palpatory Examination. In Foundations of Osteopathic Medicine, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; pp. 401–409.
- Kimberly, P.E.; Halma, K. Kirksville College of Osteopathic Medicine, Department of Osteopathic Theory and Methods. Outline of Osteopathic Manipulative Procedures: The Kimberly Manual 2006; Walsworth Pub. Co.: Marceline, MO, USA, 2008.
- Di Giovanna, E.L.; Schiowitz, S.; Dowling, D.J. An Osteopathic Approach to Diagnosis and Treatment; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; 746p.
- Giusti, R.; American Association of Colleges of Osteopathic Medicine; Educational Council on Osteopathic Principles. Glossary of Osteopathic Terminology; American Association of Colleges of Osteopathic Medicine: Chevy Chase, MD, USA, 2017.
- Licciardone, J.C.; Nelson, K.E.; Glonek, T.; Sleszynski, S.L.; Cruser, d.A. Osteopathic manipulative treatment of somatic dysfunction among patients in the family practice clinic setting: A retrospective analysis. J. Osteopath. Med. 2005, 105, 537–544.
- Tramontano, M.; Tamburella, F.; Dal Farra, F.; Bergna, A.; Lunghi, C.; Innocenti, M.; Cavera, F.; Savini, F.; Manzo, V.; D’Alessandro, G. International Overview of Somatic Dysfunction Assessment and Treatment in Osteopathic Research: A Scoping Review. Healthcare 2021, 10, 28.
- Snider, K.T.; Johnson, J.C.; Snider, E.J.; Degenhardt, B.F. Increased incidence and severity of somatic dysfunction in subjects with chronic low back pain. J. Am. Osteopath. Assoc. 2008, 108, 372–378.
- Snider, K.T.; Schneider, R.P.; Snider, E.J.; Danto, J.B.; Lehnardt, C.W.; Ngo, C.S.; Johnson, J.C.; Sheneman, T.A. Correlation of Somatic Dysfunction with Gastrointestinal Endoscopic Findings: An Observational Study. J. Am. Osteopath. Assoc. 2016, 116, 358–369.
- Waddington, E.L.; Snider, K.T.; Lockwood, M.D.; Pazdernik, V.K. Incidence of Somatic Dysfunction in Healthy Newborns. J. Am. Osteopath. Assoc. 2015, 115, 654–665.
- Ruffini, N.; D’Alessandro, G.; Cardinali, L.; Frondaroli, F.; Cerritelli, F. Osteopathic manipulative treatment in gynecology and obstetrics: A systematic review. Complement. Ther. Med. 2016, 26, 72–78.
- Lanaro, D.; Ruffini, N.; Manzotti, A.; Lista, G. Osteopathic manipulative treatment showed reduction of length of stay and costs in preterm infants: A systematic review and meta-analysis. Medicine 2017, 96, e6408.
- Cicchitti, L.; Martelli, M.; Cerritelli, F. Chronic inflammatory disease and osteopathy: A systematic review. PLoS ONE. 2015, 10, e0121327.
- Cerritelli, F.; Ruffini, N.; Lacorte, E.; Vanacore, N. Osteopathic manipulative treatment in neurological diseases: Systematic review of the literature. J. Neurol. Sci. 2016, 369, 333–341.
- Cicchitti, L.; Di Lelio, A.; Barlafante, G.; Cozzolino, V.; Di Valerio, S.; Fusilli, P.; Lucisano, G.; Renzetti, C.; Verzella, M.; Rossi, M.C. Osteopathic Manipulative Treatment in Neonatal Intensive Care Units. Med. Sci. 2020, 8, 24.
- Bagagiolo, D.; Rosa, D.; Borrelli, F. Efficacy and safety of osteopathic manipulative treatment: An overview of systematic reviews. BMJ Open 2022, 12, e053468.
- Greenman, P.E. Principles of Manual Medicine, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003.
- Di Giovanna, E.L.; Amen, C.G.; Burns, D.K. An Osteopathic Approach to Diagnosis and Treatment; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020.
- Chila, A.G. Foundations of Osteopathic Medicine; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010; 1152p.
- Nicholas Penney, J. The biopsychosocial model of pain and contemporary osteopathic practice. Int. J. Osteopath. Med. 2010, 13, 42–47.
- Lunghi, C.; Baroni, F. Cynefin Framework for Evidence-Informed Clinical Reasoning and Decision-Making. J. Am. Osteopath. Assoc. 2019, 119, 312–321.
- Lunghi, C.; Consorti, G.; Tramontano, M.; Esteves, J.E.; Cerritelli, F. Perspectives on tissue adaptation related to allostatic load: Scoping review and integrative hypothesis with a focus on osteopathic palpation. J. Bodyw. Mov. Ther. 2020, 24, 212–220.
- Esteves, J.E.; Zegarra-Parodi, R.; Dun, P.; van Cerritelli, F.; Vaucher, P. Models and theoretical frameworks for osteopathic care—A critical view and call for updates and research. Int. J. Osteopath. Med. 2020, 35, 1–4.
- Bergna, A.; Vismara, L.; Parravicini, G.; Dal Farra, F. A new perspective for Somatic Dysfunction in Osteopathy: The Variability Model. J. Bodyw. Mov. Ther. 2020, 24, 181–189.
- Hodge, L.M.; Bearden, M.K.; Schander, A.; Huff, J.B.; Williams, A.; King, H.H.; Downey, H.F. Lymphatic pump treatment mobilizes leukocytes from the gut associated lymphoid tissue into lymph. Lymphat. Res. Biol. 2010, 8, 103–110.
- Meltzer, K.R.; Standley, P.R. Modeled repetitive motion strain and indirect osteopathic manipulative techniques in regulation of human fibroblast proliferation and interleukin secretion. J. Am. Osteopath. Assoc. 2007, 107, 527–536.
- Dodd, J.G.; Good, M.M.; Nguyen, T.L.; Grigg, A.I.; Batia, L.M.; Standley, P.R. In vitro biophysical strain model for understanding mechanisms of osteopathic manipulative treatment. J. Am. Osteopath. Assoc. 2006, 106, 157–166.
- Zein-Hammoud, M.; Standley, P.R. Modeled Osteopathic Manipulative Treatments: A Review of Their in Vitro Effects on Fibroblast Tissue Preparations. J. Am. Osteopath. Assoc. 2015, 115, 490–502.
- Cao, T.V.; Hicks, M.R.; Campbell, D.; Standley, P.R. Dosed myofascial release in three-dimensional bioengineered tendons: Effects on human fibroblast hyperplasia, hypertrophy, and cytokine secretion. J. Manip. Physiol. Ther. 2013, 36, 513–521.
- Schander, A.; Downey, H.F.; Hodge, L.M. Lymphatic pump manipulation mobilizes inflammatory mediators into lymphatic circulation. Exp. Biol. Med. 2012, 237, 58–63.
- Degenhardt, B.F.; Darmani, N.A.; Johnson, J.C.; Towns, L.C.; Rhodes, D.C.; Trinh, C.; McClanahan, B.; DiMarzo, V. Role of osteopathic manipulative treatment in altering pain biomarkers: A pilot study. J. Am. Osteopath. Assoc. 2007, 107, 387–400.
- Licciardone, J.C.; Kearns, C.M.; Hodge, L.M.; Bergamini, M.V. Associations of cytokine concentrations with key osteopathic lesions and clinical outcomes in patients with nonspecific chronic low back pain: Results from the OSTEOPATHIC Trial. J. Am. Osteopath. Assoc. 2012, 112, 596–605, Erratum in: J. Am. Osteopath. Assoc. 2017, 117, 350.
- Robert, S.; Gicquel, T.; Victoni, T.; Valença, S.; Barreto, E.; Bailly-Maître, B.; Boichot, E.; Lagente, V. Involvement of matrix metalloproteinases (MMPs) and inflammasome pathway in molecular mechanisms of fibrosis. Biosci. Rep. 2016, 36, e00360.
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435.
- McEwen, B.S.; Wingfield, J.C. What is in a name? Integrating homeostasis, allostasis and stress. Horm. Behav. 2010, 57, 105–111.
- Sharma, A.; Adams, C.; Cashdollar, B.D.; Li, Z.; Nguyen, N.V.; Sai, H.; Shi, J.; Velchuru, G.; Zhu, K.Z.; Pollack, G.H. Effect of Health-Promoting Agents on Exclusion-Zone Size. Dose-Response Publ. Int. Hormesis Soc. 2018, 16, 1559325818796937.
- Sharma, A.; Pollack, G.H. Healthy fats and exclusion-zone size. Food Chem. 2020, 316, 126305.
- Tozzi, P. A unifying neuro-fasciagenic model of somatic dysfunction-underlying mechanisms and treatment-Part I. J Bodyw. Mov. Ther. 2015, 19, 310–326.
- Del Giudice, E.; Tedeschi, A. Water and autocatalysis in living matter. Electromagn. Biol. Med. 2009, 28, 46–52.
- Pollack, G.H. The Fourth Phase of Water: A role in fascia? J. Bodyw. Mov. Ther. 2013, 17, 510–511.
- Degenhardt, B.F.; Snider, K.T.; Snider, E.J.; Johnson, J.C. Interobserver reliability of osteopathic palpatory diagnostic tests of the lumbar spine: Improvements from consensus training. J. Am. Osteopath. Assoc. 2005, 105, 465–473.
- Fryer, G.; Gibbons, P.; Morris, T. The relation between thoracic paraspinal tissues and pressure sensitivity measured by a digital algometer. J. Osteopath. Med. 2004, 7, 64–69.
- Brink, R.C.; Schlösser, T.P.C.; Colo, D.; Vincken, K.L.; van Stralen, M.; Hui, S.C.N.; Chu, W.C.W.; Cheng, J.C.Y.; Castelein, R.M. Asymmetry of the Vertebral Body and Pedicles in the True Transverse Plane in Adolescent Idiopathic Scoliosis: A CT-Based Study. Spine Deform. 2017, 5, 37–45.
- Kanchan, T.; Mohan Kumar, T.S.; Pradeep Kumar, G.; Yoganarasimha, K. Skeletal asymmetry. J. Forensic Leg. Med. 2008, 15, 177–179.
- Thevenot, J.; Pulkkinen, P.; Kuhn, V.; Eckstein, F.; Jämsä, T. Structural asymmetry between the hips and its relation to experimental fracture type. Calcif. Tissue Int. 2010, 87, 203–210.
- Howell, J.N.; Willard, F. Nociception: New Understandings and Their Possible Relation to Somatic Dysfunction and Its Treatment. Ohio. Res. Clin. Rev. 2005, 15, 12–15.
- D’Alessandro, G.; Cerritelli, F.; Cortelli, P. Sensitization and Interoception as Key Neurological Concepts in Osteopathy and Other Manual Medicines. Front. Neurosci. 2016, 10, 100.
- Sorkin, L.S.; Eddinger, K.A.; Woller, S.A.; Yaksh, T.L. Origins of antidromic activity in sensory afferent fibers and neurogenic inflammation. Semin. Immunopathol. 2018, 40, 237–247.
- Xanthos, D.N.; Sandkühler, J. Neurogenic neuroinflammation: Inflammatory CNS reactions in response to neuronal activity. Nat. Rev. Neurosci. 2014, 15, 43–53.
- Brain, S.D. Sensory neuropeptides: Their role in inflammation and wound healing. Immunopharmacology 1997, 37, 133–152.
- Craig, A.D. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002, 3, 655–666.
- Wang, J.; Ren, Y.; Zou, X.; Fang, L.; Willis, W.D.; Lin, Q. Sympathetic influence on capsaicin-evoked enhancement of dorsal root reflexes in rats. J. Neurophysiol. 2004, 92, 2017–2026.
- Denslow, J.S. Pathophysiologic evidence for the osteopathic lesion: The known, unknown, and controversial. J. Am. Osteopath. Assoc. 1975, 75, 415–421.
- Korr, I.M. The neural basis of the osteopathic lesion. J. Am. Osteopath. Assoc. 1947, 47, 191–198.
- Amen, O.M.; Sarker, S.D.; Ghildyal, R.; Arya, A. Endoplasmic Reticulum Stress Activates Unfolded Protein Response Signaling and Mediates Inflammation, Obesity, and Cardiac Dysfunction: Therapeutic and Molecular Approach. Front. Pharmacol. 2019, 10, 977.
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867.
- Gusev, E.Y.; Zotova, N.V. Cellular Stress and General Pathological Processes. Curr. Pharm. Des. 2019, 25, 251–297.
- Todd, D.J.; Lee, A.-H.; Glimcher, L.H. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat. Rev. Immunol. 2008, 8, 663–674.
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462.
- Knipper, J.A.; Willenborg, S.; Brinckmann, J.; Bloch, W.; Maaß, T.; Wagener, R.; Krieg, T.; Sutherland, T.; Munitz, A.; Rothenberg, M.E.; et al. Interleukin-4 Receptor α Signaling in Myeloid Cells Controls Collagen Fibril Assembly in Skin Repair. Immunity 2015, 43, 803–816.
- Malemud, C.J. Matrix metalloproteinases (MMPs) in health and disease: An overview. Front. Biosci. J. Virtual Libr. 2006, 11, 1696–1701.
- Alameddine, H.S. Matrix metalloproteinases in skeletal muscles: Friends or foes? Neurobiol. Dis. 2012, 48, 508–518.
- Rohleder, N. Stress System Regulation of Chronic Low-grade Inflammation. Adv. Neuroimmune Biol. 2012, 3, 265–276.
- Antonelli, M.; Kushner, I. It’s time to redefine inflammation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 1787–1791.
- Walkowski, S.; Singh, M.; Puertas, J.; Pate, M.; Goodrum, K.; Benencia, F. Osteopathic manipulative therapy induces early plasma cytokine release and mobilization of a population of blood dendritic cells. PLoS ONE 2014, 9, e90132.
- Ruffini, N.; D’Alessandro, G.; Mariani, N.; Pollastrelli, A.; Cardinali, L.; Cerritelli, F. Variations of high frequency parameter of heart rate variability following osteopathic manipulative treatment in healthy subjects compared to control group and sham therapy: Randomized controlled trial. Front. Neurosci. 2015, 9, 272.
- Giles, P.D.; Hensel, K.L.; Pacchia, C.F.; Smith, M.L. Suboccipital decompression enhances heart rate variability indices of cardiac control in healthy subjects. J. Altern. Complement. Med. N. Y. 2013, 19, 92–96.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
14 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




