
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Αrgyro Papadopetraki | -- | 2629 | 2022-06-07 09:41:08 | | | |
| 2 | Lindsay Dong | -38 word(s) | 2591 | 2022-06-08 06:16:15 | | |
Video Upload Options
A growing body of in vitro and in vivo studies suggests that physical activity offers important benefits against cancer, in terms of both prevention and treatment. However, the exact mechanisms implicated in the anticancer effects of exercise remain to be further elucidated. Muscle-secreted factors in response to contraction have been proposed to mediate the physical exercise-induced beneficial effects and be responsible for the inter-tissue communications. Specifically, myokines and microRNAs (miRNAs) constitute the most studied components of the skeletal muscle secretome that appear to affect the malignancy, either directly by possessing antioncogenic properties, or indirectly by mobilizing the antitumor immune responses. Moreover, some of these factors are capable of mitigating serious, disease-associated adverse effects that deteriorate patients’ quality of life and prognosis.
1. Introduction
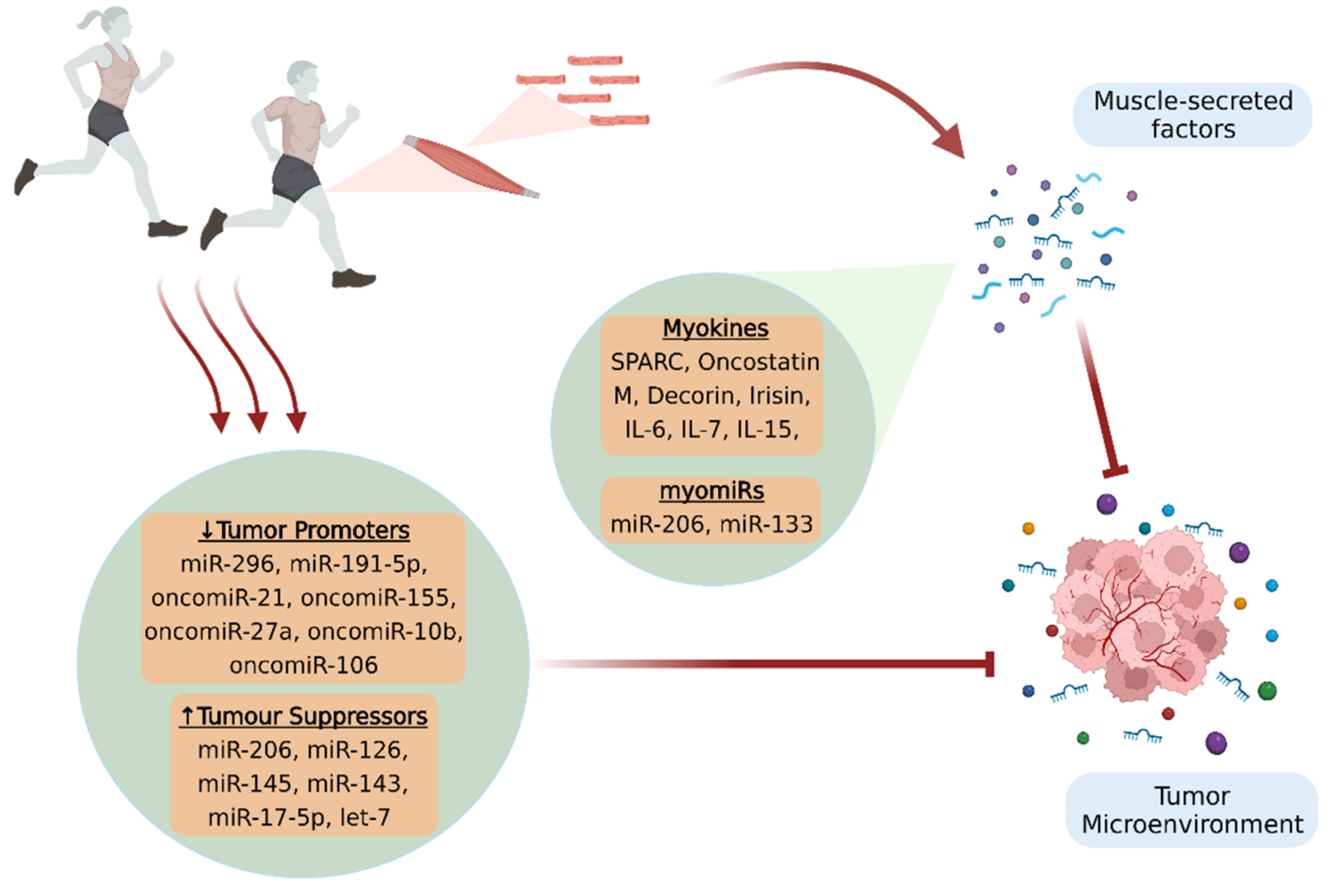
2. Myokines
2.1. Myokines and Cancer Progression
2.2. Myokines and Cancer-Associated Sarcopenia
3. Circulating microRNAs and MyomiRs
3.1. MyomiRs and Cancer Progression
3.2. MicroRNAs Regulated by Exercise
4. Intercellular Transport and Delivery of Muscle-Secreted Biomolecules: The Role of Exosomes
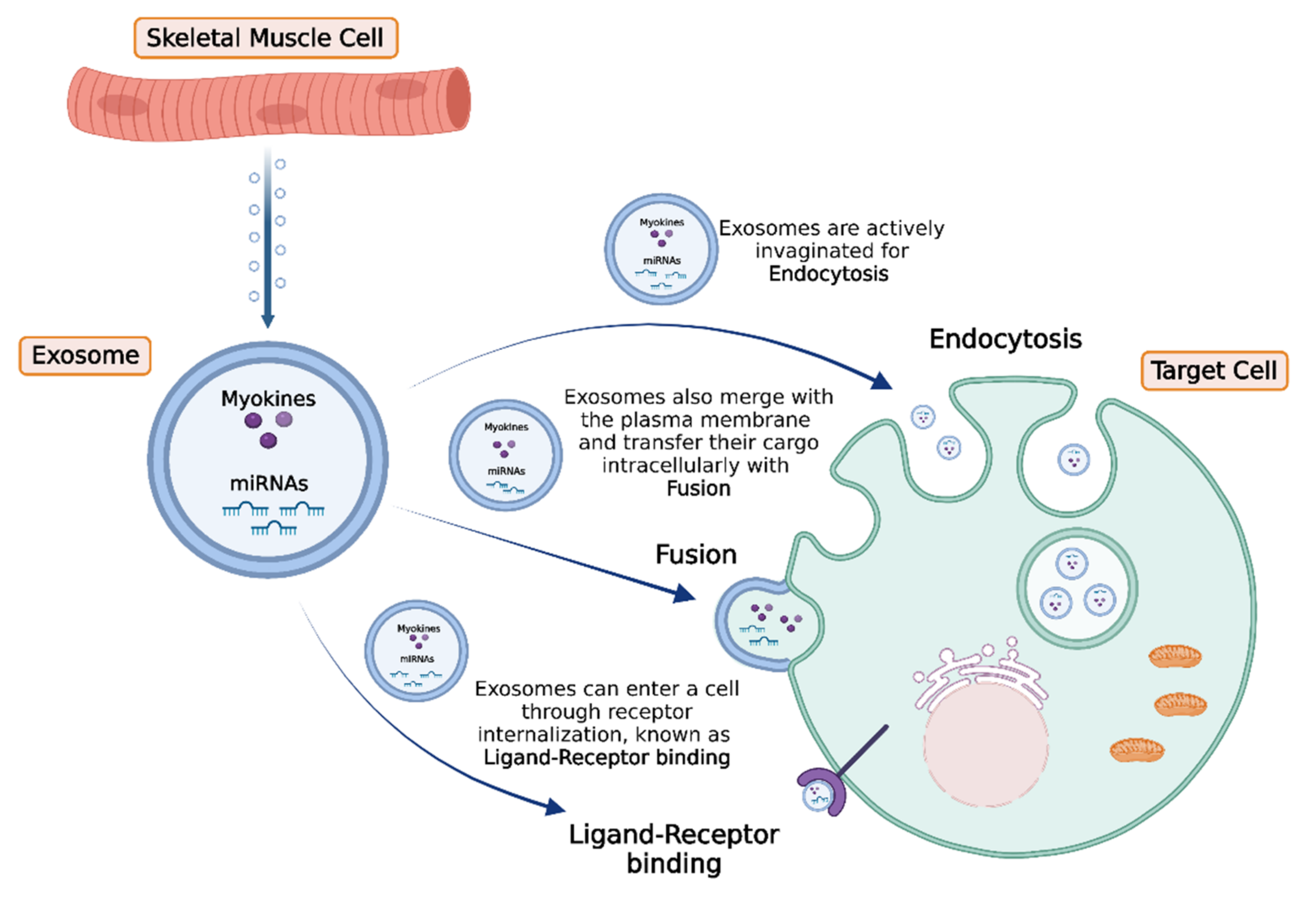
5. Conclusions and Future Perspectives
References
- Maridaki, M.; Papadopetraki, A.; Karagianni, H.; Koutsilieris, M.; Philippou, A. The Assessment and Relationship between Quality of Life and Physical Activity Levels in Greek Breast Cancer Female Patients under Chemotherapy. Sports 2020, 8, 32.
- Pedersen, B.K.; Saltin, B. Exercise as medicine-evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72.
- Hoffmann, C.; Weigert, C. Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations. Cold Spring Harb. Perspect. Med. 2017, 7, a029793.
- Soares-Miranda, L.; Lucia, A.; Silva, M.; Peixoto, A.; Ramalho, R.; da Silva, P.C.; Mota, J.; Macedo, G.; Abreu, S. Physical Fitness and Health-related Quality of Life in Patients with Colorectal Cancer. Int. J. Sports Med. 2021, 42, 924–929.
- Philippou, A.; Papadopetraki, A.; Maridaki, M.; Koutsilieris, M. Exercise as Complementary Therapy for Cancer Patients during and after Treatment. Sports Med. 2020, 1, 1–24.
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609.
- Orman, A.; Johnson, D.L.; Comander, A.; Brockton, N. Breast Cancer: A Lifestyle Medicine Approach. Am. J. Lifestyle Med. 2020, 14, 483–494.
- Koelwyn, G.J.; Quail, D.F.; Zhang, X.; White, R.M.; Jones, L.W. Exercise-dependent regulation of the tumour microenvironment. Nat. Rev. Cancer 2017, 17, 620–632.
- Hojman, P.; Dethlefsen, C.; Brandt, C.; Hansen, J.; Pedersen, L.; Pedersen, B.K. Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E504–E510.
- Adraskela, K.; Veisaki, E.; Koutsilieris, M.; Philippou, A. Physical Exercise Positively Influences Breast Cancer Evolution. Clin. Breast Cancer 2017, 17, 408–417.
- Ntanasis-Stathopoulos, J.; Tzanninis, J.G.; Philippou, A.; Koutsilieris, M. Epigenetic regulation on gene expression induced by physical exercise. J. Musculoskelet. Neuronal Interact. 2013, 13, 133–146.
- Seldin, M.M.; Wong, G.W. Regulation of tissue crosstalk by skeletal muscle-derived myonectin and other myokines. Adipocyte 2012, 1, 200–202.
- Yoshikawa, M.; Nakasa, T.; Ishikawa, M.; Adachi, N.; Ochi, M. Evaluation of autologous skeletal muscle-derived factors for regenerative medicine applications. Bone Jt. Res. 2017, 6, 277–283.
- Hong, B.S. Regulation of the Effect of Physical Activity Through MicroRNAs in Breast Cancer. Int. J. Sports Med. 2021.
- Durzynska, J.; Philippou, A.; Brisson, B.K.; Nguyen-McCarty, M.; Barton, E.R. The pro-forms of insulin-like growth factor I (IGF-I) are predominant in skeletal muscle and alter IGF-I receptor activation. Endocrinology 2013, 154, 1215–1224.
- Philippou, A.; Barton, E.R. Optimizing IGF-I for skeletal muscle therapeutics. Growth Horm. IGF Res. 2014, 24, 157–163.
- Bikle, D.D.; Tahimic, C.; Chang, W.; Wang, Y.; Philippou, A.; Barton, E.R. Role of IGF-I signaling in muscle bone interactions. Bone 2015, 80, 79–88.
- Pedersen, L.; Hojman, P. Muscle-to-organ cross talk mediated by myokines. Adipocyte 2012, 1, 164–167.
- Lightfoot, A.; Cooper, R.G. The role of myokines in muscle health and disease. Curr. Opin. Rheumatol. 2016, 28, 661–666.
- Schnyder, S.; Handschin, C. Skeletal muscle as an endocrine organ: PGC-1alpha, myokines and exercise. Bone 2015, 80, 115–125.
- Dalamaga, M. Interplay of adipokines and myokines in cancer pathophysiology: Emerging therapeutic implications. World J. Exp. Med. 2013, 3, 26–33.
- Buss, L.A.; Dachs, G.U. Effects of Exercise on the Tumour Microenvironment. Adv. Exp. Med. Biol. 2020, 1225, 31–51.
- Garneau, L.; Parsons, S.A.; Smith, S.R.; Mulvihill, E.E.; Sparks, L.M.; Aguer, C. Plasma Myokine Concentrations After Acute Exercise in Non-obese and Obese Sedentary Women. Front. Physiol. 2020, 11, 18.
- Bedore, J.; Leask, A.; Séguin, C.A. Targeting the extracellular matrix: Matricellular proteins regulate cell-extracellular matrix communication within distinct niches of the intervertebral disc. Matrix Biol. 2014, 37, 124–130.
- Liu, Y.-P.; Hsiao, M. Exercise-induced SPARC prevents tumorigenesis of colon cancer. Gut 2013, 62, 810–811.
- Aoi, W.; Naito, Y.; Takagi, T.; Tanimura, Y.; Takanami, Y.; Kawai, Y.; Sakuma, K.; Hang, L.P.; Mizushima, K.; Hirai, Y.; et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut 2013, 62, 882–889.
- Matsuo, K.; Sato, K.; Suemoto, K.; Miyamoto-Mikami, E.; Fuku, N.; Higashida, K.; Tsuji, K.; Xu, Y.; Liu, X.; Iemitsu, M.; et al. A Mechanism Underlying Preventive Effect of High-Intensity Training on Colon Cancer. Med. Sci. Sports Exerc. 2017, 49, 1805–1816.
- Akutsu, T.; Ito, E.; Narita, M.; Ohdaira, H.; Suzuki, Y.; Urashima, M. Effect of Serum SPARC Levels on Survival in Patients with Digestive Tract Cancer: A Post Hoc Analysis of the AMATERASU Randomized Clinical Trial. Cancers 2020, 12, 1465.
- Hermanns, H.M. Oncostatin M and interleukin-31: Cytokines, receptors, signal transduction and physiology. Cytokine Growth Factor Rev. 2015, 26, 545–558.
- Won Seok Hyung, W.; Gon Lee, S.; Tae Kim, K.; Soo Kim, H. Oncostatin M, a muscle-secreted myokine, recovers high-glucose-induced impairment of Akt phosphorylation by Fos induction in hippocampal neuron cells. Neuroreport 2019, 30, 765–770.
- Manzari Tavakoli, Z.; Amani Shalamzari, S.; Kazemi, A. Effects of 6 weeks’ Endurance Training on Oncostatin-M in Muscle and Tumor Tissues in mice with Breast Cancer. Iran. J. Breast Dis. 2017, 9, 50–59.
- Molanouri Shamsi, M.; Chekachak, S.; Soudi, S.; Gharakhanlou, R.; Quinn, L.S.; Ranjbar, K.; Rezaei, S.; Shirazi, F.J.; Allahmoradi, B.; Yazdi, M.H.; et al. Effects of exercise training and supplementation with selenium nanoparticle on T-helper 1 and 2 and cytokine levels in tumor tissue of mice bearing the 4 T1 mammary carcinoma. Nutrition 2019, 57, 141–147.
- Siff, T.; Parajuli, P.; Razzaque, M.S.; Atfi, A. Cancer-Mediated Muscle Cachexia: Etiology and Clinical Management. Trends. Endocrinol. Metab. 2021, 32, 382–402.
- Webster, J.M.; Kempen, L.; Hardy, R.S.; Langen, R.C.J. Inflammation and Skeletal Muscle Wasting During Cachexia. Front. Physiol. 2020, 11, 597675.
- Ciciliot, S.; Rossi, A.C.; Dyar, K.A.; Blaauw, B.; Schiaffino, S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2191–2199.
- Galvao, D.A.; Nosaka, K.; Taaffe, D.R.; Peake, J.; Spry, N.; Suzuki, K.; Yamaya, K.; McGuigan, M.R.; Kristjanson, L.J.; Newton, R.U. Endocrine and immune responses to resistance training in prostate cancer patients. Prostate Cancer Prostatic Dis. 2008, 11, 160–165.
- Daou, H.N. Exercise as an anti-inflammatory therapy for cancer cachexia: A focus on interleukin-6 regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R296–R310.
- Hoene, M.; Runge, H.; Haring, H.U.; Schleicher, E.D.; Weigert, C. Interleukin-6 promotes myogenic differentiation of mouse skeletal muscle cells: Role of the STAT3 pathway. Am. J. Physiol. Cell Physiol. 2013, 304, C128–C136.
- Cui, M.; Yao, X.; Lin, Y.; Zhang, D.; Cui, R.; Zhang, X. Interactive functions of microRNAs in the miR-23a-27a-24-2 cluster and the potential for targeted therapy in cancer. J. Cell Physiol. 2020, 235, 6–16.
- Tan, Z.; Jia, J.; Jiang, Y. MiR-150-3p targets SP1 and suppresses the growth of glioma cells. Biosci. Rep. 2018, 38, BSR20180019.
- Ling, H.; Fabbri, M.; Calin, G.A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 2013, 12, 847–865.
- Chen, H.; Gao, S.; Cheng, C. MiR-323a-3p suppressed the glycolysis of osteosarcoma via targeting LDHA. Hum. Cell 2018, 31, 300–309.
- Gareev, I.; Beylerli, O.; Yang, G.; Sun, J.; Pavlov, V.; Izmailov, A.; Shi, H.; Zhao, S. The current state of MiRNAs as biomarkers and therapeutic tools. Clin. Exp. Med. 2020, 20, 349–359.
- Dufresne, S.; Rebillard, A.; Muti, P.; Friedenreich, C.M.; Brenner, D.R. A Review of Physical Activity and Circulating miRNA Expression: Implications in Cancer Risk and Progression. Cancer Epidemiol. Biomarkers Prev. 2018, 27, 11–24.
- Kong, S.; Fang, Y.; Wang, B.; Cao, Y.; He, R.; Zhao, Z. miR-152-5p suppresses glioma progression and tumorigenesis and potentiates temozolomide sensitivity by targeting FBXL7. J. Cell Mol. Med. 2020, 24, 4569–4579.
- Khodadadi-Jamayran, A.; Akgol-Oksuz, B.; Afanasyeva, Y.; Heguy, A.; Thompson, M.; Ray, K.; Giro-Perafita, A.; Sanchez, I.; Wu, X.; Tripathy, D.; et al. Prognostic role of elevated mir-24-3p in breast cancer and its association with the metastatic process. Oncotarget 2018, 9, 12868–12878.
- Yang, Y.; Song, S.; Meng, Q.; Wang, L.; Li, X.; Xie, S.; Chen, Y.; Jiang, X.; Wang, C.; Lu, Y.; et al. miR24-2 accelerates progression of liver cancer cells by activating Pim1 through tri-methylation of Histone H3 on the ninth lysine. J. Cell Mol. Med. 2020, 24, 2772–2790.
- Organista-Nava, J.; Gomez-Gomez, Y.; Illades-Aguiar, B.; del Carmen Alarcon-Romero, L.; Saavedra-Herrera, M.V.; Rivera-Ramirez, A.B.; Garzon-Barrientos, V.H.; Leyva-Vazquez, M.A. High miR-24 expression is associated with risk of relapse and poor survival in acute leukemia. Oncol. Rep. 2015, 33, 1639–1649.
- Yan, L.; Ma, J.; Zhu, Y.; Zan, J.; Wang, Z.; Ling, L.; Li, Q.; Lv, J.; Qi, S.; Cao, Y.; et al. miR-24-3p promotes cell migration and proliferation in lung cancer by targeting SOX7. J. Cell Biochem. 2018, 119, 3989–3998.
- Siracusa, J.; Koulmann, N.; Banzet, S. Circulating myomiRs: A new class of biomarkers to monitor skeletal muscle in physiology and medicine. J. Cachexia Sarcopenia Muscle 2018, 9, 20–27.
- Li, D.; Xia, L.; Chen, M.; Lin, C.; Wu, H.; Zhang, Y.; Pan, S.; Li, X. miR-133b, a particular member of myomiRs, coming into playing its unique pathological role in human cancer. Oncotarget 2017, 8, 50193–50208.
- Liu, Y.; Han, L.; Bai, Y.; Du, W.; Yang, B. Down-regulation of MicroRNA-133 predicts poor overall survival and regulates the growth and invasive abilities in glioma. Artif. Cells Nanomed. Biotechnol. 2018, 46, 206–210.
- Chen, X.B.; Li, W.; Chu, A.X. MicroRNA-133a inhibits gastric cancer cells growth, migration, and epithelial-mesenchymal transition process by targeting presenilin 1. J. Cell. Biochem. 2019, 120, 470–480.
- Guo, J.; Xia, B.; Meng, F.; Lou, G. miR-133a suppresses ovarian cancer cell proliferation by directly targeting insulin-like growth factor 1 receptor. Tumour. Biol. 2014, 35, 1557–1564.
- Wang, D.S.; Zhang, H.Q.; Zhang, B.; Yuan, Z.B.; Yu, Z.K.; Yang, T.; Zhang, S.Q.; Liu, Y.; Jia, X.X. miR-133 inhibits pituitary tumor cell migration and invasion via down-regulating FOXC1 expression. Genet. Mol. Res. 2016, 15, gmr.15017453.
- Li, F.; Bai, M.; Xu, J.; Zhu, L.; Liu, C.; Duan, R. Long-Term Exercise Alters the Profiles of Circulating Micro-RNAs in the Plasma of Young Women. Front. Physiol. 2020, 11, 372.
- Gong, Y.; Ren, J.; Liu, K.; Tang, L.M. Tumor suppressor role of miR-133a in gastric cancer by repressing IGF1R. World J. Gastroenterol. 2015, 21, 2949–2958.
- Xu, F.; Li, F.; Zhang, W.; Jia, P. Growth of glioblastoma is inhibited by miR-133-mediated EGFR suppression. Tumour. Biol. 2015, 36, 9553–9558.
- Liu, S.; Chen, J.; Zhang, T.; Chen, H. MicroRNA-133 inhibits the growth and metastasis of the human lung cancer cells by targeting epidermal growth factor receptor. J. Buon 2019, 24, 929–935.
- Pulliero, A.; You, M.; Chaluvally-Raghavan, P.; Marengo, B.; Domenicotti, C.; Banelli, B.; Degan, P.; Molfetta, L.; Gianiorio, F.; Izzotti, A. Anticancer effect of physical activity is mediated by modulation of extracellular microRNA in blood. Oncotarget 2020, 11, 2106–2119.
- Rahimi, M.; Sharifi-Zarchi, A.; Zarghami, N.; Geranpayeh, L.; Ebrahimi, M.; Alizadeh, E. Down-Regulation of miR-200c and Up-Regulation of miR-30c Target both Stemness and Metastasis Genes in Breast Cancer. Cell J. 2020, 21, 467–478.
- Yen, M.C.; Shih, Y.C.; Hsu, Y.L.; Lin, E.S.; Lin, Y.S.; Tsai, E.M.; Ho, Y.W.; Hou, M.F.; Kuo, P.L. Isolinderalactone enhances the inhibition of SOCS3 on STAT3 activity by decreasing miR-30c in breast cancer. Oncol. Rep. 2016, 35, 1356–1364.
- Nasiri, M.; Peeri, M.; Matinhomaei, H. Endurance Training Attenuates Angiogenesis Following Breast Cancer by Regulation of MiR-126 and MiR-296 in Breast Cancer Bearing Mice. Int. J. Cancer Manag. 2017, in press.
- Yang, N.J.; Hinner, M.J. Getting across the cell membrane: An overview for small molecules, peptides, and proteins. Methods Mol. Biol. 2015, 1266, 29–53.
- Barlowe, C.; Helenius, A. Cargo Capture and Bulk Flow in the Early Secretory Pathway. Annu. Rev. Cell Dev. Biol. 2016, 32, 197–222.
- Trovato, E.; di Felice, V.; Barone, R. Extracellular Vesicles: Delivery Vehicles of Myokines. Front. Physiol. 2019, 10, 522.
- Manou, D.; Caon, I.; Bouris, P.; Triantaphyllidou, I.E.; Giaroni, C.; Passi, A.; Karamanos, N.K.; Vigetti, D.; Theocharis, A.D. The Complex Interplay Between Extracellular Matrix and Cells in Tissues. Methods Mol. Biol. 2019, 1952, 1–20.
- Yue, B.; Yang, H.; Wang, J.; Ru, W.; Wu, J.; Huang, Y.; Lan, X.; Lei, C.; Chen, H. Exosome biogenesis, secretion and function of exosomal miRNAs in skeletal muscle myogenesis. Cell Prolif. 2020, 53, e12857.
- Whitham, M.; Febbraio, M.A. The ever-expanding myokinome: Discovery challenges and therapeutic implications. Nat. Rev. Drug Discov. 2016, 15, 719–729.
- Bei, Y.; Xu, T.; Lv, D.; Yu, P.; Xu, J.; Che, L.; Das, A.; Tigges, J.; Toxavidis, V.; Ghiran, I.; et al. Exercise-induced circulating extracellular vesicles protect against cardiac ischemia-reperfusion injury. Basic Res. Cardiol. 2017, 112, 38.
- Romancino, D.P.; Paterniti, G.; Campos, Y.; de Luca, A.; di Felice, V.; d’Azzo, A.; Bongiovanni, A. Identification and characterization of the nano-sized vesicles released by muscle cells. FEBS Lett. 2013, 587, 1379–1384.
- Moustogiannis, A.; Philippou, A.; Zevolis, E.; Taso, O.; Chatzigeorgiou, A.; Koutsilieris, M. Characterization of Optimal Strain, Frequency and Duration of Mechanical Loading on Skeletal Myotubes’ Biological Responses. Vivo 2020, 34, 1779–1788.




