
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shuen-Iu Hung | -- | 1985 | 2022-05-31 11:15:20 | | | |
| 2 | Vivi Li | -1 word(s) | 1984 | 2022-06-01 04:52:23 | | |
Video Upload Options
As the world deals with the COVID-19 pandemic, vaccination remains vital to successfully end this crisis. However, COVID-19-vaccine-induced immediate hypersensitivity reactions presenting with potentially life-threatening systemic anaphylactic reactions are one of the reasons for vaccine hesitancy. Studies have suggested that different mechanisms, including IgE-mediated and non-IgE-mediated mast cell activation, may be involved in immediate hypersensitivity. The main culprits triggering hypersensitivity reactions have been suggested to be the excipients of vaccines, including polyethylene glycol and polysorbate 80. Patients with a history of allergic reactions to drugs, foods, or other vaccines may have an increased risk of hypersensitivity reactions to COVID-19 vaccines. Various strategies have been suggested to prevent hypersensitivity reactions, including performing skin tests or in vitro tests before vaccination, administering different vaccines for the primary and following boosters, changing the fractionated doses, or pretreating the anti-IgE antibody.
1. Introduction
2. Clinical Phenotypes of Vaccine-Induced Immediate Hypersensitivity Reactions
3. Epidemiology of Immediate Hypersensitivity Induced by Vaccines
| Type of Reaction | Number of Participants | Number of Anaphylactic Reactions | Type of Vaccine | Incidence of Reactions (per One Million) | Reference |
|---|---|---|---|---|---|
| anaphylactic | |||||
| 890,604 | 15 | mRNA-1273; BNT162b2 | 17 | [35] | |
| 4,041,396 | 10 | mRNA-1273 | 37.1 | [29] | |
| 1,893,360 | 21 | BNT162b2 | 11 | [36] | |
| 1116 | 1 | BNT162b2; mRNA-1273 | 890 | [37] | |
| 283 | 5 | mRNA-1273 and AZD1222 | 17,668 | [38] | |
| nonanaphylactic | |||||
| 277 | 14 | BNT162b2 | 50,540 | [39] | |
| 5589 | 1391 | AZD1222 (Astra Zeneca) |
248,880 | [39] | |
| 5574 | 6 | BNT162b2 | 1070 | [40] | |
| 3170 | 11 | BNT162b2 | 3470 | * [41] | |
| 1,893,360 | 83 | BNT162b2 | 43.8 | * [36] | |
| 877 | 10 | BNT162b2 | 11,400 | [42] | |
| 1116 | 7 | BNT162b2; mRNA-1273 | 6270 | [37] | |
| 74 | 35 | BNT162b2 | 472,973 | [23] |
4. Causality of Vaccine-Induced Immediate Hypersensitivity Reactions
5. Proposed Immune Mechanisms for Vaccine-Induced Immediate Hypersensitivity Reactions
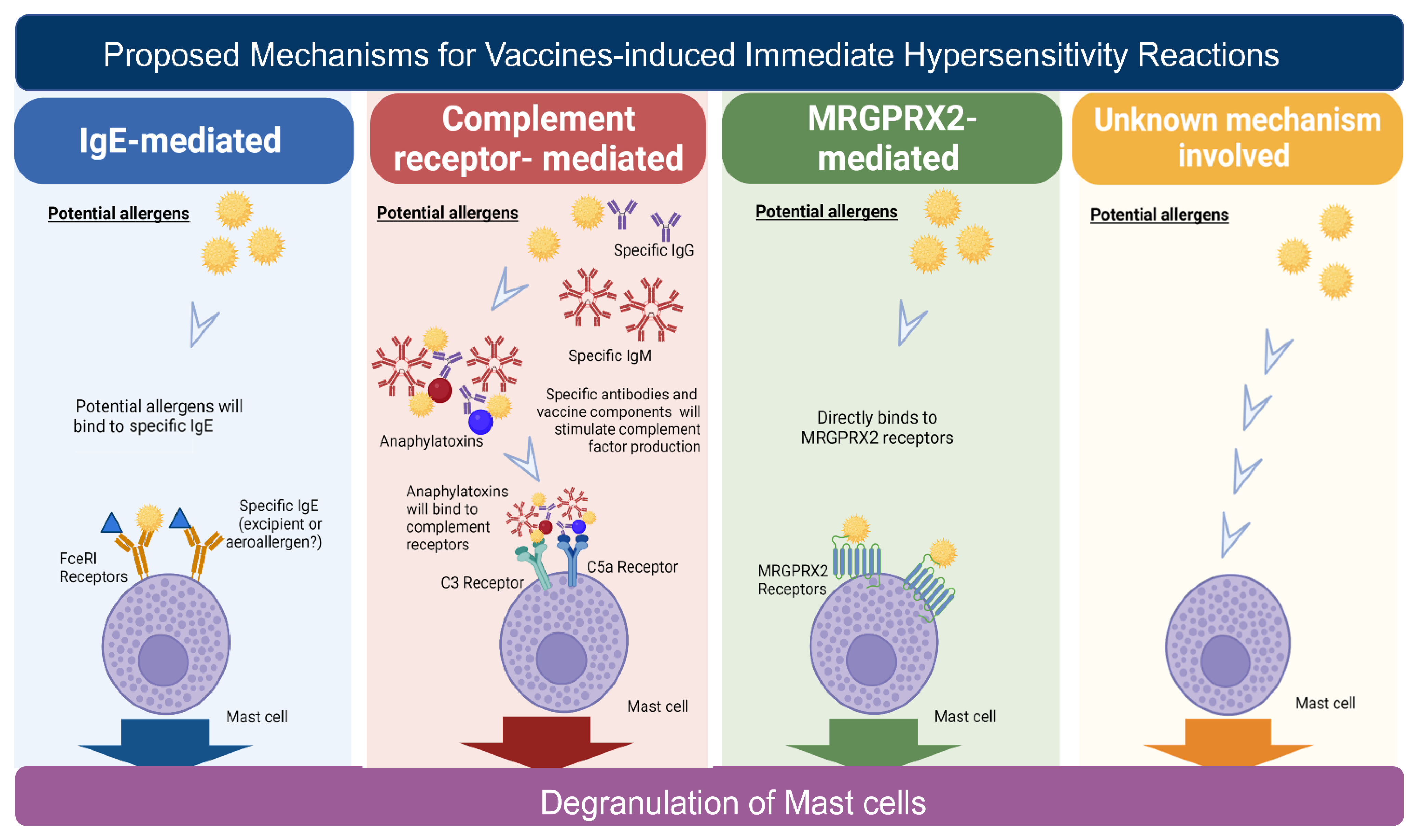
References
- Etienne, C.F. COVID-19 has revealed a pandemic of inequality. Nat. Med. 2022, 28, 17.
- Graham, F. Daily briefing: COVID-19 vaccine development—Where we are now. Nature 2020. Online ahead of print.
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. Publisher correction: COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2021, 590, E17.
- Arunachalam, P.S.; Walls, A.C.; Golden, N.; Atyeo, C.; Fischinger, S.; Li, C.; Aye, P.; Navarro, M.J.; Lai, L.; Edara, V.V.; et al. Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature 2021, 594, 253–258.
- Heitmann, J.S.; Bilich, T.; Tandler, C.; Nelde, A.; Maringer, Y.; Marconato, M.; Reusch, J.; Jager, S.; Denk, M.; Richter, M.; et al. A COVID-19 peptide vaccine for the induction of SARS-CoV-2 T cell immunity. Nature 2022, 601, 617–622.
- Cui, X.; Wang, P.; Wei, Z. Emergency use of COVID-19 vaccines recommended by the World Health Organization (WHO) as of June 2021. Drug Discov. Ther. 2021, 15, 222–224.
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416.
- Ali, K.; Berman, G.; Zhou, H.; Deng, W.; Faughnan, V.; Coronado-Voges, M.; Ding, B.; Dooley, J.; Girard, B.; Hillebrand, W.; et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N. Engl. J. Med. 2021, 385, 2241–2251.
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Munoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 COVID-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 35–46.
- Mascellino, M.T.; Di Timoteo, F.; De Angelis, M.; Oliva, A. Overview of the main anti-SARS-CoV-2 vaccines: Mechanism of action, efficacy and safety. Infect. Drug Resist. 2021, 14, 3459–3476.
- Storlie, C.B.; Pollock, B.D.; Rojas, R.L.; Demuth, G.O.; Johnson, P.W.; Wilson, P.M.; Heinzen, E.P.; Liu, H.; Carter, R.E.; Habermann, E.B.; et al. Quantifying the importance of COVID-19 vaccination to our future outlook. Mayo. Clin. Proc. 2021, 96, 1890–1895.
- Abrams, E.M.; Shaker, M.; Sinha, I.; Greenhawt, M. COVID-19 vaccines: Addressing hesitancy in young people with allergies. Lancet. Respir. Med. 2021, 9, 1090–1092.
- Digregorio, M.; Van Ngoc, P.; Delogne, S.; Meyers, E.; Deschepper, E.; Duysburgh, E.; De Rop, L.; De Burghgraeve, T.; Coen, A.; De Clercq, N.; et al. Vaccine hesitancy towards the COVID-19 vaccine in a random national sample of belgian nursing home staff members. Vaccines 2022, 10, 598.
- Nilsson, L.; Brockow, K.; Alm, J.; Cardona, V.; Caubet, J.C.; Gomes, E.; Jenmalm, M.C.; Lau, S.; Netterlid, E.; Schwarze, J.; et al. Vaccination and allergy: EAACI position paper, practical aspects. Pediatr. Allergy Immunol. 2017, 28, 628–640.
- Castells, M.C.; Phillips, E.J. Maintaining safety with SARS-CoV-2 vaccines. N. Engl. J. Med. 2021, 384, 643–649.
- Shimabukuro, T.; Nair, N. Allergic reactions including anaphylaxis after receipt of the first dose of pfizer-BioNTech COVID-19 vaccine. JAMA 2021, 325, 780–781.
- Dreskin, S.C.; Halsey, N.A.; Kelso, J.M.; Wood, R.A.; Hummell, D.S.; Edwards, K.M.; Caubet, J.C.; Engler, R.J.; Gold, M.S.; Ponvert, C.; et al. International consensus (ICON): Allergic reactions to vaccines. World Allergy Organ. J. 2016, 9, 32.
- Zuberbier, T.; Abdul Latiff, A.H.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J.A.; et al. The international EAACI/GA(2)LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy 2021, 77, 734–766.
- Cheng, D.R.; Perrett, K.P.; Choo, S.; Danchin, M.; Buttery, J.P.; Crawford, N.W. Pediatric anaphylactic adverse events following immunization in Victoria, Australia from 2007 to 2013. Vaccine 2015, 33, 1602–1607.
- Poziomkowska-Gesicka, I.; Kurek, M. Clinical manifestations and causes of anaphylaxis. analysis of 382 cases from the anaphylaxis registry in west Pomerania Province in Poland. Int. J. Environ. Res. Public Health 2020, 17, 109.
- Alhumaid, S.; Al Mutair, A.; Al Alawi, Z.; Rabaan, A.A.; Tirupathi, R.; Alomari, M.A.; Alshakhes, A.S.; Alshawi, A.M.; Ahmed, G.Y.; Almusabeh, H.M.; et al. Anaphylactic and nonanaphylactic reactions to SARS-CoV-2 vaccines: A systematic review and meta-analysis. Allergy Asthma Clin. Immunol. 2021, 17, 109.
- Cabanillas, B.; Novak, N. Allergy to COVID-19 vaccines: A current update. Allergol. Int. 2021, 70, 313–318.
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021, 85, 46–55.
- Kohl, K.S.; Bonhoeffer, J.; Braun, M.M.; Chen, R.T.; Duclos, P.; Heijbel, H.; Heininger, U.; Loupi, E.; Marcy, S.M. The brighton collaboration: Creating a global standard for case definitions (and guidelines) for adverse events following immunization. In Advances in Patient Safety: From Research to Implementation; Henriksen, K., Battles, J.B., Marks, E.S., Lewin, D.I., Eds.; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2005; Volume 2.
- Laisuan, W. COVID-19 vaccine anaphylaxis: Current evidence and future approaches. Front Allergy 2021, 2, 801322.
- McNeil, M.M.; Weintraub, E.S.; Duffy, J.; Sukumaran, L.; Jacobsen, S.J.; Klein, N.P.; Hambidge, S.J.; Lee, G.M.; Jackson, L.A.; Irving, S.A.; et al. Risk of anaphylaxis after vaccination in children and adults. J. Allergy Clin. Immunol. 2016, 137, 868–878.
- Micheletti, F.; Peroni, D.; Piacentini, G.; Schweiger, V.; Mirandola, R.; Chiesa, E.; Zanoni, G. Vaccine allergy evaluation and management at the specialized green channel consultation clinic. Clin. Exp. Allergy 2012, 42, 1088–1096.
- Robinson, L.B.; Fu, X.; Hashimoto, D.; Wickner, P.; Shenoy, E.S.; Landman, A.B.; Blumenthal, K.G. Incidence of cutaneous reactions after messenger RNA COVID-19 vaccines. JAMA Dermatol 2021, 157, 1000–1002.
- Shimabukuro, T. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine—United States, December 21, 2020-January 10, 2021. Am. J. Transplant. 2021, 21, 1326–1331.
- Shimabukuro, T. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14–23, 2020. Am. J. Transplant. 2021, 21, 1332–1337.
- Sampath, V.; Rabinowitz, G.; Shah, M.; Jain, S.; Diamant, Z.; Jesenak, M.; Rabin, R.; Vieths, S.; Agache, I.; Akdis, M.; et al. Vaccines and allergic reactions: The past, the current COVID-19 pandemic, and future perspectives. Allergy 2021, 76, 1640–1660.
- Pool, V.; Braun, M.M.; Kelso, J.M.; Mootrey, G.; Chen, R.T.; Yunginger, J.W.; Jacobson, R.M.; Gargiullo, P.M.; VAERS Team. Prevalence of anti-gelatin IgE antibodies in people with anaphylaxis after measles-mumps rubella vaccine in the United States. Pediatrics 2002, 110, e71.
- Chu, D.K.; Abrams, E.M.; Golden, D.B.K.; Blumenthal, K.G.; Wolfson, A.R.; Stone, C.A., Jr.; Krantz, M.S.; Shaker, M.; Greenhawt, M. Risk of second allergic reaction to SARS-CoV-2 vaccines: A Systematic review and meta-analysis. JAMA Intern. Med. 2022, 182, 376–385.
- Greenhawt, M.; Abrams, E.M.; Shaker, M.; Chu, D.K.; Khan, D.; Akin, C.; Alqurashi, W.; Arkwright, P.; Baldwin, J.L.; Ben-Shoshan, M.; et al. The Risk of Allergic Reaction to SARS-CoV-2 Vaccines and recommended evaluation and management: A systematic review, meta-analysis, GRADE assessment, and international consensus approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 3546–3567.
- Ontario Agency for Health Protection and Promotion (Public Health Ontario). Reports of Events Managed as Anaphylaxis following COVID-19 Vaccines in Ontario: December 13, 2020 to March 6, 2021. Toronto, ON: Queen’s Printer for Ontario. 2021. Available online: https://www.publichealthontario.ca/-/media/documents/ncov/epi/covid-19-anaphylaxis-epi-summary.pdf?la=en (accessed on 16 March 2022).
- COVID; CDC; Response Team. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 Vaccine—United States, December 14–23, 2020. Morb. Mortal. Wkly. Rep. 2021, 70, 46–51.
- Kadali, R.A.K.; Janagama, R.; Peruru, S.; Gajula, V.; Madathala, R.R.; Chennaiahgari, N.; Malayala, S.V. Non-life-threatening adverse effects with COVID-19 mRNA-1273 vaccine: A randomized, cross-sectional study on healthcare workers with detailed self-reported symptoms. J. Med. Virol. 2021, 93, 4420–4429.
- Mathioudakis, A.G.; Ghrew, M.; Ustianowski, A.; Ahmad, S.; Borrow, R.; Papavasileiou, L.P.; Petrakis, D.; Bakerly, N.D. Self-reported real-world safety and reactogenicity of COVID-19 vaccines: A vaccine recipient survey. Life 2021, 11, 249.
- Bae, S.; Lee, Y.W.; Lim, S.Y.; Lee, J.H.; Lim, J.S.; Lee, S.; Park, S.; Kim, S.K.; Lim, Y.J.; Kim, E.O.; et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J. Korean Med. Sci. 2021, 36, e115.
- Bianchi, L.; Biondi, F.; Hansel, K.; Murgia, N.; Tramontana, M.; Stingeni, L. Skin tests in urticaria/angioedema and flushing to Pfizer-BioNTech SARS-CoV-2 vaccine: LIMITS of intradermal testing. Allergy 2021, 76, 2605–2607.
- Corbeddu, M.; Diociaiuti, A.; Vinci, M.R.; Santoro, A.; Camisa, V.; Zaffina, S.; El Hachem, M. Transient cutaneous manifestations after administration of Pfizer-BioNTech COVID-19 Vaccine: An Italian single-centre case series. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e483–e485.
- Riad, A.; Pokorna, A.; Attia, S.; Klugarova, J.; Koscik, M.; Klugar, M. Prevalence of COVID-19 vaccine side effects among healthcare workers in the Czech Republic. J. Clin. Med. 2021, 10, 1428.
- Dunkle, L.M.; Kotloff, K.L.; Gay, C.L.; Anez, G.; Adelglass, J.M.; Barrat Hernandez, A.Q.; Harper, W.L.; Duncanson, D.M.; McArthur, M.A.; Florescu, D.F.; et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N. Engl. J. Med. 2022, 386, 531–543.
- Stuart, A.S.V.; Shaw, R.H.; Liu, X.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): A single-blind, randomised, phase 2, non-inferiority trial. Lancet 2022, 399, 36–49.
- Han, B.; Song, Y.; Li, C.; Yang, W.; Ma, Q.; Jiang, Z.; Li, M.; Lian, X.; Jiao, W.; Wang, L.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: A double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 1645–1653.
- Blumenthal, K.G.; Robinson, L.B.; Camargo, C.A., Jr.; Shenoy, E.S.; Banerji, A.; Landman, A.B.; Wickner, P. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA 2021, 325, 1562–1565.
- Amanzio, M.; Mitsikostas, D.D.; Giovannelli, F.; Bartoli, M.; Cipriani, G.E.; Brown, W.A. Adverse events of active and placebo groups in SARS-CoV-2 vaccine randomized trials: A systematic review. Lancet Reg. Health Eur. 2022, 12, 100253.
- Caubet, J.C.; Ponvert, C. Vaccine allergy. Immunol. Allergy Clin. North. Am. 2014, 34, 597–613.
- McNeil, M.M.; DeStefano, F. Vaccine-associated hypersensitivity. J. Allergy. Clin. Immunol. 2018, 141, 463–472.
- Kelso, J.M.; Greenhawt, M.J.; Li, J.T.; Nicklas, R.A.; Bernstein, D.I.; Blessing-Moore, J.; Cox, L.; Khan, D.; Lang, D.M.; Oppenheimer, J.; et al. Adverse reactions to vaccines practice parameter 2012 update. J. Allergy Clin. Immunol. 2012, 130, 25–43.
- Leventhal, J.S.; Berger, E.M.; Brauer, J.A.; Cohen, D.E. Hypersensitivity reactions to vaccine constituents: A case series and review of the literature. Dermatitis 2012, 23, 102–109.
- Kounis, N.G.; Koniari, I.; de Gregorio, C.; Velissaris, D.; Petalas, K.; Brinia, A.; Assimakopoulos, S.F.; Gogos, C.; Kouni, S.N.; Kounis, G.N.; et al. Allergic reactions to current available COVID-19 vaccinations: Pathophysiology, causality, and therapeutic considerations. Vaccines 2021, 9, 221.
- Nakayama, T.; Kumagai, T. Gelatin allergy. Pediatrics 2004, 113, 170–171.
- Shah, R.R.; Hassett, K.J.; Brito, L.A. Overview of vaccine adjuvants: Introduction, history, and current status. Methods Mol. Biol. 2017, 1494, 1–13.
- Kutlu, A.; Ucar, R.; Aydin, E.; Arslan, S.; Caliskaner, A.Z. Could aluminum be a new hidden allergen in type 1 hypersensitivity reactions when used as a drug additive? Postepy Dermatol. Alergol. 2016, 33, 243–245.
- Rouleau, I.; De Serres, G.; Drolet, J.P.; Skowronski, D.M.; Ouakki, M.; Toth, E.; Landry, M.; Menard, S.; Gagnon, R. Increased risk of anaphylaxis following administration of 2009 AS03-adjuvanted monovalent pandemic A/H1N1 (H1N1pdm09) vaccine. Vaccine 2013, 31, 5989–5996.
- Rouleau, I.; De Serres, G.; Skowronski, D.M.; Drolet, J.P.; Lemire, C.; Toth, E.; Landry, M. Risk factors associated with anaphylaxis and other allergic-like events following receipt of 2009 monovalent AS03-adjuvanted pandemic influenza vaccine in Quebec, Canada. Vaccine 2014, 32, 3480–3487.
- Justiz Vaillant, A.A.; Vashisht, R.; Zito, P.M. Immediate hypersensitivity reactions. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454.
- Kneilling, M.; Rocken, M. Mast cells: Novel clinical perspectives from recent insights. Exp. Dermatol. 2009, 18, 488–496.
- Elieh Ali Komi, D.; Shafaghat, F.; Kovanen, P.T.; Meri, S. Mast cells and complement system: Ancient interactions between components of innate immunity. Allergy 2020, 75, 2818–2828.
- Nguyen, S.M.T.; Rupprecht, C.P.; Haque, A.; Pattanaik, D.; Yusin, J.; Krishnaswamy, G. Mechanisms governing anaphylaxis: Inflammatory cells, mediators, endothelial gap junctions and beyond. Int. J. Mol. Sci. 2021, 22, 7785.
- Kumar, M.; Duraisamy, K.; Chow, B.K. Unlocking the non-IgE-mediated pseudo-allergic reaction puzzle with mas-related g-protein coupled receptor member X2 (MRGPRX2). Cells 2021, 10, 1033.
- Porebski, G.; Kwiecien, K.; Pawica, M.; Kwitniewski, M. Mas-related G protein-coupled receptor-X2 (MRGPRX2) in drug hypersensitivity reactions. Front. Immunol. 2018, 9, 3027.
- Caballero, M.L.; Krantz, M.S.; Quirce, S.; Phillips, E.J.; Stone, C.A., Jr. Hidden dangers: Recognizing excipients as potential causes of drug and vaccine hypersensitivity reactions. J. Allergy Clin. Immunol. Pract. 2021, 9, 2968–2982.
- Kelso, J.M. Potential food allergens in medications. J. Allergy. Clin. Immunol. 2014, 133, 1509–1518.
- Ponvert, C.; Ardelean-Jaby, D.; Colin-Gorski, A.M.; Soufflet, B.; Hamberger, C.; de Blic, J.; Scheinmann, P. Anaphylaxis to the 23-valent pneumococcal vaccine in child: A case-control study based on immediate responses in skin tests and specific IgE determination. Vaccine 2001, 19, 4588–4591.
- Ponvert, C.; Scheinmann, P.; de Blic, J. Anaphylaxis to the 23-valent pneumococcal vaccine: A second explored case by means of immediate-reading skin tests with pneumococcal vaccines. Vaccine 2010, 28, 8256–8257.





