
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Salvatore Cozzi | -- | 5487 | 2022-06-09 10:32:01 | | | |
| 2 | Vivi Li | -3699 word(s) | 1788 | 2022-06-10 03:16:22 | | |
Video Upload Options
Breast cancer represents the second leading cause of cancer-related death in the female population, despite continuing advances in treatment options that have significantly accelerated in recent years. Conservative treatments have radically changed the concept of healing, also focusing on the psychological aspect of oncological treatments. In this scenario, radiotherapy plays a key role. Brachytherapy is an extremely versatile radiation technique that can be used in various settings for breast cancer treatment. Although it is invasive, technically complex, and requires a long learning curve, the dosimetric advantages and sparing of organs at risk are unequivocal. Literature data support muticatheter interstitial brachytherapy as the only method with strong scientific evidence to perform partial breast irradiation and reirradiation after previous conservative surgery and external beam radiotherapy, with longer follow-up than new, emerging radiation techniques, whose effectiveness is proven by over 20 years of experience.
1. Introduction
2. Implant Technique and Treatment Delivery
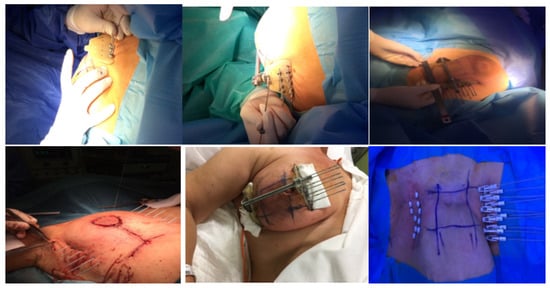
2.1. Catheter Insertion
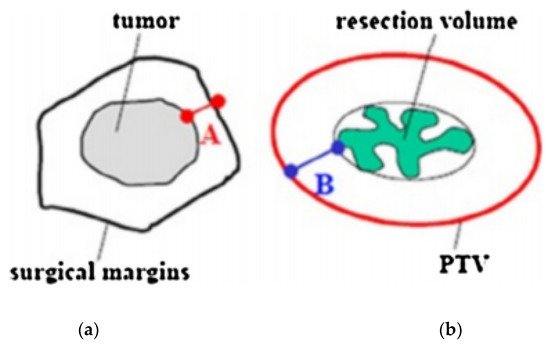
2.2. Target Definition and Delineation
2.3. Dosimetry
| Organs | Constraints |
|---|---|
| Ipsilateral no target breast tissue | V90 < 10% V50 < 40% |
| Skin | D1 cm3 < 90% D0.2 cm3 < 100% |
| Ribs | D0.1 cm3 < 90% D1 cm3 < 80% |
| Heart | MHD < 8% D0.1 cm3 < 50% |
| Ipsilateral lung | MLD < 8% D0.1 cm3 < 60% |
3. Brachytherapy Doses
4. Advantages and Disadvantages of the Technique
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33.
- Fisher, B.; Bauer, M.; Margolese, R.; Poisson, R.; Pylch, Y.; Redmond, C.; Fisher, E.; Wolmark, N.; Deutsch, M.; Montague, E.; et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N. Engl. J. Med. 1985, 312, 665–673.
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.; Deutch, M.; Fisher, E.R.; Jeong, J.H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 347, 1233–1241.
- Veronesi, U.; Saccozzi, R.; Del Vecchio, M.; Banfi, A.; Clemente, C.; De Lena, M.; Gallus, G.; Greco, M.; Luini, A.; Marubini, E.; et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N. Engl. J. Med. 1981, 305, 6–11.
- Veronesi, U.; Cascinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubuni, E. Twenty year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 2002, 347, 1227–1232.
- Keynes, G. The place of radium in the treatment of cancer of the breast. Ann. Surg. 1937, 106, 619–630.
- Polgar, C.; Fodor, J.; Major, T.; Sulyok, Z.; Kásler, M. Breast-conserving therapy with partial or whole breast irradiation: Ten-year results of the Budapest randomized trial. Radiother. Oncol. 2013, 108, 197–202.
- Ott, O.J.; Hildebrandt, G.; Potter, R.; Hammer, J.; Lotter, M.; Resch, A.; Sauer, R.; Strnad, V. Accelerated partial breast irradiation with multi-catheter brachytherapy: Local control, side effects and cosmetic outcome for 274 patients. Results of the GermanAustrian multi-centre trial. Radiother. Oncol. 2007, 82, 281–286.
- Strnad, V.; Hildebrandt, G.; Pötter, R.; Hammer, J.; Hindemith, M.; Resch, A.; Spiegl, K.; Lotter, M.; Uter, W.; Bani, M.; et al. Accelerated partial breast irradiation: 5-year results of the German-ustrian multicenter phase II trial using interstitial multicatheter brachytherapy alone after breast-conserving surgery. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 17–24.
- Polgar, C.; Major, T.; Fodor, J.; Sulyok, Z.; Somogyi, A.; Lovey, K.; Nemeth, G.; Klaser, M. Accelerated partial-breast irradiation using high-dose-rate interstitial brachytherapy: 12-year update of a prospective clinical study. Radiother. Oncol. 2010, 94, 274–279.
- Hannoun-Levi, J.M.; Resch, A.; Gal, J.; Kauer-Dorner, D.; Strnad, V.; Niehoff, P.; Loessl, K.; Kovács, G.; Van Lim-bergen, E.; Polgár, C.; et al. Accelerated partial breast irradiation with interstitial brachytherapy as second conservative treatment for ipsilateral breast tumour recurrence: Multicentric study of the GEC-ESTRO Breast Cancer Working Group. Radiother. Oncol. 2013, 108, 226–231.
- Pierquin, B.; Wilson, J.-F.; Chassagne, D. The Paris System. In Modern Brachytherapy; Pierquin, B., Wilson, J.F., Chassagne, D., Eds.; Masson: Paris, France, 1987.
- Major, T.; Fröhlich, G.; Lövey, K.; Fodor, J.; Polgár, C. Dosimetric experience with accelerated partial breastirradiation using image-guided interstitial brachytherapy. Radiother. Oncol. 2009, 90, 48–55.
- Polgar, C.; Strnad, V.; Major, T. Brachytherapy for partial breast irradiation: The European experience. Semin. Radiat. Oncol. 2005, 15, 116–122.
- Polgar, C.; Van Limbergen, E.; Potter, R.; Kovacs, G.; Polo, A.; Lyczek, J.; Hildebrandt, G.; Niehoff, P.; Guinot, J.L.; Guedea, F.; et al. Patient selection for accelerated partial-breast irradiation (APBI) after breastconserving surgery: Recommendations of the Group Europeen de Curietherapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother. Oncol. 2010, 94, 264–273.
- Strnad, V.; Hannoun-Lévi, J.-M.; Guinot, J.-L.; Lössl, K.; Kauer-Dorner, D.; Resch, A.; Kovacs, G.; Major, T.; Van Limnergen, E. Recommendations from GEC ESTRO Breast Cancer Working Group (I): Target definition and target delineation for accelerated or boost partial breast irradiation using multicatheter interstitial brachytherapy after breast conserving closed cavity surgery. Radiother. Oncol. 2015, 115, 342–348.
- Major, T.; Gutiérrez, C.; Guix, B.; van Limbergen, E.; Strnad, V.; Polgar, C. Recommendations from GEC ESTRO Breast Cancer Working Group (II): Target definition and target delineation for accelerated or boost partial breast irradiation using multicatheter interstitial brachytherapy after breast conserving open cavity surgery. Radiother. Oncol. 2016, 118, 199–204.
- Strnad, V.; Ott, O.J.; Hildebrandt, G.; Kauer-Dorner, D.; Knauerhase, H.; Major, T.; Lyczek, J.; Guinot, J.L.; Dunst, J.; Gutierrez miguelez, C.; et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: A randomised, phase 3, non-inferiority trial. Lancet 2016, 387, 229–238.
- Sato, K.; Shimo, T.; Fuchikami, H.; Takeda, N.; Kato, M.; Okawa, T. Catheter-based delineation of lumpectomy cavity for accurate target definition in partial-breast irradiation with multicatheter interstitial brachytherapy. J. Contemp. Brachyther. 2019, 11, 108–115.
- Strnad, V.; Major, T.; Polgar, C.; Lotter, M.; Guinot, J.L. ESTRO-ACROP guideline: Interstitial multi-catheter breast brachytherapy as Accelerated Partial Breast Irradiation alone or as boost—GEC-ESTRO Breast Cancer Working Group practical recommendations. Radiother. Oncol. 2018, 128, 411–420.
- Chargari, C.; Van Limbergen, E.; Mahantshetty, U.; Deutsch, E.; Haie-Meder, C. Radiobiology of brachytherapy: The historical view based on linear quadratic model and perspectives for optimization. Cancer Radiother. 2018, 22, 312–318.
- Georg, D.; Kirisits, C.; Hillbrand, M.; Dimopoulos, J.; Potter, R. Image-guided radiotherapy for cervix cancer: High-tech external beam therapy versus high-tech brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1272–1278.
- Yanez, L.; Ciudad, A.M.; Mehta, M.P.; Marisglia, H. What is the evidence for the clinical value of SBRT in cancer of the cervix? Rep. Pract. Oncol. Radiother. 2018, 23, 574–579.
- Otahal, B.; Dolezel, M.; Cvek, J.; Simetka, O.; Klat, J.; Knibel, L.; MOlenda, L.; Skacelikova, E.; Hlavka, A.; Felti, D. Dosimetric comparison of MRI-based HDR brachytherapy and stereotactic radiotherapy in patients with advanced cervical cancer: A virtual brachytherapy study. Rep. Pract. Oncol. Radiother. 2014, 19, 399–404.
- Chargari, C.; Deutsch, E.; Blanchard, P.; Gouy, S.; Martelli, H.; Guerin, F.; Dumas, I.; Bossi, A.; MOrice, P.; Viswanathan, A.; et al. Brachytherapy: An overview for clinicians. CA Cancer J. Clin. 2019, 69, 386–401.
- Sato, K.; Shimo, T.; Fuchikami, H.; Takeda, N.; Kato, M.; Okawa, T. Predicting adherence of dose-volume constraints for personalized partial-breast irradiation technique. Brachytherapy 2021, 20, 163–170.




