Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Luca Di Pietrantonio.
Somatic dysfunction (SD) is classified by the ICD 11 as a “Biomechanical lesion, not elsewhere classified”; however, the definitions are not equally shared and codified by osteopathic professionals.
- exclusion zone water
- interstitial fluid pressure
- water
- somatic dysfunction
1. Introduction
The main means available to osteopathic medicine is to assess tissues by palpating, in particular tissues of the musculoskeletal system, with the aim of diagnosing a possible somatic dysfunction (SD).
By underscoring some contradictory aspects, several reseauthorchers have called into question SD, by defining it as a nosological entity detectable on palpation [1,2,3][1][2][3].
SD is classified by the ICD 11 [4] as a “Biomechanical lesion, not elsewhere classified”; however, the definitions are not equally shared and codified by osteopathic professionals [1,5,6,7][1][5][6][7].
SD presents the characteristics of impaired or altered function of components related to the somatic system, involving skeletal, arthrodial, and myofascial structures, and osteopathic manipulative treatment (OMT) is aimed at the treatment of SD [8,9,10][8][9][10].
The osteopathic literature describes the relationship between SD and OMT in many studies [10,11,12,13][10][11][12][13].
OMT is a drug-free manual medicine, a patient-centered, whole-body intervention. OMT has shown positive effects in different fields such as gynecology and obstetrics, neonatology, chronic inflammatory disease management, and musculoskeletal disorders [14,15,16,17,18,19][14][15][16][17][18][19].
There are many aspects to consider regarding the etiology and diagnosis of SD, and the osteopathic literature provides details on the signs that characterize it, including tissue texture changes [8,20,21,22][8][20][21][22].
Over the last few years, some reseauthorchers have proposed a variety of interpretation models in order to clarify the mechanisms of onset and the inherent characteristics of tissue alterations concerning SD. Among such models, there are also clinical reasoning and decision-making procedures suitable to establish a treatment routine [23,24,25,26,27][23][24][25][26][27].
Recent knowledge suggests that tissue, and, in particular, connective tissue, may react by modulating the inflammation degree. This issue should also be extended to any response to OMT, and several studies show the efficacy of OMT on inflammatory tissue levels [28,29,30,31,32,33,34,35][28][29][30][31][32][33][34][35].
LGI would act on the ECM, and alter its structure, such as in fibrosis, which is defined as a lesion of the connective component in an organ or tissue [36].
These alterations occur through mechanisms mediated by the environment in which the tissues are placed, namely water [37,38,39,40,41][37][38][39][40][41].
The water under consideration is water present in living matter. It has particular biophysical characteristics, which could exemplify the functioning of both healthy and injured tissues [39,42,43][39][42][43].
2. New Model Regarding the Characteristics of Somatic Dysfunction
With the acronym TART (tenderness, asymmetry, range of motion abnormality, and tissue texture changes), osteopathic literature accurately provides the characteristic elements of SD, at which OMT is aimed [7,8,21][7][8][21]. However, some reseauthorchers disagree on the relevance to be attributed to different clinical signs: some indicate the range of motion abnormality as fundamental for a diagnosis of SD, but there is no univocal evidence on the reproducibility in the evaluation [114][44]. Other reseauthorchers suggest the need for the presence of at least 2 of these 4 signs; still, others do not consider the sign of hypersensitivity or tenderness [1,5,6,7,115][1][5][6][7][45]. Regarding the asymmetries of the musculoskeletal structures, these can occur for a variety of causes, and are, therefore, difficult to attribute solely to SD [116,117,118][46][47][48]. In light of the results of this revisew, wearch, researchers believe that among the 4 clinical signs considered, tissue texture changes are the most significant to define an SD, thus proposing the hypothesis that SD can be compared to a condition of LGI. The mechanisms underlying SD are still widely discussed in the literature, but it is reasonable to think that without first having tissue texture changes, caused by inflammatory phenomena, the presence of the other three clinical signs is not possible. WThe researchers suggest that an inflammatory phenomenon could determine an alteration of the tissue as described in the chapters above, and only subsequently tenderness, altered movement, and asymmetry of the musculoskeletal structures can occur (Figure 21D).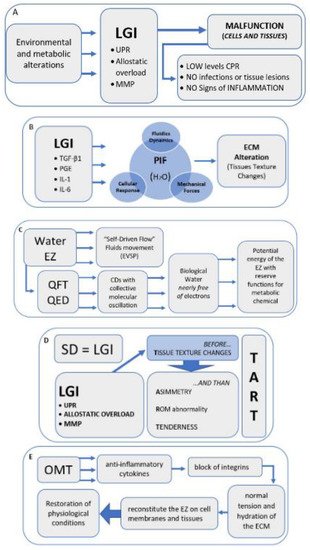
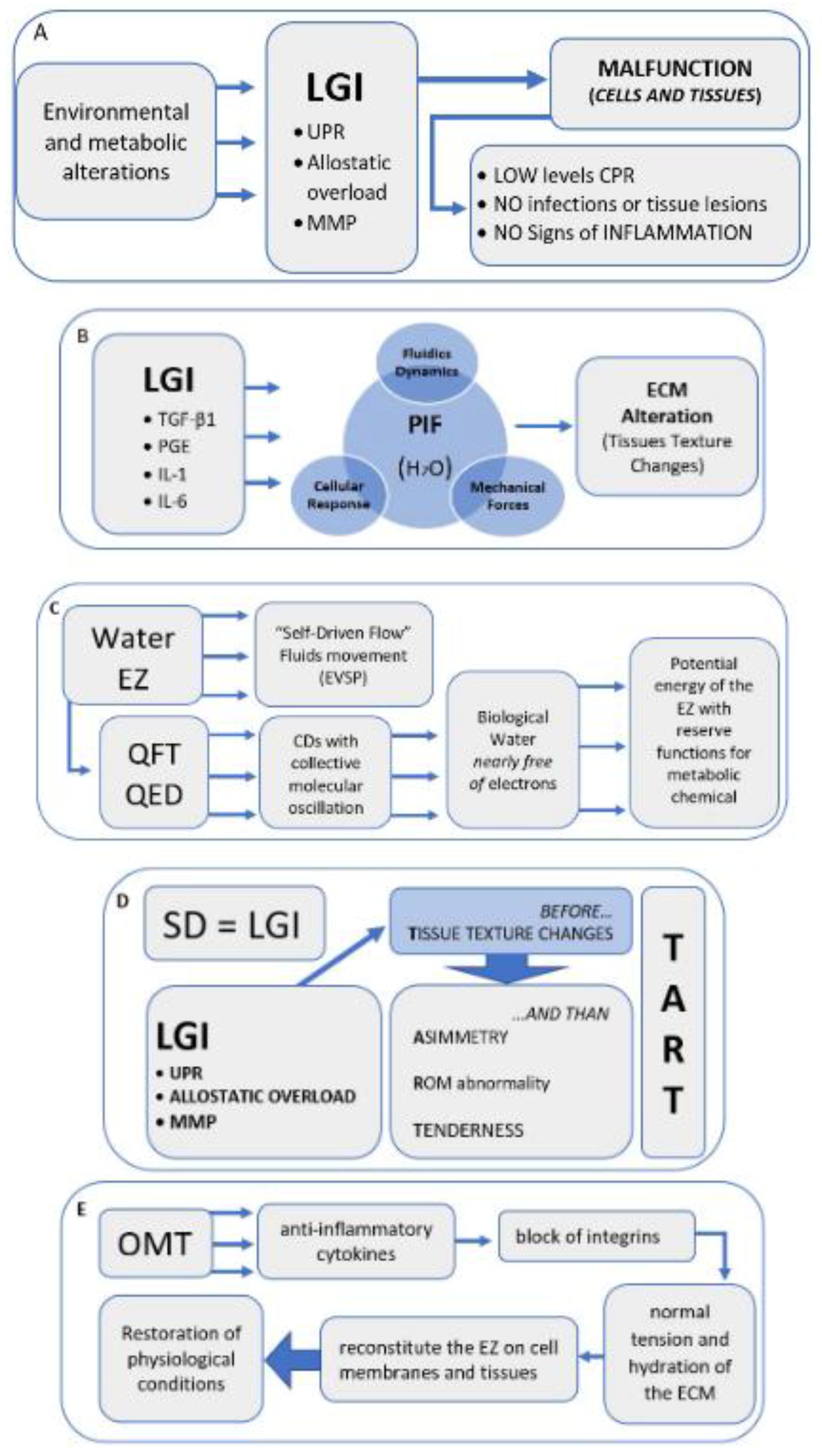
Figure 21. Narrative flow charts.
Narrative flow charts.
References
- Fryer, G. Somatic dysfunction: An osteopathic conundrum. Int. J. Osteopath. Med. 2016, 22, 52–63.
- Chaitow, L. Somatic dysfunction and fascia’s gliding-potential. J. Bodyw. Mov. Ther. 2014, 18, 1–3.
- Moran, R. Somatic dysfunction—Conceptually fascinating, but does it help us address health needs? Int. J. Osteopath. Med. 2016, 22, 1–2.
- World Health Organization (WHO). International Statistical Classification of Diseases and Related Health Problems (ICD 11); WHO: Geneva, Switzerland, 2018.
- Ehrenfeuchter, W.C.; Kappler, R.E. Palpatory Examination. In Foundations of Osteopathic Medicine, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; pp. 401–409.
- Kimberly, P.E.; Halma, K. Kirksville College of Osteopathic Medicine, Department of Osteopathic Theory and Methods. Outline of Osteopathic Manipulative Procedures: The Kimberly Manual 2006; Walsworth Pub. Co.: Marceline, MO, USA, 2008.
- Di Giovanna, E.L.; Schiowitz, S.; Dowling, D.J. An Osteopathic Approach to Diagnosis and Treatment; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; 746p.
- Giusti, R.; American Association of Colleges of Osteopathic Medicine; Educational Council on Osteopathic Principles. Glossary of Osteopathic Terminology; American Association of Colleges of Osteopathic Medicine: Chevy Chase, MD, USA, 2017.
- Licciardone, J.C.; Nelson, K.E.; Glonek, T.; Sleszynski, S.L.; Cruser, d.A. Osteopathic manipulative treatment of somatic dysfunction among patients in the family practice clinic setting: A retrospective analysis. J. Osteopath. Med. 2005, 105, 537–544.
- Tramontano, M.; Tamburella, F.; Dal Farra, F.; Bergna, A.; Lunghi, C.; Innocenti, M.; Cavera, F.; Savini, F.; Manzo, V.; D’Alessandro, G. International Overview of Somatic Dysfunction Assessment and Treatment in Osteopathic Research: A Scoping Review. Healthcare 2021, 10, 28.
- Snider, K.T.; Johnson, J.C.; Snider, E.J.; Degenhardt, B.F. Increased incidence and severity of somatic dysfunction in subjects with chronic low back pain. J. Am. Osteopath. Assoc. 2008, 108, 372–378.
- Snider, K.T.; Schneider, R.P.; Snider, E.J.; Danto, J.B.; Lehnardt, C.W.; Ngo, C.S.; Johnson, J.C.; Sheneman, T.A. Correlation of Somatic Dysfunction with Gastrointestinal Endoscopic Findings: An Observational Study. J. Am. Osteopath. Assoc. 2016, 116, 358–369.
- Waddington, E.L.; Snider, K.T.; Lockwood, M.D.; Pazdernik, V.K. Incidence of Somatic Dysfunction in Healthy Newborns. J. Am. Osteopath. Assoc. 2015, 115, 654–665.
- Ruffini, N.; D’Alessandro, G.; Cardinali, L.; Frondaroli, F.; Cerritelli, F. Osteopathic manipulative treatment in gynecology and obstetrics: A systematic review. Complement. Ther. Med. 2016, 26, 72–78.
- Lanaro, D.; Ruffini, N.; Manzotti, A.; Lista, G. Osteopathic manipulative treatment showed reduction of length of stay and costs in preterm infants: A systematic review and meta-analysis. Medicine 2017, 96, e6408.
- Cicchitti, L.; Martelli, M.; Cerritelli, F. Chronic inflammatory disease and osteopathy: A systematic review. PLoS ONE. 2015, 10, e0121327.
- Cerritelli, F.; Ruffini, N.; Lacorte, E.; Vanacore, N. Osteopathic manipulative treatment in neurological diseases: Systematic review of the literature. J. Neurol. Sci. 2016, 369, 333–341.
- Cicchitti, L.; Di Lelio, A.; Barlafante, G.; Cozzolino, V.; Di Valerio, S.; Fusilli, P.; Lucisano, G.; Renzetti, C.; Verzella, M.; Rossi, M.C. Osteopathic Manipulative Treatment in Neonatal Intensive Care Units. Med. Sci. 2020, 8, 24.
- Bagagiolo, D.; Rosa, D.; Borrelli, F. Efficacy and safety of osteopathic manipulative treatment: An overview of systematic reviews. BMJ Open 2022, 12, e053468.
- Greenman, P.E. Principles of Manual Medicine, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003.
- Di Giovanna, E.L.; Amen, C.G.; Burns, D.K. An Osteopathic Approach to Diagnosis and Treatment; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020.
- Chila, A.G. Foundations of Osteopathic Medicine; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010; 1152p.
- Nicholas Penney, J. The biopsychosocial model of pain and contemporary osteopathic practice. Int. J. Osteopath. Med. 2010, 13, 42–47.
- Lunghi, C.; Baroni, F. Cynefin Framework for Evidence-Informed Clinical Reasoning and Decision-Making. J. Am. Osteopath. Assoc. 2019, 119, 312–321.
- Lunghi, C.; Consorti, G.; Tramontano, M.; Esteves, J.E.; Cerritelli, F. Perspectives on tissue adaptation related to allostatic load: Scoping review and integrative hypothesis with a focus on osteopathic palpation. J. Bodyw. Mov. Ther. 2020, 24, 212–220.
- Esteves, J.E.; Zegarra-Parodi, R.; Dun, P.; van Cerritelli, F.; Vaucher, P. Models and theoretical frameworks for osteopathic care—A critical view and call for updates and research. Int. J. Osteopath. Med. 2020, 35, 1–4.
- Bergna, A.; Vismara, L.; Parravicini, G.; Dal Farra, F. A new perspective for Somatic Dysfunction in Osteopathy: The Variability Model. J. Bodyw. Mov. Ther. 2020, 24, 181–189.
- Hodge, L.M.; Bearden, M.K.; Schander, A.; Huff, J.B.; Williams, A.; King, H.H.; Downey, H.F. Lymphatic pump treatment mobilizes leukocytes from the gut associated lymphoid tissue into lymph. Lymphat. Res. Biol. 2010, 8, 103–110.
- Meltzer, K.R.; Standley, P.R. Modeled repetitive motion strain and indirect osteopathic manipulative techniques in regulation of human fibroblast proliferation and interleukin secretion. J. Am. Osteopath. Assoc. 2007, 107, 527–536.
- Dodd, J.G.; Good, M.M.; Nguyen, T.L.; Grigg, A.I.; Batia, L.M.; Standley, P.R. In vitro biophysical strain model for understanding mechanisms of osteopathic manipulative treatment. J. Am. Osteopath. Assoc. 2006, 106, 157–166.
- Zein-Hammoud, M.; Standley, P.R. Modeled Osteopathic Manipulative Treatments: A Review of Their in Vitro Effects on Fibroblast Tissue Preparations. J. Am. Osteopath. Assoc. 2015, 115, 490–502.
- Cao, T.V.; Hicks, M.R.; Campbell, D.; Standley, P.R. Dosed myofascial release in three-dimensional bioengineered tendons: Effects on human fibroblast hyperplasia, hypertrophy, and cytokine secretion. J. Manip. Physiol. Ther. 2013, 36, 513–521.
- Schander, A.; Downey, H.F.; Hodge, L.M. Lymphatic pump manipulation mobilizes inflammatory mediators into lymphatic circulation. Exp. Biol. Med. 2012, 237, 58–63.
- Degenhardt, B.F.; Darmani, N.A.; Johnson, J.C.; Towns, L.C.; Rhodes, D.C.; Trinh, C.; McClanahan, B.; DiMarzo, V. Role of osteopathic manipulative treatment in altering pain biomarkers: A pilot study. J. Am. Osteopath. Assoc. 2007, 107, 387–400.
- Licciardone, J.C.; Kearns, C.M.; Hodge, L.M.; Bergamini, M.V. Associations of cytokine concentrations with key osteopathic lesions and clinical outcomes in patients with nonspecific chronic low back pain: Results from the OSTEOPATHIC Trial. J. Am. Osteopath. Assoc. 2012, 112, 596–605, Erratum in: J. Am. Osteopath. Assoc. 2017, 117, 350.
- Robert, S.; Gicquel, T.; Victoni, T.; Valença, S.; Barreto, E.; Bailly-Maître, B.; Boichot, E.; Lagente, V. Involvement of matrix metalloproteinases (MMPs) and inflammasome pathway in molecular mechanisms of fibrosis. Biosci. Rep. 2016, 36, e00360.
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435.
- McEwen, B.S.; Wingfield, J.C. What is in a name? Integrating homeostasis, allostasis and stress. Horm. Behav. 2010, 57, 105–111.
- Sharma, A.; Adams, C.; Cashdollar, B.D.; Li, Z.; Nguyen, N.V.; Sai, H.; Shi, J.; Velchuru, G.; Zhu, K.Z.; Pollack, G.H. Effect of Health-Promoting Agents on Exclusion-Zone Size. Dose-Response Publ. Int. Hormesis Soc. 2018, 16, 1559325818796937.
- Sharma, A.; Pollack, G.H. Healthy fats and exclusion-zone size. Food Chem. 2020, 316, 126305.
- Tozzi, P. A unifying neuro-fasciagenic model of somatic dysfunction-underlying mechanisms and treatment-Part I. J Bodyw. Mov. Ther. 2015, 19, 310–326.
- Del Giudice, E.; Tedeschi, A. Water and autocatalysis in living matter. Electromagn. Biol. Med. 2009, 28, 46–52.
- Pollack, G.H. The Fourth Phase of Water: A role in fascia? J. Bodyw. Mov. Ther. 2013, 17, 510–511.
- Degenhardt, B.F.; Snider, K.T.; Snider, E.J.; Johnson, J.C. Interobserver reliability of osteopathic palpatory diagnostic tests of the lumbar spine: Improvements from consensus training. J. Am. Osteopath. Assoc. 2005, 105, 465–473.
- Fryer, G.; Gibbons, P.; Morris, T. The relation between thoracic paraspinal tissues and pressure sensitivity measured by a digital algometer. J. Osteopath. Med. 2004, 7, 64–69.
- Brink, R.C.; Schlösser, T.P.C.; Colo, D.; Vincken, K.L.; van Stralen, M.; Hui, S.C.N.; Chu, W.C.W.; Cheng, J.C.Y.; Castelein, R.M. Asymmetry of the Vertebral Body and Pedicles in the True Transverse Plane in Adolescent Idiopathic Scoliosis: A CT-Based Study. Spine Deform. 2017, 5, 37–45.
- Kanchan, T.; Mohan Kumar, T.S.; Pradeep Kumar, G.; Yoganarasimha, K. Skeletal asymmetry. J. Forensic Leg. Med. 2008, 15, 177–179.
- Thevenot, J.; Pulkkinen, P.; Kuhn, V.; Eckstein, F.; Jämsä, T. Structural asymmetry between the hips and its relation to experimental fracture type. Calcif. Tissue Int. 2010, 87, 203–210.
- Howell, J.N.; Willard, F. Nociception: New Understandings and Their Possible Relation to Somatic Dysfunction and Its Treatment. Ohio. Res. Clin. Rev. 2005, 15, 12–15.
- D’Alessandro, G.; Cerritelli, F.; Cortelli, P. Sensitization and Interoception as Key Neurological Concepts in Osteopathy and Other Manual Medicines. Front. Neurosci. 2016, 10, 100.
- Sorkin, L.S.; Eddinger, K.A.; Woller, S.A.; Yaksh, T.L. Origins of antidromic activity in sensory afferent fibers and neurogenic inflammation. Semin. Immunopathol. 2018, 40, 237–247.
- Xanthos, D.N.; Sandkühler, J. Neurogenic neuroinflammation: Inflammatory CNS reactions in response to neuronal activity. Nat. Rev. Neurosci. 2014, 15, 43–53.
- Brain, S.D. Sensory neuropeptides: Their role in inflammation and wound healing. Immunopharmacology 1997, 37, 133–152.
- Craig, A.D. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002, 3, 655–666.
- Wang, J.; Ren, Y.; Zou, X.; Fang, L.; Willis, W.D.; Lin, Q. Sympathetic influence on capsaicin-evoked enhancement of dorsal root reflexes in rats. J. Neurophysiol. 2004, 92, 2017–2026.
- Denslow, J.S. Pathophysiologic evidence for the osteopathic lesion: The known, unknown, and controversial. J. Am. Osteopath. Assoc. 1975, 75, 415–421.
- Korr, I.M. The neural basis of the osteopathic lesion. J. Am. Osteopath. Assoc. 1947, 47, 191–198.
- Amen, O.M.; Sarker, S.D.; Ghildyal, R.; Arya, A. Endoplasmic Reticulum Stress Activates Unfolded Protein Response Signaling and Mediates Inflammation, Obesity, and Cardiac Dysfunction: Therapeutic and Molecular Approach. Front. Pharmacol. 2019, 10, 977.
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867.
- Gusev, E.Y.; Zotova, N.V. Cellular Stress and General Pathological Processes. Curr. Pharm. Des. 2019, 25, 251–297.
- Todd, D.J.; Lee, A.-H.; Glimcher, L.H. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat. Rev. Immunol. 2008, 8, 663–674.
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462.
- Knipper, J.A.; Willenborg, S.; Brinckmann, J.; Bloch, W.; Maaß, T.; Wagener, R.; Krieg, T.; Sutherland, T.; Munitz, A.; Rothenberg, M.E.; et al. Interleukin-4 Receptor α Signaling in Myeloid Cells Controls Collagen Fibril Assembly in Skin Repair. Immunity 2015, 43, 803–816.
- Malemud, C.J. Matrix metalloproteinases (MMPs) in health and disease: An overview. Front. Biosci. J. Virtual Libr. 2006, 11, 1696–1701.
- Alameddine, H.S. Matrix metalloproteinases in skeletal muscles: Friends or foes? Neurobiol. Dis. 2012, 48, 508–518.
- Rohleder, N. Stress System Regulation of Chronic Low-grade Inflammation. Adv. Neuroimmune Biol. 2012, 3, 265–276.
- Antonelli, M.; Kushner, I. It’s time to redefine inflammation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 1787–1791.
- Walkowski, S.; Singh, M.; Puertas, J.; Pate, M.; Goodrum, K.; Benencia, F. Osteopathic manipulative therapy induces early plasma cytokine release and mobilization of a population of blood dendritic cells. PLoS ONE 2014, 9, e90132.
- Ruffini, N.; D’Alessandro, G.; Mariani, N.; Pollastrelli, A.; Cardinali, L.; Cerritelli, F. Variations of high frequency parameter of heart rate variability following osteopathic manipulative treatment in healthy subjects compared to control group and sham therapy: Randomized controlled trial. Front. Neurosci. 2015, 9, 272.
- Giles, P.D.; Hensel, K.L.; Pacchia, C.F.; Smith, M.L. Suboccipital decompression enhances heart rate variability indices of cardiac control in healthy subjects. J. Altern. Complement. Med. N. Y. 2013, 19, 92–96.
More
